Efficacy of Polymer-Based Nanomedicine for the Treatment of Brain Cancer
Abstract
1. Introduction
2. Brain Targeting
2.1. Transport Mechanisms
2.1.1. Pathways of Nasal Transportation to the Brain
2.1.2. Olfactory Neuronal Pathways
2.1.3. Trigeminal Nerve Pathways
2.1.4. Cerebrospinal Fluid (CSF) Pathways
3. Drug Delivery to the Brain
3.1. Nanodelivery Platforms Developed for Drug Delivery
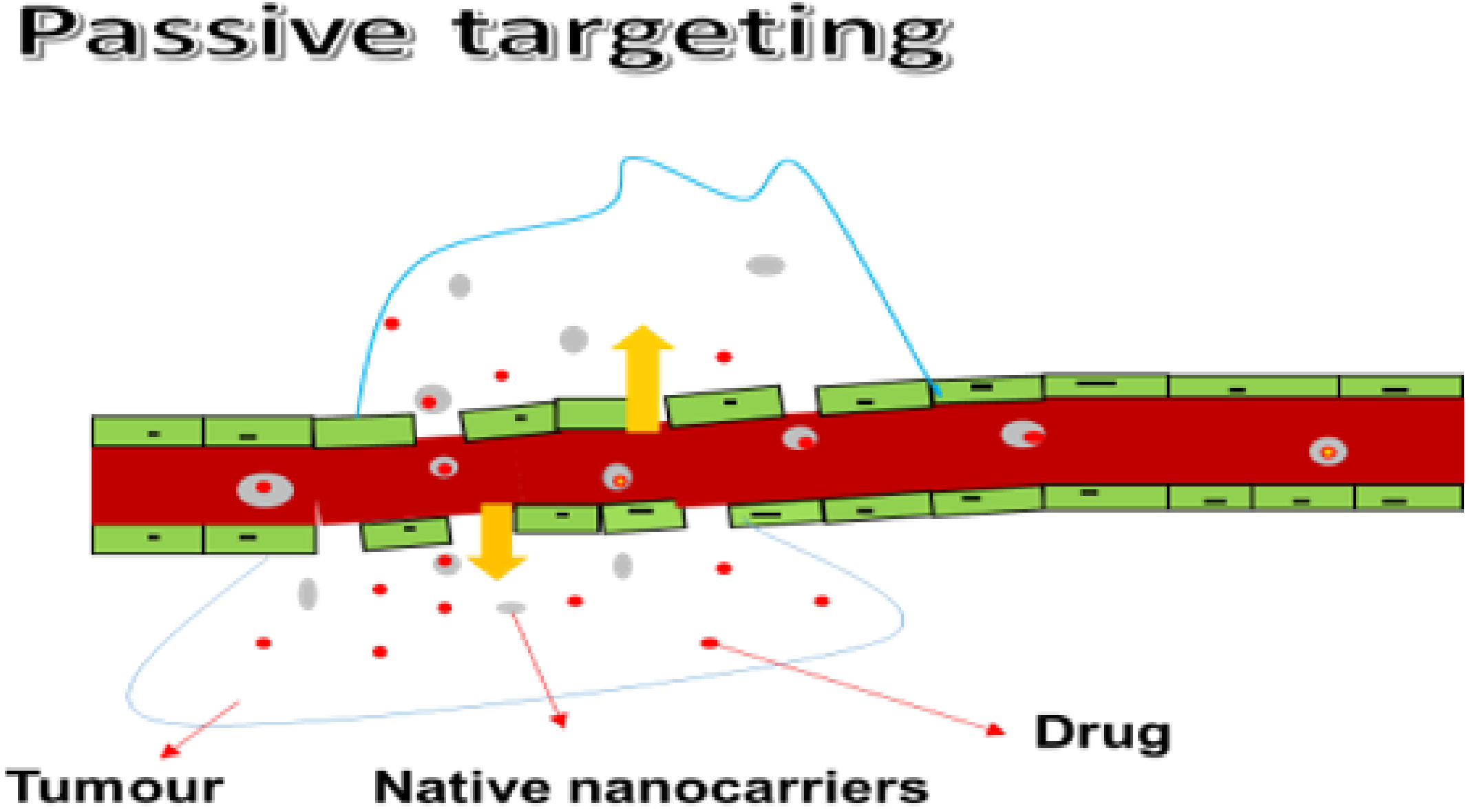
3.1.1. Passive Targeting
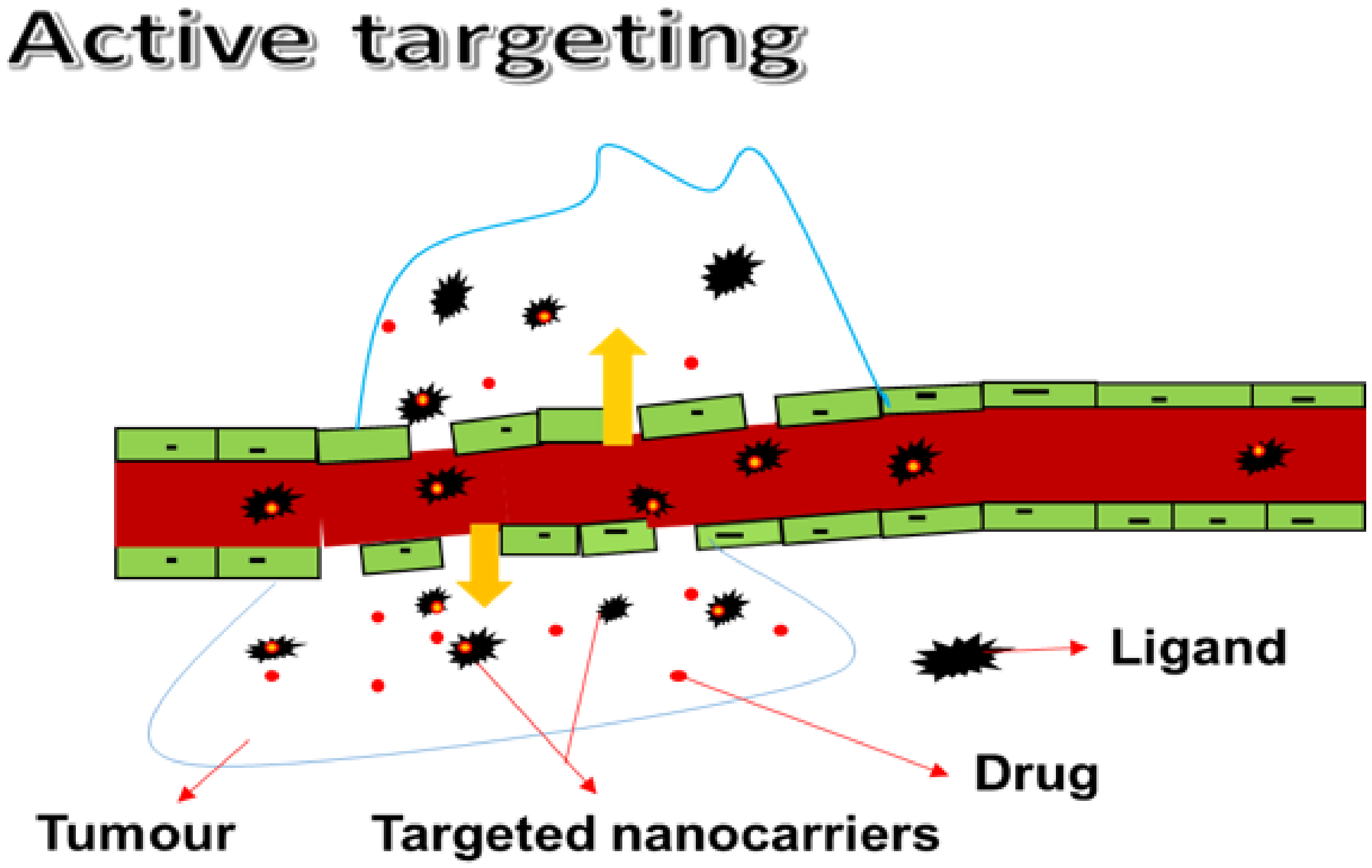
3.1.2. Active Targeting
3.1.3. Mechanisms of Efflux in Drug Transport to the Brain
4. Mode of Transportation across the Brain
4.1. Suggested Mechanisms of Transport through the Blood–Brain Barrier
4.1.1. Paracellular Transport
4.1.2. Transcellular Diffusion
5. The Different Nanomedicines Designed for Brain Cancer Therapy
5.1. Polymer-Based Nanoparticles
5.2. Nanoliposomes
5.3. Polymer–Drug Conjugates
5.4. Solid Lipid Nanoparticles
5.5. Nanostructured Lipid Carriers (NLCs)
5.6. Thermosensitive Gel
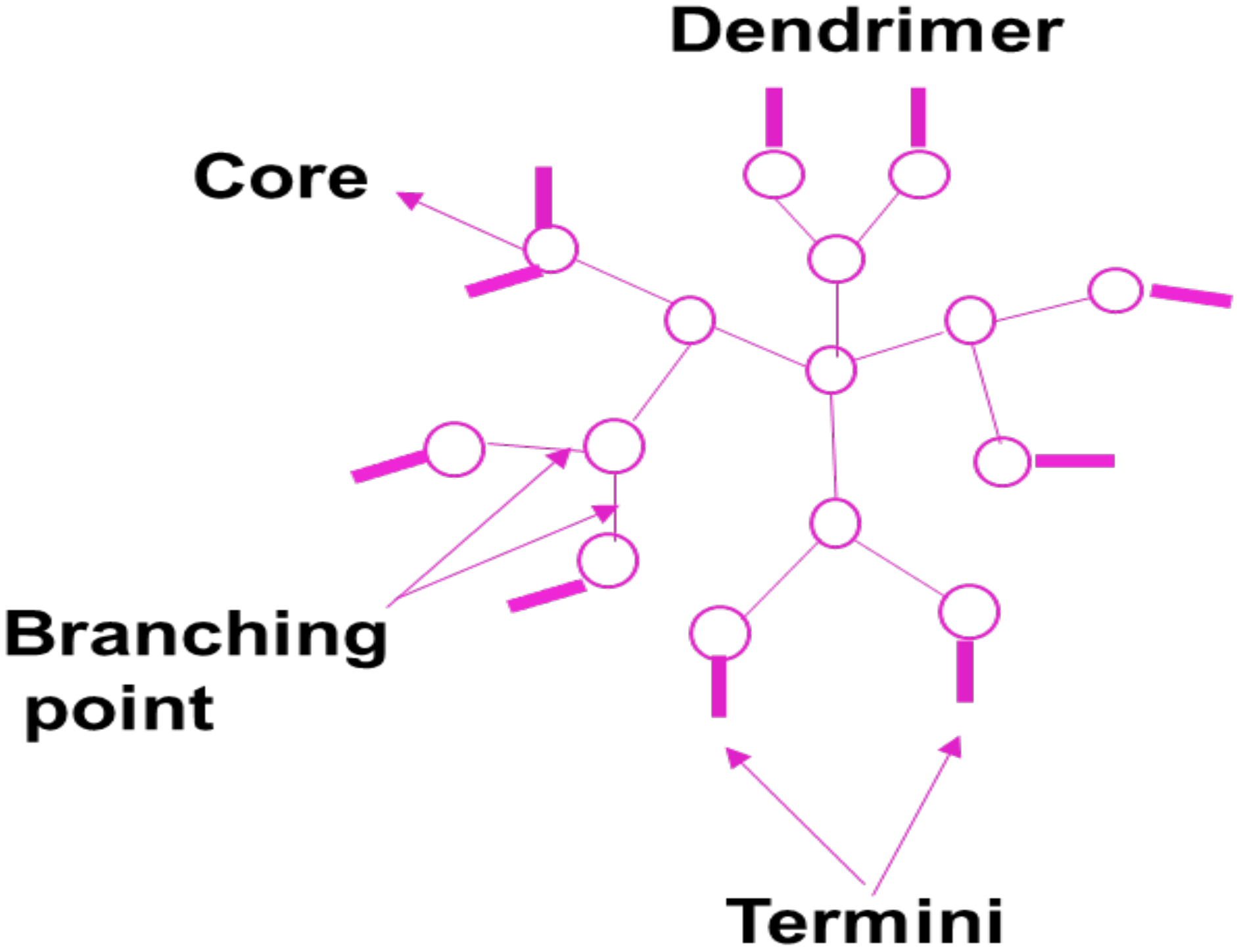
5.7. Dendrimers
5.8. Micelles
6. The Limitations of Nanomedicine
7. Strategies under Development to Overcome Limitations of Nanomedicine
8. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Muhammad, I.; Tariq Tahir, B.; Syed, W.A.S.; Muhammad, S.; Arif, M.; Srijit, D.; Hnin, E.T.; Aishah, A.; Zahid, H. Curcumin based nanomedicines as efficient nanoplatform for treatment of cancer: New developments in reversing cancer drug resistance, rapid internalization, and improved anticancer efficacy. Trends Food Sci. Technol. 2018, 80, 8–22. [Google Scholar] [CrossRef]
- Tereza, C.; Marie, S.; Vojtech, A.; Rene, K.; Tomas, E. Nanocarrier drugs in the treatment of brain tumors. J. Cancer Metastasis Treat. 2016, 2, 407–416. [Google Scholar]
- Available online: https://www.cancer.org.au/about-cancer/types-of-cancer/brain-cancer.html (accessed on 5 October 2019).
- Available online: https://abc2.org/guidance/brain-cancer-facts/tumor-grades-and-types (accessed on 5 October 2019).
- Markman, M. Brain cancer grades. CTCA 2022, 844, 240–6471. Available online: https://www.cancercenter.com/cancer-types/brain-cancer/grades (accessed on 15 March 2022).
- Available online: http://www.cbtrus.org/factsheet/factsheet.html (accessed on 1 October 2019).
- Khazaei, Z.; Goodarzi, E.; Borhaninejad, V.; Iranmanesh, F.; Mirshekarpour, H.; Mirzaei, B.; Naemi, H.; Bechashk, S.M.; Darvishi, I.; Sarabi, R.E.; et al. The association between incidence and mortality of brain cancer and human development index (HDI): An ecological study. BMC Public Health 2020, 20, 1696. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Haley, G.; Alexander, B.; Quinn, T.O.; Gabrielle, T.; Yi., F.; Carol, K.; Jill, S.B.S. Survivorship in adults with malignant brain and other central nervous system tumor from 2000–2014. Neuro-oncology 2018, 20 (Suppl. 7), 6–16. [Google Scholar]
- Van’t, R.M.; Lowik, C.; Mezzanotte, L. Targeting Nanomedicine to Brain Tumors: Latest Progress and Achievements. Curr. Pharm. Des. 2017, 23, 1953–1962. [Google Scholar]
- Andreas, W.; Dominik, W.; Vimalkumar, B.; Jörg, H. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar]
- Kazumi, S.; Yutaka, M.; Yuki, M.; Takuya, M.; Kazuko, T.; Yasutaka, A.; Vinicio, M.; Xueying, L.; Takehiko, I.; Osamu, N.; et al. Glucose transporter 1-mediated vascular translocation of nanomedicines enhances accumulation and efficacy in solid tumors. J. Control. Release 2019, 301, 28–41. [Google Scholar]
- Huanli, S.; Yangyang, D.; Jan, F.; Zhiyuan, Z. Peptide-decorated polymeric nanomedicines for precision cancer therapy. J. Control. Release 2018, 290, 11–27. [Google Scholar]
- Tyler, P.C.; Heather, M.W.G.; Anumantha, G.; Kanthasamy, W.H.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar]
- Maoxiang, L.; Jack, R.H.; Zahidul, I.; Chistopher, F.C.; James, J.P. T-2 toxin impairs murine immune response to respiratory reovirus and exacerbates viral bronchiolitis. Toxicol. Appl. Pharmacol. 2006, 217, 76–85. [Google Scholar]
- Timothy, P.C.; Stanley, D.A.; John, F.K.; David, R.L.; Narasimhulu, B.N.; Manoj, P.; Kevin, P.; Michael, A.W.; Zhongyu, W.; Zhizhen, B.Z. Pyrazolopyrimidine Macrocycles as Inhibitors of Human Immunodeficiency Virus Replication. U.S. Patent 10,125,148 B2, 13 November 2018. [Google Scholar]
- Eleni, S.; Maria, J.A. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar]
- Erdo, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Hemant, M.; Rajeev, J.; Balbir, S.K.; Arjun, M.; Suprakas, S.R. Synthesis and flocculation properties of gum ghatti and poly(acrylamide-co-acrylonitrile) based biodegradable hydrogels. Carbohydr. Polym. 2014, 114, 321–329. [Google Scholar]
- Alexander, E.B.; Evgeny, V.G.; Irina, V.B.; Anastassia, E.K.; Shilpi, A.; Alexey, G.T.; Vinod, K.G. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar]
- Ramanatham, V.; Sanjay, D.V.; Bobba, V.S.K.; Umesh, N.B.; Shekhar, B.B.; Uday, C.M. Synthesis, anti-bacterial, anti-asthmatic and anti-diabetic activities of novel N-substituted-2-(4-phenylethynyl-phenyl)-1H-benzimidazoles and N-substituted 2[4-(4,4-dimethyl-thiochroman-6-yl-ethynyl)-phenyl)-1Hbenzimidazoles. Eur. J. Med. Chem. 2008, 43, 986–995. [Google Scholar]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cereb. Fluid Res. 2004, 1, 2. [Google Scholar]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Liu, X.F.; Fawcett, J.R.; Hanson, L.R.; Frey, W.H. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J. Stroke Cerebrovasc. Dis. 2004, 13, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Roland, N.; Fritz, S.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar]
- Mengke, Q.; Qing, L.; Shanshan, H.; Luyao, W.; Yao, F.; Zhirong, Z.; Ling, Z. A brain targeting functionalized liposomes of the dopamine derivative N-3,4-bis(pivaloyloxy)-dopamine for treatment of Parkinson’s disease. J. Control. Release 2018, 277, 173–182. [Google Scholar]
- Weijun, W.; Steve, S.; Hee-Yeon, C.; Florence, M.H.; Axel, H.S.; Thomas, C.C. Efficient brain targeting and therapeutic intracranial activity of bortezomib through intranasal co-delivery with NEO100 in rodent glioblastoma models. JNS 2019, 132, 1. [Google Scholar]
- Nikhil, R.B.; Pramod, S.S. Selegiline nanoparticle embedded transdermal film: An alternative approach for brain targeting in Parkinson’s disease. J. Drug Deliv. Sci. Technol. 2019, 54, 101299. [Google Scholar]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef]
- Khaled, M.H.; Zainy, M.B. The formulation of a nasal nanoemulsion zaleplon in situ gel for the treatment of insomnia. Expert Opin. Drug Deliv. 2013, 10, 1033–1041. [Google Scholar]
- Wang, S.; Chen, P.; Zhang, L.; Yang, C.; Zhai, G. Formulation and evaluation of microemulsion-based in situ ion-sensitive gelling systems for intranasal administration of curcumin. J. Drug Target. 2012, 20, 831–840. [Google Scholar] [CrossRef]
- Marazioti, A.; Papadia, K.; Kannavou, M.; Spella, M.; Basta, A.; de Lastic, A.-L.; Rodi, M.; Mouzaki, A.; Samiotaki, M.; Panayotou, G.; et al. Cellular Vesicles: New insights in engineering methods, interaction with cells and potential for brain targeting. ASPET J. 2019, 370, 772–785. [Google Scholar] [CrossRef]
- Kirthivasan, B.; Singh, D.; Bommana, M.M.; Raut, S.L.; Squillante, E.; Sadoqi, M. Active brain targeting of a fluorescent P-gp substrate using polymeric magnetic nanocarrier system. Nanotechnology 2012, 23, 255102. [Google Scholar] [CrossRef] [PubMed]
- Hongchen, H.; Xuemei, Z.; Hongjie, M.; Qingqing, M.; Ying, J.; Yiyun, W.; Xiaoyan, L.; Aiping, W.; Sha, L.; Yaping, Z.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar]
- Zi-Ying, W.; Sravan, G.S.; Ju-Xian, S.; Jing-Yi, L.; Min, L. Strategies for brain-targeting liposomal delivery of small hydrophobic molecules in the treatment of neurodegenerative diseases. Drug Discov. Today 2019, 24, 595–605. [Google Scholar]
- Bushra, N.; Saleha, R.; Saba, K.; Sanjula, B.; Javed, A. Ligand conjugation: An emerging platform for enhanced brain drug delivery. Brain Res. Bull. 2018, 142, 384–393. [Google Scholar]
- Jason, M.L.; Eric, V.S. Targeting Receptor-Mediated Transport for Delivery of Biologics Across the Blood-Brain Barrie. BJP 2015, 55, 613–631. [Google Scholar]
- Yang, T.; Fogarty, B.; LaForge, B.; Salma, A.; Pham, T.; Lai, L.; Bai, S. Delivery of small interfering rna to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017, 19, 475–478. [Google Scholar] [CrossRef]
- Jinbing, X.; Zheyu, S.; Yasutaka, A.; Kazunori, K.; Xiaoyuan, C. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar]
- Luca, A.B.; Franciska, E. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87(1), 6. [Google Scholar]
- Sang-Soo, K.; Joe, B.H.; Kathleen, F.P.; Esther, H.C. Effective treatment of glioblastoma requires crossing the blood– brain barrier and targeting tumors including cancer stem cells: The promise of nanomedicine. Biochem. Biophys. Res. Commun. 2015, 468, 485–489. [Google Scholar]
- Maria, M.; Joao, J.S.; Alberto, P.; Carla, V. Targeted theranostic nanoparticles for brain tumor treatment. Pharmaceutics 2018, 10, 181. [Google Scholar]
- Rachel, A.K.; Resham, B.; Priyabrata, M. Cancer Nanotechnology: Emerging Role of Gold Nanoconjugates. Anticancer Agents Med. Chem. 2011, 11, 965–973. [Google Scholar]
- Drazen, R.; Sonja, D.; Jungsu, R. Macromolecular drug carriers for targeted Glioblastoma therapy: Preclinical studies, challenges, and Future Perspectives. Front. Oncol. 2018, 8, 624. [Google Scholar]
- Be´duneaua, A.; Saulniera, P.; Benoita, J.P. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, H.E.; Abdelrahman, I.A.; Ahmed, H.E.H.; Hadeer, M.L.; Amira, B.; Shurouk, A.M.; Hossam, A.-E.; Somia, A.; Aya, S.; Nouran, A.; et al. Nanomedicine as a putative approach for active targeting of hepatocellular carcinoma. Semin. Cell Biol. 2019, 61, 91–99. [Google Scholar]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.W.; Xia, Y. Stimuli-Responsive Materials for Controlled Release of Theranostic Agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Chen, W.C.; Zhang, A.X.; Li1, S.D. Limitations and niches of the active targeting approach for nanoparticle drug delivery. Eur. J. Nanomed. 2012, 4, 89–93. [Google Scholar] [CrossRef]
- Sandipan, R. Strategic Drug Delivery Targeted to the Brain: A Review. Der. Pharm. Sin. 2012, 3, 76–92. [Google Scholar]
- Georgieva, J.V.; Dick, H.; Inge, S.H. Ligand-mediated transport of drug delivery devices across the blood-brain barrier. APA 2012, 1–33. Available online: https://pure.rug.nl/ws/portalfiles/portal/25703785/02_c2.pdf (accessed on 26 March 2022).
- Julia, V.G.; Dick, H.; Inge, S.Z. Smuggling drugs into the Brain: An overview of ligands targerting transcytosis for drug delivery across the BBB. Pharmaceutics 2014, 6, 557–583. [Google Scholar]
- Jaleh, B.; Mohammad, A.R.; Mohammad, M.P.; Yadollah, O. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts 2016, 6, 225–248. [Google Scholar]
- Abdur, R.K.; Mengrui, L.; Muhammad, W.K.; Guangxi, Z. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar]
- Michelle, A.E.; William, A.B. Neuroimmune Axes of the Blood–Brain Barriers and Blood–Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar]
- Marijke, D.B.; Valérie, V.H.; Roosmarijn, E.V.; Elke, D.; Nan, W.; Luc, L. Into rather unexplored terrain—Transcellular transport across the blood–brain barrier. GLIA 2016, 64, 1097–1123. [Google Scholar]
- Sailaja, A.K.; Amareshwar, P.; Chakravarty, P. Different Technique Used for the Preparation of Nanoparticles Using Natural Polymers and Their Application. Int. J. Pharm. Pharm. Sci. 2011, 3, 45–50. [Google Scholar]
- Sams, M.A.S.; Sheikh, T.J.; Azita, H. Effects of Size and Surface Charge of Polymeric Nanoparticles on in Vitro and in Vivo Applications. JBNB 2016, 7, 91. [Google Scholar]
- Saucier-Sawyer, J.K.; Seo, Y.E.; Gaudin, A.; Quijano, E.; Song, E.; Sawyer, A.J.; Deng, Y.; Huttener, A.; Saltzman, W.M. Distribution of polymer nanoparticles by convection-enhanced delivery to brain tumors. JCR 2016, 232, 103–112. [Google Scholar] [CrossRef]
- Navya, P.N.; Anubhav, K.; Srinivas, S.P.; Suresh, K.B.; Vincent, M.R.; Hemant, K.D. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Chenlu, L.; Pooya, D.; Wenbo, Z.; Kah-Hoe, P.C.; Chi-Hwa, W. Development of Nanoparticles for Drug Delivery to Brain Tumor: The Effect of Surface Materials on Penetration into Brain Tissue. J. Pharm. Sci. 2019, 108, 1736–1745. [Google Scholar]
- Eleonora, C.; Alessio, C.; Alice, P.; Alessandro, D.M.; Brunella, T.; Carla, E. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar]
- Daniel, M.T.; Christina, C.; Alexandru, M.G.; Adrian, V.; Raluca, I.T. Impact of Nanoparticles on Brain Health: An Up to date review. J. Clin. Med. 2018, 7, 490. [Google Scholar]
- Corey, J.B.; Stephany, Y.T.; Jordan, J.G. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015, 11, 393–403. [Google Scholar]
- Chunsheng, H.; Ping, C.; Jason, L.; Tian, Z.; Lucy, L.; Azhar, Z.A.; Jeffrey, T.H.; Andrew, M.R.; Xiao, Y.W. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J. Control. Release 2017, 246, 98–109. [Google Scholar]
- John, C.; Yuan, R.; Jayoung, K.; Noah, G.; David, R.W.; Kristen, K.; Antonella, M.; Eric, S.; Henry, B.; Betty, T.; et al. Nonviral polymeric nanoparticles for gene therapy in pediatric CNS malignanciesâ. Nanomed. Nanotechnol. Biol. Med. 2019, 23, 102115. [Google Scholar]
- Dou, Y.; Omar, F.K.; Mario, L.S.; Biqin, D.; Wojciech, K.P.; Ting, X.; Meijing, W.; Yu, H.; Atique, U.A.; Irina, V.B.; et al. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. USA 2017, 114, E6147–E6156. [Google Scholar]
- Ahmed, T.; Liu, F.C.F.; He, C.; Abbasi, A.Z.; Cai, P.; Rauth, A.M.; Henderson, J.T.; Wu, X.Y. Optimizing the Design of Blood–Brain Barrier-Penetrating Polymer-Lipid-Hybrid Nanoparticles for Delivering Anticancer Drugs to Glioblastoma. Pharm. Res. 2021, 38, 1897–1914. [Google Scholar] [CrossRef]
- Paus, C.; Voort, R.; Cambil, A. Nanomedicine in cancer therapy: Promises and hurdles of polymeric nanoparticles. Explor. Med. 2021, 2, 167–185. [Google Scholar] [CrossRef]
- Danaei, M.; Kalantari, M.; Raji, M.; Samareh, F.H.; Saber, R.; Asnani, G.P.; Mortazavi, S.M.; Mozafari, M.R.; Rasti, B.; Taheriazam, A. Probing nanoliposomes using single particle analytical techniques:Effect of excipients, solvents, phase transition and zeta potential. Heliyon 2018, 4, e01088. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Yang, G.Y.; Du, S.M.; Zeng, N.; Li, D.S.; Li, R.M.; Chen, J.Y.; Feng, J.B.; Yuan, S.H.; et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int. J. Nanomed. 2012, 7, 271–280. [Google Scholar]
- Lundy, D.J.; Nguyễn, H.; Hsieh, P.C.H. Emerging Nano-Carrier Strategies for Brain Tumor Drug Delivery and Considerations for Clinical Translation. Pharmaceutics 2021, 13, 1193. [Google Scholar] [CrossRef] [PubMed]
- Mojarad-Jabali, S.; Farshbaf, M.; Walker, P.R.; Hemmati, S.; Fatahi, Y.; Zakeri-Milani, P.; Sarfraz, M.; Valizadeh, H. An update on actively targeted liposomes in advanced drug delivery to glioma. Int. J. Pharm. 2021, 602, 120645. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Benny, O.b.; Joki, T.; Menon, L.G.; Machluf, M.b.; Abe, T.; Carroll, R.S. Novel local drug delivery system using thermoreversible gel in combination with polymeric microspheres or liposomes. Anticancer Res. 2010, 30, 1057–1064. [Google Scholar] [PubMed]
- Vimalkumar, J.M.; Nidhi, R.; Piyush, G.; Vishakha, T.; Kiran, K.; Rakesh, K.T. Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar]
- Erica, L.; Maria, N.; Chiara, U.; George, L.; Eirini, F.; Valerio, M.; Andrea, P.; Theodoros, T.; Dimitrios, P.; Jessica, P.; et al. Targeted delivery of silver nanoparticles and alisertib: In vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 2014, 9, 839–849. [Google Scholar]
- Elvira, C.; Gallardo, A.; San Roman, J.; Cifuentes, A. Covalent polymer-drug conjugates. Molecules 2005, 10, 114–125. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Buyana, B.; Aderibigbe, B.A. Polymer-Drug Conjugate, a Potential Therapeutic to Combat Breast and Lung Cancer. Pharmaceutics 2020, 12, 406. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Raosaheb, H.S.; Mohanty, P.; Jain, A. Solid lipids nanoparticles for brain targerting. J. Drug Deliv. 2019, 9, 248–252. [Google Scholar]
- Hodoshima, N.; Udagawa, C.; Ando, T.; Fukuyasu, H.; Watanabe, S.; Nakabayashi, S. Lipid nanoparticles for delivering antitumor drugs. Int. J. Pharm. 1997, 146, 81–92. [Google Scholar] [CrossRef]
- Yu, B.T.; Sun, X.; Zhang, Z.R. Enhanced liver targeting by synthesis of N1-stearyl-5 Fu and incorporation into solid lipid nanoparticles. Arch. Pharm. Res. 2003, 26, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, K.; Suresh, J.R.; Venkateswarlu, V. Solid Lipid Nanoparticles as Drug Delivery Systems. Exp. Clin. Pharmacol. 2005, 27, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pandey, V.; Soni, V. Surface Modified Solid Lipid Nanoparticles for Brain Cancer Treatment. Asian J. Pharm. Sci. 2019, 13, 119–124. [Google Scholar]
- Kakkar, V.; Mishra, A.K.; Chuttani, K.; Kaur, I.P. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int. J. Pharm. 2013, 448, 354–359. [Google Scholar] [CrossRef]
- Göppert, T.M.; Müller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J. Drug Target. 2005, 13, 179–187. [Google Scholar] [CrossRef]
- Agarwal, A.; Majumder, S.; Agrawal, H.; Majumdar, S.; Agrawal, G.P. Cationized Albumin Conjugated Solid Lipid Nanoparticles as Vectors for Brain Delivery of an Anti-Cancer Drug. Curr. Nanosci. 2011, 7, 71–80. [Google Scholar] [CrossRef]
- Gupta, Y.; Jain, A.; Jain, S.K. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J. Pharm. Pharmacol. 2007, 59, 935–940. [Google Scholar] [CrossRef]
- Dal Magro, R.; Ornaghi, F.; Cambianica, I.; Beretta, S.; Re, F.; Musicanti, C.; Rigolio, R.; Donzelli, E.; Canta, A.; Ballarini, E.; et al. Nanomedicine for the Treatment of Alzheimer’s Disease. J. Control. Release 2017, 249, 1997–2024. [Google Scholar]
- Sonia, M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, W.; Gan, L.; Zhu, C.; Gan, Y.; Nie, S. Preparation of a Dispersible PEGylate Nanostructured Lipid Carriers (NLC) Loaded with 10-Hydroxycamptothecin by Spray-Drying. Chem. Pharm. Bull. 2008, 56, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lai, X.; Song, S.; Zhu, X.; Zhu, J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2016, 23, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Sa, C.K.; Goh, M.S.; Lee, E.S.; Kang, T.H.; Yoon, H.Y.; Battogtokh, G.; Ko, Y.T.; Choi, Y.W. pH-sensitive PEGylation of RIPL peptide-conjugated nanostructured lipid carriers: Design and in vitro evaluation. Int. J. Nanomed. 2018, 13, 6661–6675. [Google Scholar] [CrossRef] [PubMed]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Pharmaceutics 2020, 12, 860. [Google Scholar]
- Tsai, M.J.; Wuc, P.C.; Huang, Y.B.; Changc, J.S.; Linc, C.L.; Tsai, Y.H.; Fange, J.Y. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int. J. Pharm. 2012, 423, 461–470. [Google Scholar] [CrossRef]
- Hsu, S.H.; Wen, C.J.; Al-Suwayeh, S.A.; Chang, H.W.; Yen, T.C.; Fang, J.Y. Physicochemical characterization and in vivo bioluminescence imaging of nanostructured lipid carriers (NLCs) for targeting the brain: Apomorphine as a model drug. Nanotechnology 2010, 21, 405101. [Google Scholar] [CrossRef][Green Version]
- Flavia, S.; Harkiranpreet, K.D.; Florence, G.; Bruno, S.; Mansoor, M.A. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar]
- Alven, S.; Aderibigbe, B.A. Combination Therapy Strategies for the Treatment. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Q.; Ming, S.; Ying, S.; Xiangyu, Z.; Can, H.; Jianhua, C.; Rongxin, L.; Yourong, D. Thermoresponsive nanocomposite gel for local drug delivery to suppress the growth of glioma by inducing autophagy. Autophagy 2017, 13, 1176–1190. [Google Scholar] [CrossRef]
- Kim, J.; Chang, J.C.; Bora, K.; Hong, J.M.; Jung-Kyo, C.; Seung, H.L.; Soo-Chang, S. Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform. Biomaterials 2012, 33, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Fakhrossadat, E.; Sooyeun, L.; Jee-Heon, J.; Simmyung, Y. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar]
- Mehmood, R.; Rashid, F.; Mansoor, Z. Thermosensitive Liposome Formulation with Topotecan and Doxorubicin as Drug Payload for Delivery Coupled with High Intensity Focused Ultrasound. Clin. Pharm. Biopharm. 2020, 9, 199. [Google Scholar]
- Alven, S.; Aderibigbe, B.A. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; pp. 378–406. [Google Scholar] [CrossRef]
- Beezer, A.K.; King, A.S.H.; Martin, I.K.; Mitchel, J.C.; Twyman, L.J.; Wain, C.F. Dendrimers as Potential Drug Carriers; Encapsulation of Acidic Hydrophobes within Water Soluble PAMAM Derivatives. Tetrahedron 2003, 34, 3873–3880. [Google Scholar] [CrossRef]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Sousa, A.A.; Wilson, C.M.; Aronova, M.A.; Griths, G.L.; Leapman, R.D.; Vo, H.Q. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J. Transl. Med. 2009, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, B.; Shen, S.; Chen, J.; Zhang, Q.; Jiang, X.; Pang, Z. CREKA peptide-conjugated dendrimer nanoparticles for glioblastoma multiforme delivery. J. Colloid Interface Sci. 2015, 450, 396–403. [Google Scholar] [CrossRef]
- Mishra, V.; Kesharwani, P. Dendrimer technologies for brain tumor. Drug Discov. Today 2016, 21, 766–778. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Qian, L.; Pei, Y.; Qiu, Y.; Jiang, Y. RGD-modified PEG–PAMAM–DOX conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharm. Biopharm. 2011, 79, 232–240. [Google Scholar] [CrossRef]
- Dongdong, M.; Xiuqin, C.; Yuhua, W.; Qiumei, G.; Qiuhao, Y.; Ruotao, G.; Shuanghuang, X.; Qing, Y.; Yide, H.; Yiru, P. Benzyl ester dendrimer silicon phthalocyanine based polymeric nanoparticle for in vitro photodynamic therapy of glioma. J. Lumin. 2019, 207, 597–601. [Google Scholar]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Zhang, F.; Mishra, M.K.; Zhang, Z.; Kambhampati, S.P.; Kannan, R.M.; Kannan, S. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 2016, 101, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Wasiak, T.; Ionov, M.; Fernandez-Villamarin, M.; Sousa-Herves, A.; Correa, J.; Riguera, R.; Fernandez-Megia, E. Dendrimers reduce toxicity of Abeta 1−28 peptide during aggregation and accelerate fibril formation. Nanomedicine 2012, 8, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer advances for the Central Nervous System delivery of therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef]
- Dhanikula, R.S.; Hildgen, P. Synthesis and evaluation of novel dendrimers with a hydrophilic interior as nanocarriers for drug delivery. Bioconjug. Chem. 2006, 17, 29–41. [Google Scholar] [CrossRef]
- Yang, H. Nanoparticle-mediated Brain-Specific Drug Delivery, imaging, and diagnosis. Pharm. Res. 2010, 27, 1759–1771. [Google Scholar] [CrossRef]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, M.A.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef]
- Jie, Q.; Liangqiao, Z.; Zhihua, C.; Guohua, M.; Ziyun, G.; Xianling, L.; Xingen, Z.; Jianming, Z. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar]
- Valenzuela-Oses, J.K.; García, M.C.; Feitosa, V.A.; Pachioni-Vasconcelos, J.A.; Gomes-Filho, S.M.; Lourenço, F.R.; Cerize, N.N.P.; Bassères, D.S.; Rangel-Yagui, C.O. Development and characterization of miltefosine-loaded polymeric micelles for cancer treatment. Mater. Sci. Eng. C 2017, 81, 327–333. [Google Scholar] [CrossRef]
- Giovanni, T.; Teresa, M.; Barbara, R.; Claudia, C.; Daniela, B.; Rosario, P.; Maria, A.V.; Giovanni, P. The “fate” of polymeric and lipid nanoparticles for brain delivery and targeting: Strategies and mechanism of blood–brain barrier crossing and trafficking into the central nervous system. J. Drug Deliv. Sci. Technol. 2016, 32, 66–76. [Google Scholar]
- Soni, S.; Babbar, A.K.; Sharma, R.K.; Maitra, A. Delivery of hydrophobised 5-fluorouracil derivative to brain tissue through intravenous route using surface modified nanogels. J. Drug Target. 2006, 14, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Yamashita, Y.; Nishihara, M.; Sugiyama, S.; Sonoda, Y.; Kumabe, T.; Yokoyama, M.; Tominaga, T. Therapeutic efficacy of a polymeric micellar doxorubicin infused by convection-enhanced delivery against intracranial 9L brain tumor models. Neuro-Oncol. 2009, 11, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Taki, H.; Tanaka, K.; Takashima, Y.; Okada, H. Cell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administration. Pharm. Res. 2011, 28, 2130–2139. [Google Scholar] [CrossRef]
- Sosnik, A.; Raskin, M.M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol. Adv. 2015, 33, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, S. Nanotechnology and medicine—The upside and the downside. IJDDR 2013, 5, 0975–9344. [Google Scholar]
- De Jong, W.; Borm, P. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Singh, O.P.; Nchru, R.M. Nanotechnology and Cancer treatment. Asian J. Exp. Sci. 2008, 22, 45–50. [Google Scholar]
- Nicole, H.; Poh, C. Nanomedicine and Cancer. Food Sci. Technol. 2005. Available online: http://www.tahan.com/charlie/nanosociety/course201/nanos/NH.pdf (accessed on 26 March 2022).
- Dong, X.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug de-livery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Murakami, M.; Cabral, H.; Matsumoto, Y.; Wu, S.; Kano, M.R.; Yamori, T.; Nishiyama, N.; Kataoka, K. Improving drug potency and efficacy by nanocarrier-mediated subcellular targeting. Sci. Transl. Med. 2011, 3, 64ra2. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wu, Z.; Wu, C.; Wang, X.; Liow, S.S.; Li, Z.; Wu, Y.L. Overcoming STC2 mediated drug resistance through drug and gene co-delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater. Sci. Eng. C 2018, 83, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Soma, C.E.; Dubernet, C.; Bentolila, D.; Benita, S.; Couvreur, P. Reversion of multidrug re-sistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials 2000, 21, 1–7. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yu, S.; Yuan, X. Effects of hypoxia on expression of P-gp and mutltidrug resistance protein in human lung adenocarcinoma A549 cell line. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 279–281. [Google Scholar]
- Vadde, R.; Vemula, S.; Jinka, R.; Merchant, N.; Bramhachari, P.V.; Nagaraju, G.P. Role of hypox-ia-inducible factors (HIF) in the maintenance of stemness and malignancy of colorectal cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 22–27. [Google Scholar] [CrossRef]
- Rey, S.; Schito, L.; Wouters, B.G.; Eliasof, S.; Kerbel, R.S. Targeting Hypoxia-inducible factors for anti-angiogenic cancer therapy. Trends Cancer 2017, 3, 529–541. [Google Scholar] [CrossRef]
- Luan, X.; Guan, Y.Y.; Liu, H.J.; Lu, Q.; Zhao, M.; Sun, D.; Lovell, J.F.; Sun, P.; Chen, H.Z.; Fang, C. A tumor vascular-targeted interlocking trimodal nanosystem that induces and exploits hypoxia. Adv. Sci. 2018, 5, 1800034. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Ardebili, S.M.; Moornani, M.B.; Masjedi, A.; Atyabi, F.; Kiani, M.; Namdar, A.; Karpisheh, V.; Izadi, S.; Baradaran, B.; et al. Silencing of HIF-1α/CD73 axis by siRNA-loaded TAT-chitosan-spion nanoparticles ro-bustly blocks cancer cell progression. Eur. J. Pharmacol. 2020, 5, 882–173235. [Google Scholar]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2019, 2, 26–41. [Google Scholar] [CrossRef]
- Hashizume, R.; Ozawa, T.; Gryaznov, S.M.; Bollen, A.W.; Lamborn, K.R.; Frey, W.H.; Deen, D.F. New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163. Neurooncology 2008, 10, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, X.; Yue, Y.; Raliya, R.; Biswas, P.; Taylor, S.; Tai, y.; Rubin, J.B.; Liu, Y.; Chen, H. Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. J. Control. Release 2018, 286, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Nicolas, H.; Voelcker, H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; Camus, P.; Abergel, A.; Pageaux, G.P.; Masliah, C.; Bronowicki, J.P.; Zarski, J.P.; Pelletier, G.; Bouattour, M.; Farloux, L.; et al. Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO Open 2017, 2, e000238. [Google Scholar] [CrossRef]
- Hu, Y.; Hammarlund-udenaes, M. Perspectives on Nanodelivery to the Brain: Prerequisites for Successful Brain Treatment. Mol. Pharm. 2020, 17, 4029–4039. [Google Scholar] [CrossRef]
- Chu, K.S.; Hasan, W.; Rawal, S.; Walsh, M.D.; Enlow, E.M.; Luft, J.C.; Bridges, A.S.; Kuijer, J.L.; Napier, M.E.; Zamboni, W.C.; et al. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomedicine 2013, 9, 686. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H.J. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Vykhodtseva, N.; McDannold, N.; Hynynen, K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 2008, 48, 279–296. [Google Scholar] [CrossRef]
- Pernot, M.; Aubry, J.F.; Tanter, M.; Thomas, J.L.; Fink, M. High power transcranial beam steering for ultrasonic brain therapy. Phys. Med. Biol. 2003, 48, 2577–2589. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Lipsman, N.; Kordower, J.H. Focused ultrasound opening of the blood–brain barrier for treatment of Parkinson’s disease [published online 28 May 2019]. Mov. Disord. 2019, 34, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N. The Future of Focused Ultrasound: Movement Disorders and Beyond. NeurologyLive 2019, 2. Available online: https://www.neurologylive.com/view/the-future-of-focused-ultrasound-movement-disorders-and-beyond (accessed on 15 March 2022).
- Taira, T.; Lozano, A.M.; Obeso, J.A. MRI-Guided Focused Ultrasound (FUS) to Treat Movement Disorders. Int. Parkinson Mov. Disord. Soc. 2018. Available online: https://www.movementdisorders.org/MDS/Scientific-Issues-Committee-Blog/MRI-guided-focused-ultrasound-to-treat-movement-disorders.htm (accessed on 15 March 2022).
- Elhelf, I.A.S.; Albahar, H.; Shahd, U.; Otoe, A.; Cressman, E.; Almekkawy, M. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagn. Interv. Imaging 2018, 99, 349–359. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, J.P.; Lee, E.W.; Yamamoto, S.; Loh, C.T.; Kee, S.T. Image-guided tumor ablation: Emerging technologies and futuredirections. Semin. Interv. Radiol. 2010, 27, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Kocbek, P.; Obermajer, N.; Cegnar, M.; Kos, J.; Kristl, J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J. Control. Release 2007, 120, 18. [Google Scholar] [CrossRef]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 Nanoparticles for Blood–Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef]
- Huang, N.; Lu, S.; Liu, X.G.; Zhu, J.; Wang, Y.J.; Liu, R.T. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017, 8, 81001. [Google Scholar] [CrossRef]
- Varan, C.; Bilensoy, E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293. [Google Scholar] [CrossRef]
- Nowak, M.; Brown, T.D.; Graham, A.; Helgeson, M.E.; Mitragotri, S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng. Transl. Med. 2020, 5, e10153. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.G.; Youn, Y.S. Rabies Virus-Inspired Silica-Coated Gold Nanorods as a Photothermal Therapeutic Platform for Treating Brain Tumors. Adv. Mater. 2017, 29, 1605563. [Google Scholar] [CrossRef] [PubMed]
- Black, K.C.L.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198Au-Doped Nanostructures with Different Shapes for In Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385. [Google Scholar] [CrossRef] [PubMed]
- Thangudu, S.; Cheng, F.Y.; Su, C.H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. Polymers 2020, 12, 3055. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A promising approach for delivery of neuroprotective drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naki, T.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanomedicine for the Treatment of Brain Cancer. Pharmaceutics 2022, 14, 1048. https://doi.org/10.3390/pharmaceutics14051048
Naki T, Aderibigbe BA. Efficacy of Polymer-Based Nanomedicine for the Treatment of Brain Cancer. Pharmaceutics. 2022; 14(5):1048. https://doi.org/10.3390/pharmaceutics14051048
Chicago/Turabian StyleNaki, Tobeka, and Blessing A. Aderibigbe. 2022. "Efficacy of Polymer-Based Nanomedicine for the Treatment of Brain Cancer" Pharmaceutics 14, no. 5: 1048. https://doi.org/10.3390/pharmaceutics14051048
APA StyleNaki, T., & Aderibigbe, B. A. (2022). Efficacy of Polymer-Based Nanomedicine for the Treatment of Brain Cancer. Pharmaceutics, 14(5), 1048. https://doi.org/10.3390/pharmaceutics14051048





