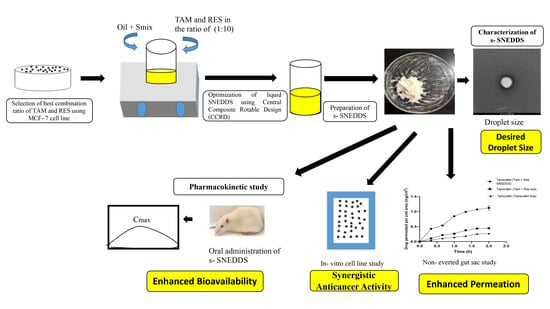
Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Combination Index (CI)
2.3. Selection of Excipients
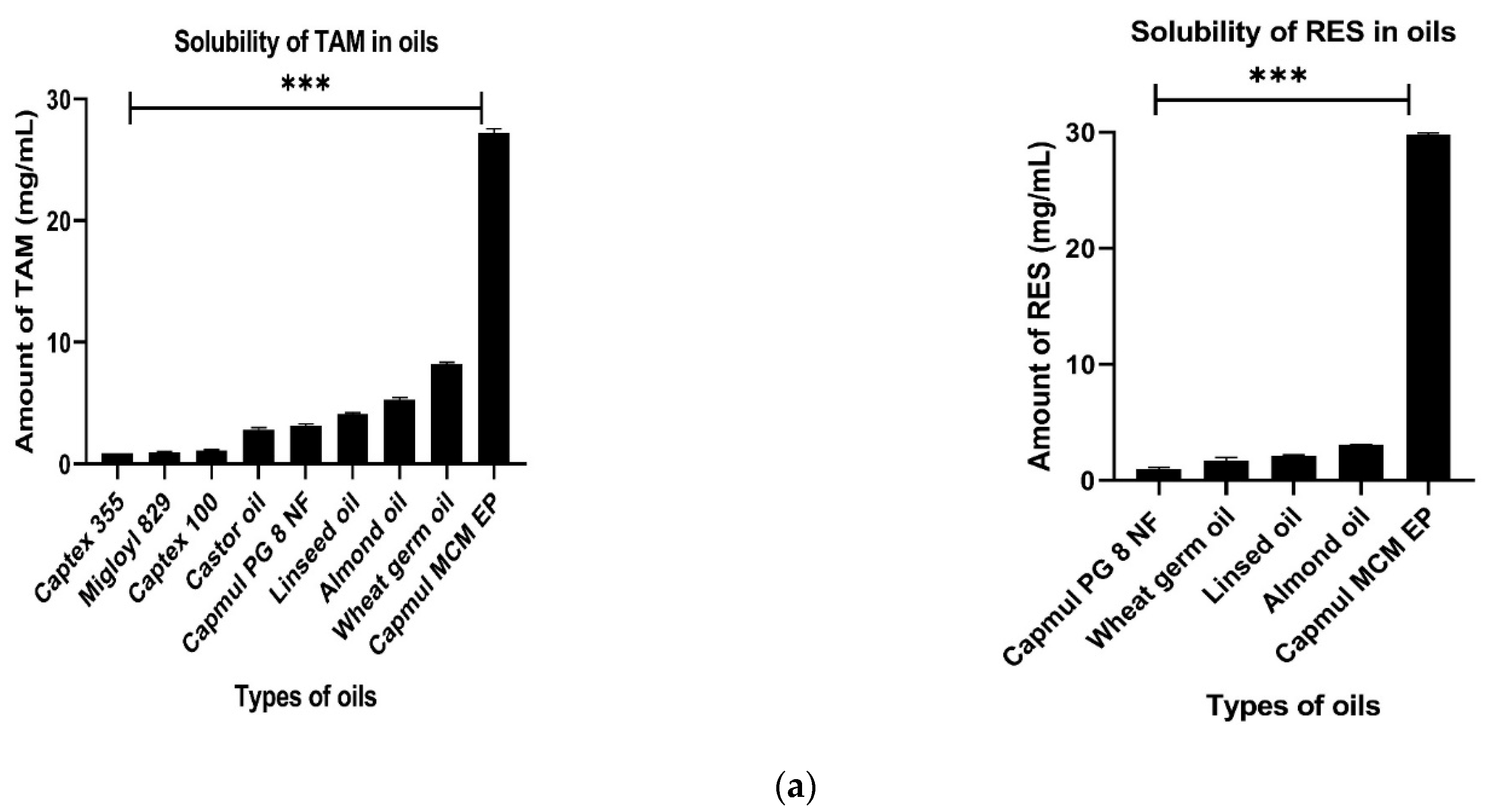
2.3.1. Selection of Oil
2.3.2. Selection of Surfactants and Co- Surfactants
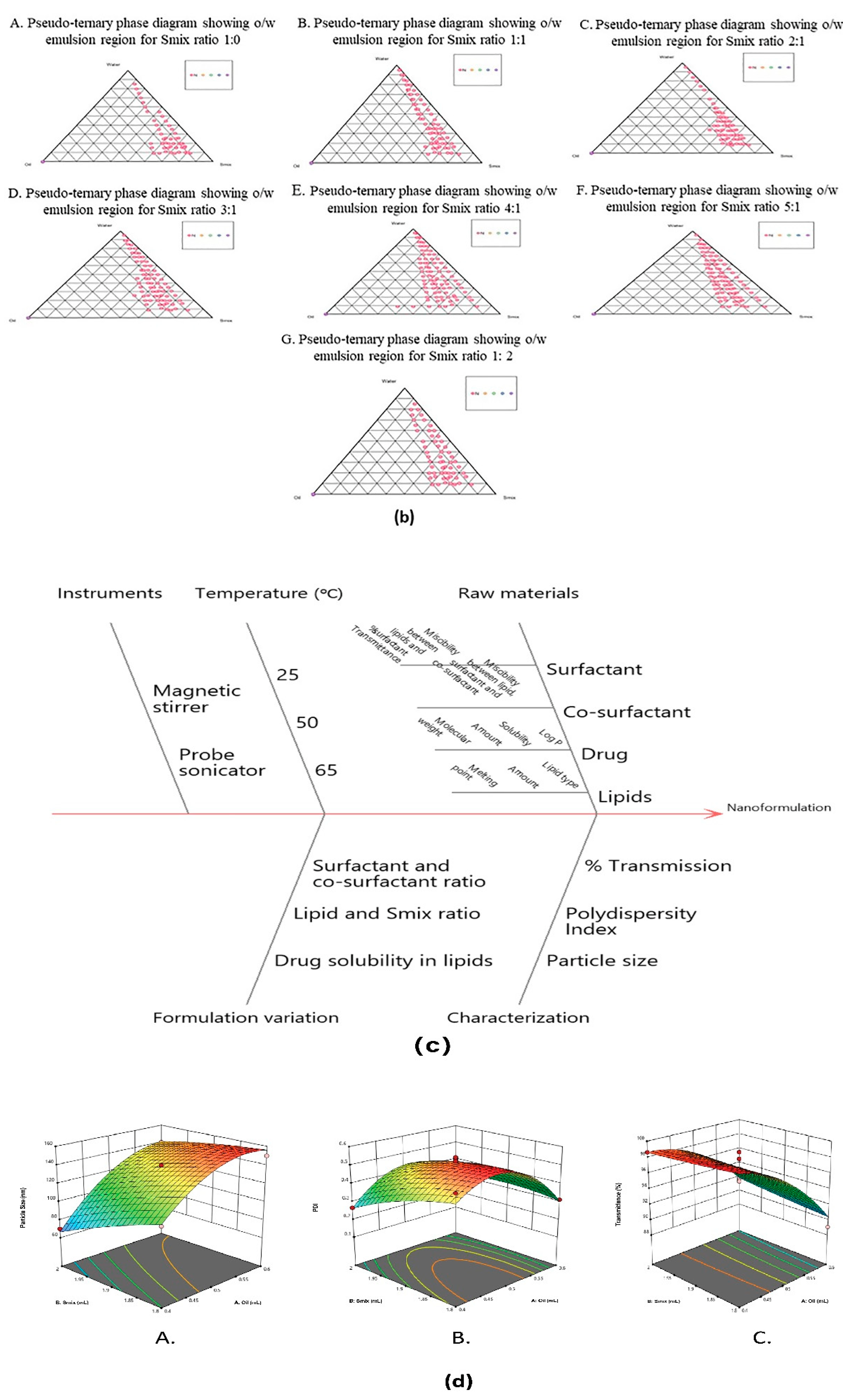
2.3.3. Construction of Pseudoternary Phase Diagram
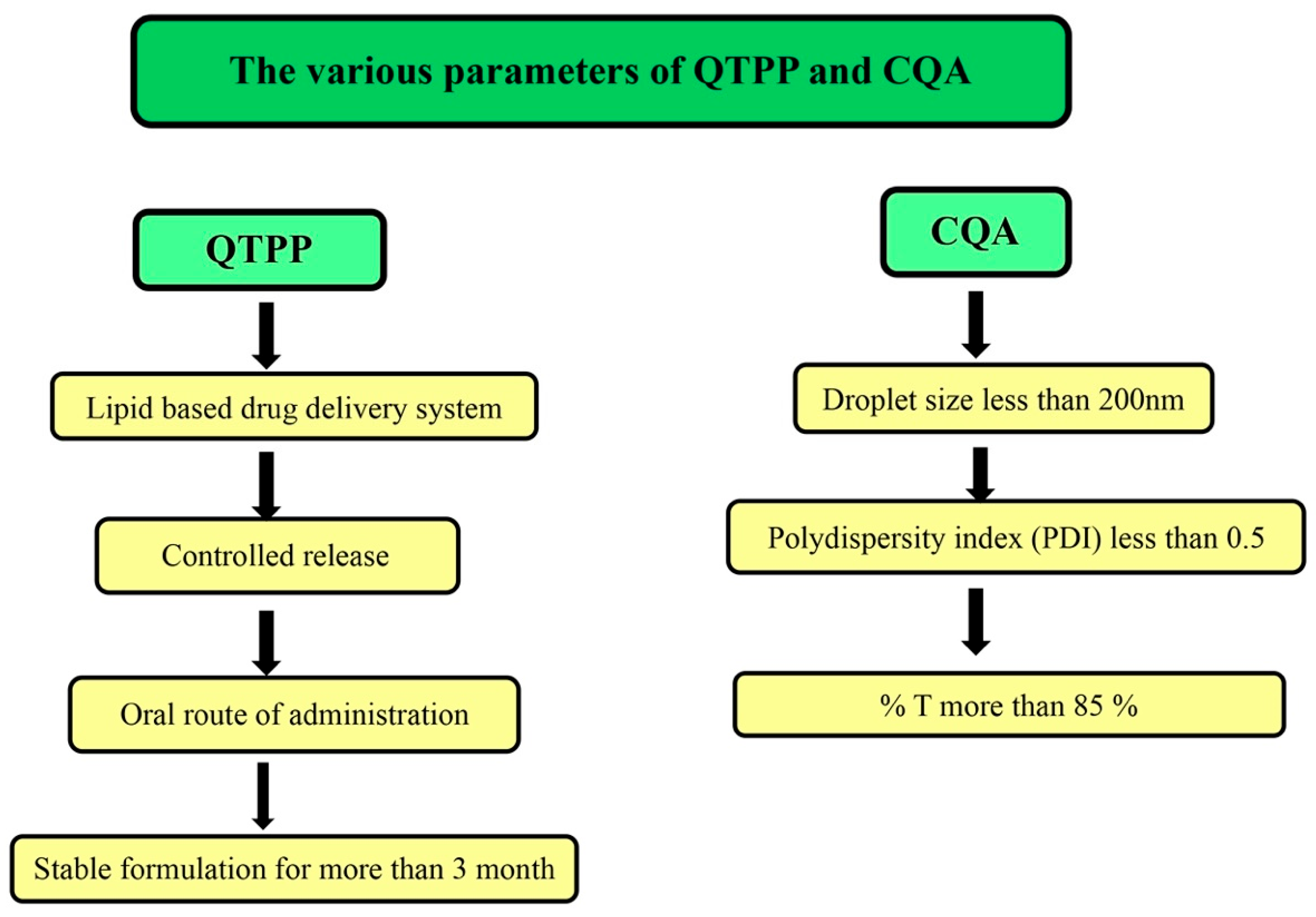
2.4. Application of Quality by Design (QbD)
2.5. Formulation and Optimization of SNEDDS
2.6. Preparation of TAM and RES-Loaded Liquid SNEDDS
2.7. Conversion of SNEDDS into Solid SNEDDS
2.8. Characterization of s-SNEDDS
2.8.1. Droplet Size and PDI
2.8.2. Percentage Transmittance
2.8.3. Zeta Potential
2.8.4. Robustness to Dilutions
2.8.5. Drugs Content Analysis
2.8.6. Morphological Analysis
Scanning Electron Microscope (SEM)
Transmission Electron Microscope (TEM)
2.8.7. Solid State Characterization
Differential Scanning Calorimetry (DSC)
X-ray Diffraction (XRD)
Fourier Transform Infrared (FTIR)
2.9. In Vitro Studies
2.9.1. Reconstitution Ability and Stability of s-SNEDDS in Simulated Gastrointestinal Fluids
2.9.2. Release Studies Using Dialysis Membrane
2.9.3. Non-Everted Gut Sac Permeability Study
2.9.4. Assessment of Depth of Permeation Using Confocal Laser Scanning Microscopy (CLSM)
2.9.5. In Vitro Lipolysis by pH Stat Method
2.9.6. Hemolysis Test
2.10. Cell Line Studies
2.10.1. Cytotoxicity Studies
2.10.2. Cellular Uptake Studies
2.10.3. Estimation of Intracellular Antioxidant
Reactive Oxygen Species (ROS) in MCF-7 Cells by DCFDA Assay
Assessment of Superoxide Dismutase Activity (SOD)
2.11. Pharmacokinetic Study
2.12. Statistical Analysis
3. Results and Discussions
3.1. Determination of Combination Index (CI)
3.2. Selection of Excipients
3.2.1. Selection of Oil
3.2.2. Selection of Surfactant
3.2.3. Pseudoternary Phase Diagram
3.3. QbD Approach
3.4. Optimization of SNEDDS Using CCRD
3.4.1. Effect of Independent Variables on Droplet Size
3.4.2. Effect of Independent Variables on Polydispersity (PDI)
3.4.3. Effect of Independent Variables on Percentage of Transmittance
3.4.4. Validation of Experimental Design
3.5. Conversion of SNEDDS into Solid SNEDDS
3.6. Characterization of s-SNEDDS
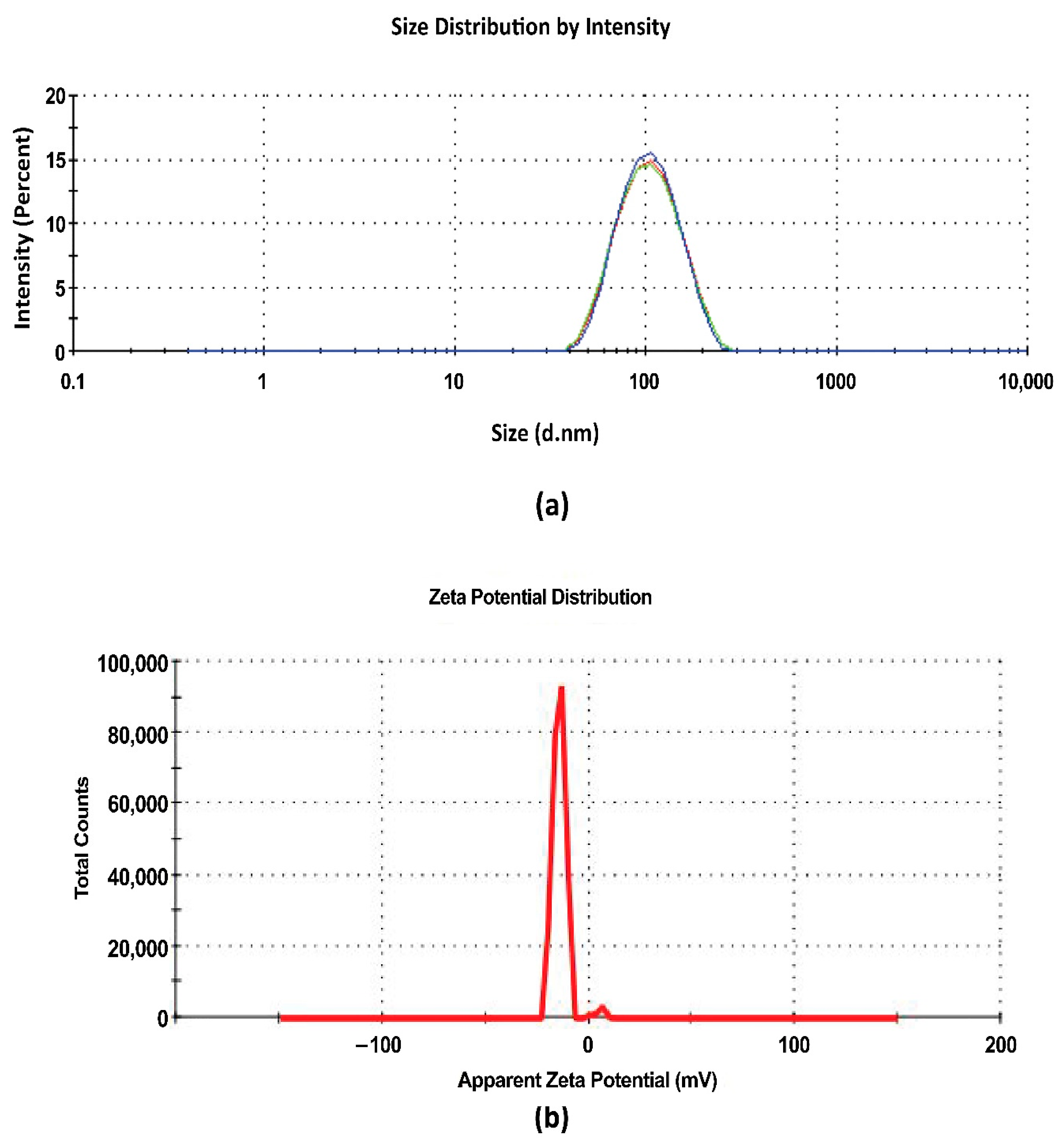
3.6.1. Droplet Size and PDI
3.6.2. Percentage Transmittance
3.6.3. Zeta Potential
3.6.4. Robustness to Dilutions
3.6.5. Drugs Content Analysis
3.6.6. Morphological Analysis
Scanning Electron Microscope (SEM)
Transmission Electron Microscope (TEM)
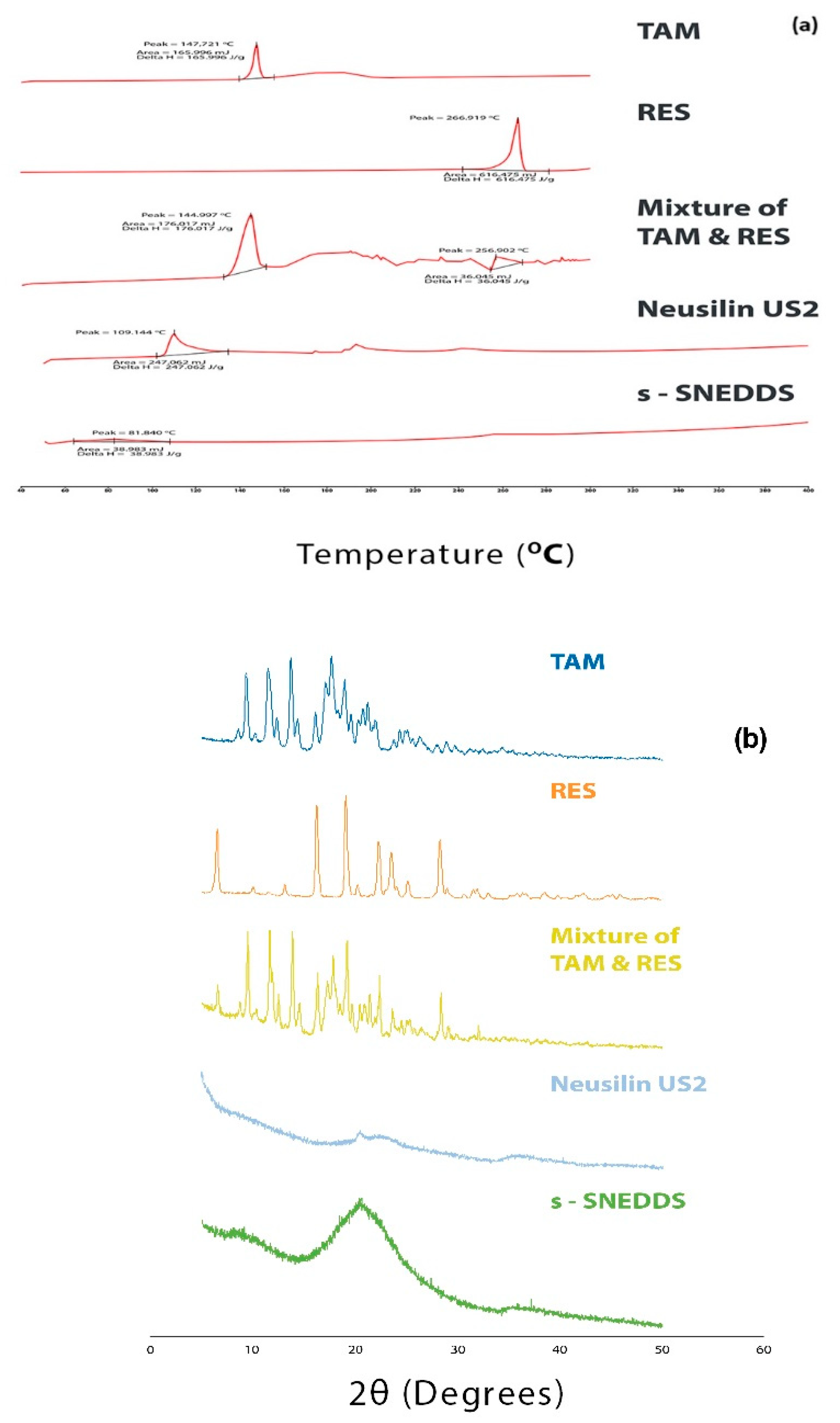
3.6.7. Solid State Characterization
Differential Scanning Calorimetry (DSC)
X-ray Diffraction (XRD)
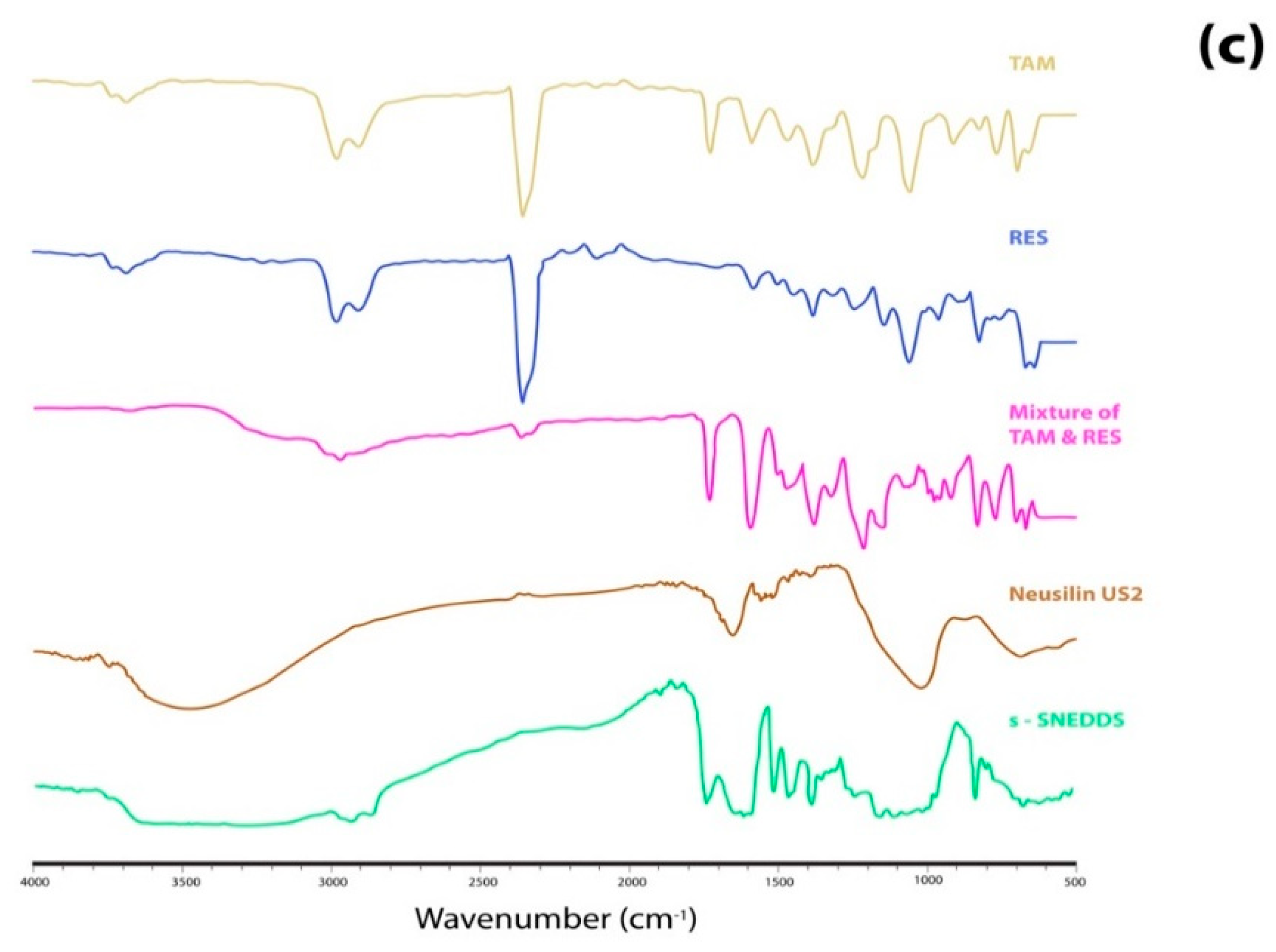
Fourier Transform Infrared (FTIR)
3.7. In Vitro Studies
3.7.1. Reconstitution Ability and Stability of s-SNEDDS in Simulated Gastrointestinal Fluids
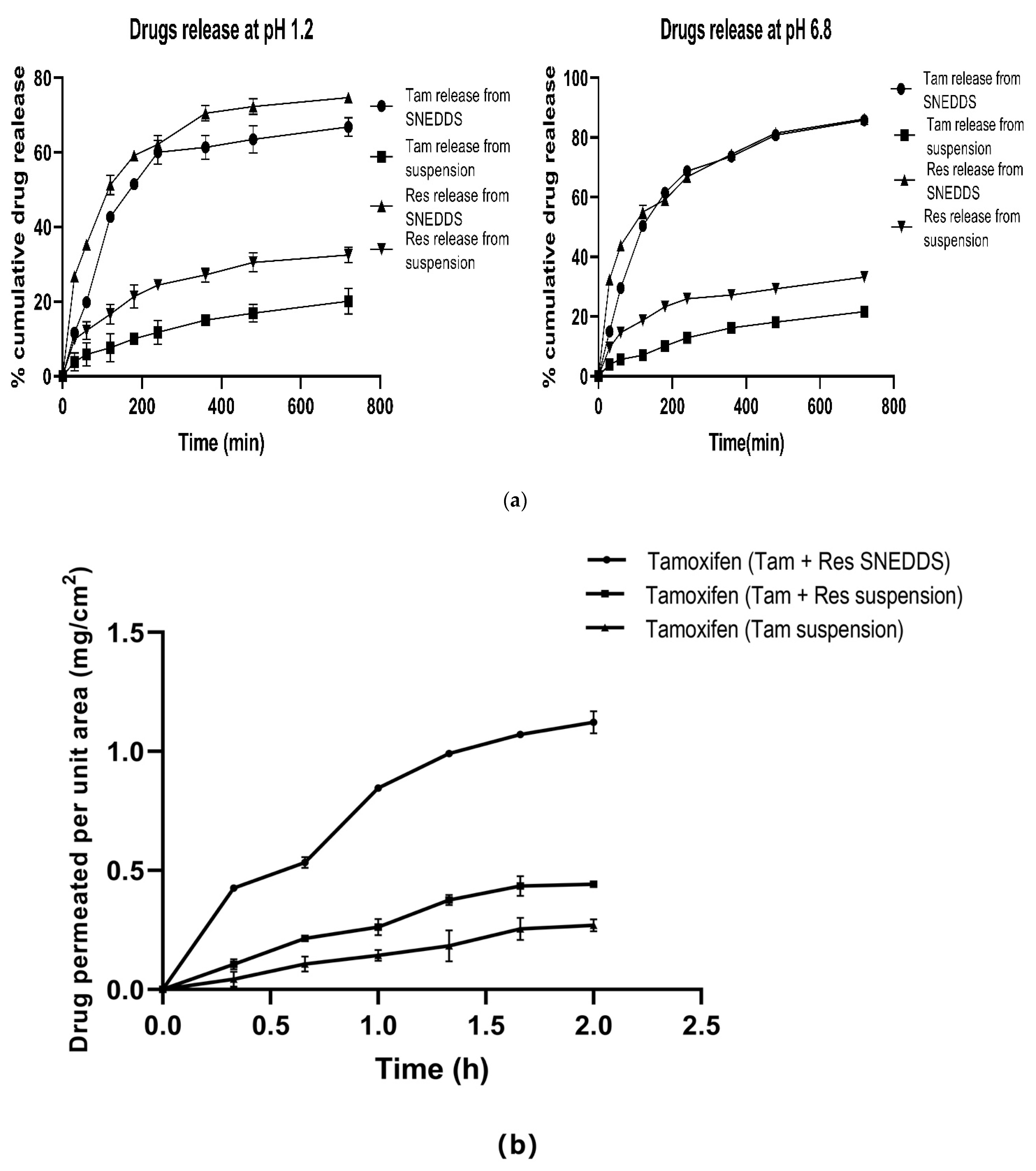
3.7.2. Release Studies Using Dialysis Membrane
3.7.3. Non-Everted Gut Sac Permeability Study
3.7.4. Assessment of Depth of Permeation Using Confocal Laser Scanning Microscopy (CLSM)
3.7.5. Lipolysis by pH Stat Method
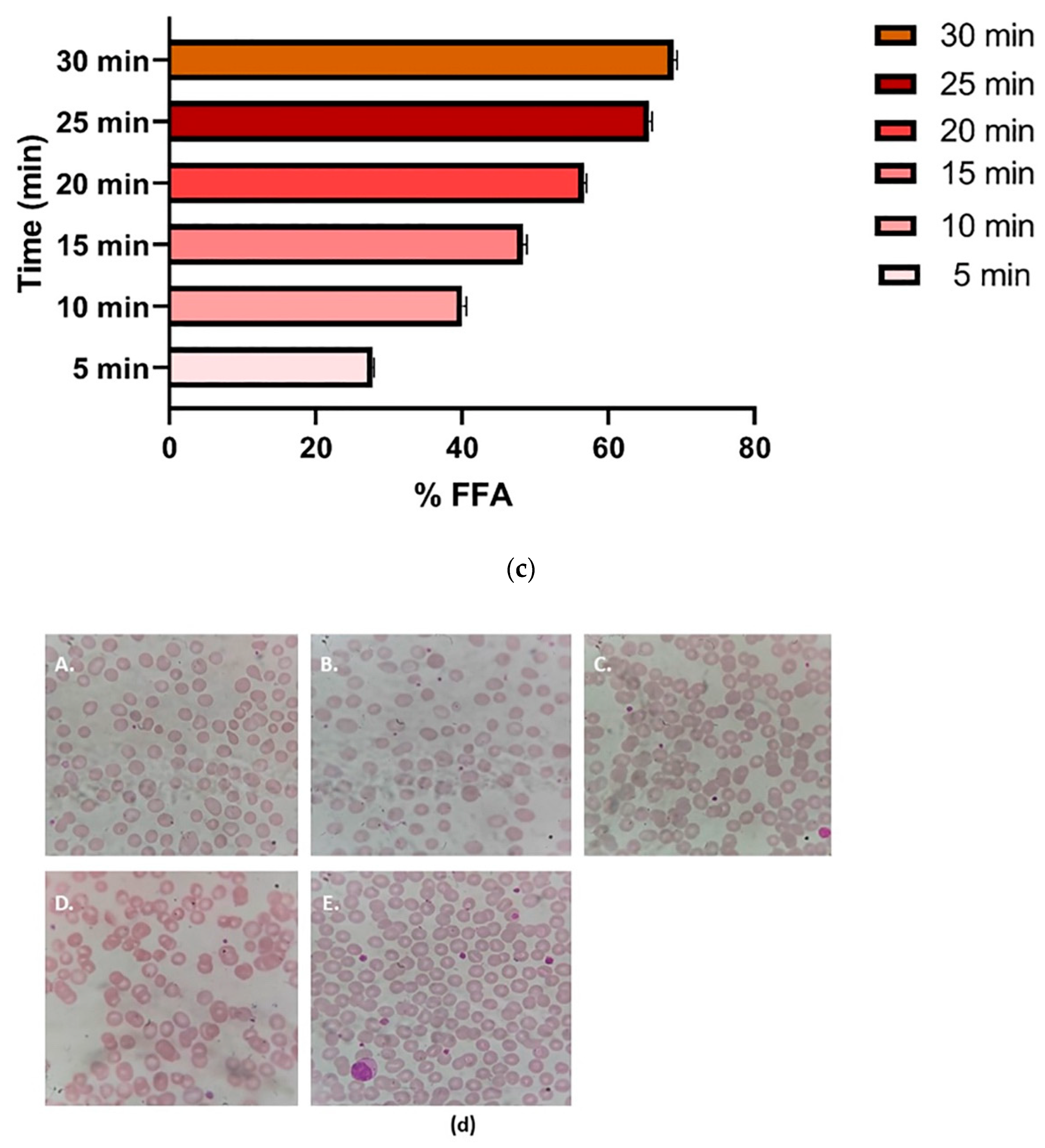
3.7.6. Hemolysis Test
3.8. Cell Line Studies
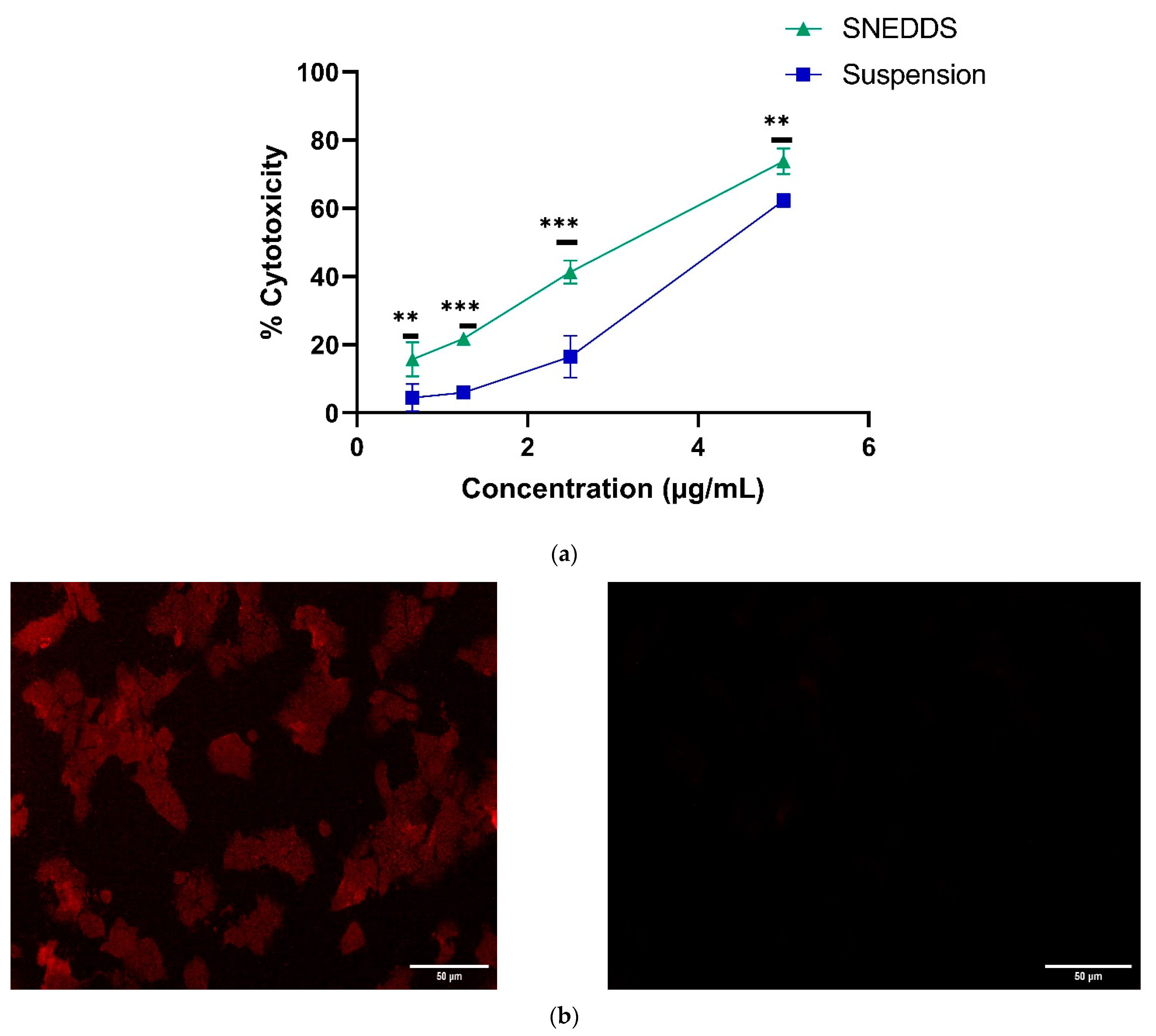
3.8.1. Cytotoxicity Studies
3.8.2. Cellular Uptake Studies
3.8.3. Estimation of Intracellular Antioxidant
Reactive Oxygen Species (ROS) in MCF-7 Cells by DCFDA Assay
Assessment of Superoxide Dismutase Activity (SOD)
3.9. Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landeros-Martínez, L.-L.; Chavez-Flores, D.; Orrantia-Borunda, E.; Flores-Holguin, N. Construction of a Nanodiamond–Tamoxifen Complex as a Breast Cancer Drug Delivery Vehicle. J. Nanomater. 2016, 2016, 2682105. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Vinodh, N.; Badwe, R.A.; Deo, S.V.S.; Manoharan, N.; Malik, R.; Panse, N.S.; Ramesh, C.; Shrivastava, A.; Swaminathan, R.; et al. Trends in Breast and Cervical Cancer in India under National Cancer Registry Programme: An Age-Period-Cohort Analysis. Cancer Epidemiol. 2021, 74, 101982. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Hassan, M.; Ullah, S.; Abbas, H.; Tawakkal, F.; Khan, M.A. Breast Cancer; Discovery of Novel Diagnostic Biomarkers, Drug Resistance, and Therapeutic Implications. Front. Mol. Biosci. 2022, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen Synthesis and Signaling Pathways during Aging: From Periphery to Brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Yue, W.; Wang, J.P.; Li, Y.; Fan, P.; Liu, G.; Zhang, N.; Conaway, M.; Wang, H.; Korach, K.S.; Bocchinfuso, W.; et al. Effects of Estrogen on Breast Cancer Development: Role of Estrogen Receptor Independent Mechanisms. Int. J. Cancer 2010, 127, 1748–1757. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, E.; Chakravarti, D.; Guttenplan, J.; Hart, E.; Ingle, J.; Jankowiak, R.; Muti, P.; Rogan, E.; Russo, J.; Santen, R.; et al. Catechol Estrogen Quinones as Initiators of Breast and Other Human Cancers: Implications for Biomarkers of Susceptibility and Cancer Prevention. Biochim. Biophys. Acta-Rev. Cancer 2006, 1766, 63–78. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wang, X. The Metabolic Mechanisms of Breast Cancer Metastasis. Front. Oncol. 2021, 10, 2942. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef] [Green Version]
- An, K.C. Selective Estrogen Receptor Modulators. Asian Spine J. 2016, 10, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Fontana, G.; Maniscalco, L.; Schillaci, D.; Cavallaro, G.; Giammona, G. Solid Lipid Nanoparticles Containing Tamoxifen Characterization and in Vitro Antitumoral Activity. Drug Deliv. 2005, 12, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Shete, H.; Chatterjee, S.; De, A.; Patravale, V. Long Chain Lipid Based Tamoxifen NLC. Part II: Pharmacokinetic, Biodistribution and in Vitro Anticancer Efficacy Studies. Int. J. Pharm. 2013, 454, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thanki, K.; Jain, S. Solidified Self-Nanoemulsifying Formulation for Oral Delivery of Combinatorial Therapeutic Regimen: Part I. Formulation Development, Statistical Optimization, and in Vitro Characterization. Pharm. Res. 2014, 31, 923–945. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, K.P.L.; Lantvit, D.; Christov, K.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Estrogenic and Antiestrogenic Properties of Resveratrol in Mammary Tumor Models. Cancer Res. 2001, 61, 7456–7463. [Google Scholar] [PubMed]
- Lu, R.; Serrero, G. Resveratrol, a Natural Product Derived from Grape, Exhibits Antiestrogenic Activity and Inhibits the Growth of Human Breast Cancer Cells. J. Cell. Physiol. 1999, 179, 297–304. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, J.; Xia, C.; Shi, X.; Jiang, B.H. Trans-3,4,5’-Trihydroxystibene Inhibits Hypoxia-Inducible Factor 1alpha and Vascular Endothelial Growth Factor Expression in Human Ovarian Cancer Cells. Clin. Cancer Res. 2004, 10, 5253–5263. [Google Scholar] [CrossRef] [Green Version]
- El-Mowafy, A.M.; Alkhalaf, M. Resveratrol Activates Adenylyl-Cyclase in Human Breast Cancer Cells: A Novel, Estrogen Receptor-Independent Cytostatic Mechanism. Carcinogenesis 2003, 24, 869–873. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Chowdhury, N.; Vhora, I.; Patel, K.; Bagde, A.; Kutlehria, S.; Singh, M. Development of Hot Melt Extruded Solid Dispersion of Tamoxifen Citrate and Resveratrol for Synergistic Effects on Breast Cancer Cells. AAPS PharmSciTech 2018, 19, 3287–3297. [Google Scholar] [CrossRef]
- Shi, X.P.; Miao, S.; Wu, Y.; Zhang, W.; Zhang, X.F.; Ma, H.Z.; Xin, H.L.; Feng, J.; Wen, A.D.; Li, Y. Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features. Int. J. Mol. Sci. 2013, 14, 15655–15668. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Kaur, P.; Gulati, M.; Singh, S.K.; Kumar, B.; Pandey, N.K.; Yadav, A.K.; Kumar, R.; Kuppusamy, G.; De, A.; et al. Coadministration of Polypeptide-k and Curcumin Through Solid Self-Nanoemulsifying Drug Delivery System for Better Therapeutic Effect Against Diabetes Mellitus: Formulation, Optimization, Biopharmaceutical Characterization, and Pharmacodynamic Assessment. Assay Drug Dev. Technol. 2019, 17, 201–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barani, M.; Sargazi, S.; Hajinezhad, M.R.; Rahdar, A.; Sabir, F.; Pardakhty, A.; Zargari, F.; Anwer, M.K.; Aboudzadeh, M.A. Preparation of PH-Responsive Vesicular Deferasirox: Evidence from in Silico, in Vitro, and in Vivo Evaluations. ACS Omega 2021, 6, 24218–24232. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K. Novel Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Oral Delivery of Cinnarizine: Design, Optimization, and In-Vitro Assessment. AAPS PharmSciTech 2012, 13, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Chime, S.; Kenechukwu, F.; Attama, A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery. In Application of Nanotechnology in Drug Delivery; Intech Open: London, UK, 2014; pp. 77–126. [Google Scholar]
- Pouton, C.W. Lipid Formulations for Oral Administration of Drugs: Non-Emulsifying, Self-Emulsifying and “self-Microemulsifying” Drug Delivery Systems. Eur. J. Pharm. Sci. 2000, 11, 93–98. [Google Scholar] [CrossRef]
- Tang, B.; Cheng, G.; Gu, J.C.; Xu, C.H. Development of Solid Self-Emulsifying Drug Delivery Systems: Preparation Techniques and Dosage Forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef]
- Schmidt, B.; Luan, C.; Passos, A.; Ferreira, C.; da Silva, J.L.; Fialho, E. Synergistic Effect of Curcumin, Piperine and Resveratrol in MCF-7 and MDA-MB-231 Breast Cancer Cells. Biomed. Res. 2020, 31, 113–118. [Google Scholar]
- Singh, A.; Neupane, Y.R.; Mangla, B.; Kohli, K. Nanostructured Lipid Carriers for Oral Bioavailability Enhancement of Exemestane: Formulation Design, In Vitro, Ex Vivo, and In Vivo Studies. J. Pharm. Sci. 2019, 108, 3382–3395. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Zhou, L.; Yang, L.; Tilton, S.; Wang, J. Development of a High Throughput Equilibrium Solubility Assay Using Miniaturized Shake-Flask Method in Early Drug Discovery. J. Pharm. Sci. 2007, 96, 3052–3071. [Google Scholar] [CrossRef]
- Villar, A.M.S.; Naveros, B.C.; Campmany, A.C.C.; Trenchs, M.A.; Rocabert, C.B.; Bellowa, L.H. Design and Optimization of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Enhanced Dissolution of Gemfibrozil. Int. J. Pharm. 2012, 431, 161–175. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Shi, C.H.; Zhang, X.; Tang, X.; Suo, H.; Yang, L.; Zhao, Y. Self-Microemulsifying Drug-Delivery System for Improved Oral Bioavailability of 20(S)-25-Methoxyl-Dammarane-3β, 12β, 20-Triol: Preparation and Evaluation. Int. J. Nanomed. 2014, 9, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, A.R.; Rahim-Bata, R.; Freeman, A.B.; Clarke, C.D.; Howard, R.L.; Strobl, J.S. Differentiation-Inducing Quinolines as Experimental Breast Cancer Agents in the MCF-7 Human Breast Cancer Cell Model. Biochem. Pharmacol. 2004, 68, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kos̈nik, A.; Szekalska, M.; Amelian, A.; Szymańska, E.; Winnicka, K. Development and Evaluation of Liquid and Solid Self-Emulsifying Drug Delivery Systems for Atorvastatin. Molecules 2015, 20, 21010–21022. [Google Scholar] [CrossRef] [Green Version]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In Statistical Approaches With Emphasis on Design of Experiments Applied to Chemical Processes; InTech: London, UK, 2018. [Google Scholar]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; García, M.L. Optimizing Flurbiprofen-Loaded NLC by Central Composite Factorial Design for Ocular Delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef]
- Seo, Y.G.; Kim, D.H.; Ramasamy, T.; Kim, J.H.; Marasini, N.; Oh, Y.K.; Kim, D.W.; Kim, J.K.; Yong, C.S.; Kim, J.O.; et al. Development of Docetaxel-Loaded Solid Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Enhanced Chemotherapeutic Effect. Int. J. Pharm. 2013, 452, 412–420. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Singh, H.P.; Patra, C.N.; Rao, M.B. Development, Optimization, and Characterization of Solid Self-Nanoemulsifying Drug Delivery Systems of Valsartan Using Porous Carriers. AAPS PharmSciTech 2012, 13, 1416–1427. [Google Scholar] [CrossRef] [Green Version]
- Muta, T.; Parikh, A.; Kathawala, K.; Haidari, H.; Song, Y.; Thomas, J.; Garg, S. Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties. Pharmaceutics 2020, 12, 1091. [Google Scholar] [CrossRef]
- Jain, K.; Kumar, R.; Sood, S.; Dhyanandhan, G. Betaxolol Hydrochloride Loaded Chitosan Nanoparticles for Ocular Delivery and Their Anti-Glaucoma Efficacy. Curr. Drug Deliv. 2013, 10, 493–499. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Odeberg, J.M.; Kaufmann, P.; Kroon, K.G.; Höglund, P. Lipid Drug Delivery and Rational Formulation Design for Lipophilic Drugs with Low Oral Bioavailability, Applied to Cyclosporine. Eur. J. Pharm. Sci. 2003, 20, 375–382. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Chung, R.; Song, Y.; Nagalingam, G.; Triccas, J.; Wang, L.; Liu, L.; Hayball, J.D.; et al. Synthesis and Characterization of PH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery. Pharmaceutics 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, N.; Madni, A.; Balasubramanian, V.; Rehman, M.; Correia, A.; Kashif, P.M.; Mäkilä, E.; Salonen, J.; Santos, H.A. Development and Optimization of Methotrexate-Loaded Lipid-Polymer Hybrid Nanoparticles for Controlled Drug Delivery Applications. Int. J. Pharm. 2017, 533, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thanki, K.; Jain, S. Solidified Self-Nanoemulsifying Formulation for Oral Delivery of Combinatorial Therapeutic Regimen: Part II. In vivo Pharmacokinetics, Antitumor Efficacy and Hepatotoxicity. Pharm. Res. 2014, 31, 946–958. [Google Scholar] [CrossRef]
- Balakumar, K.; Raghavan, C.V.; Selvan, N.T.; Prasad, R.H.; Abdu, S. Self Nanoemulsifying Drug Delivery System (SNEDDS) of Rosuvastatin Calcium: Design, Formulation, Bioavailability and Pharmacokinetic Evaluation. Colloids Surfaces B Biointerfaces 2013, 112, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Nabi, B.; Baboota, S.; Ali, J. Tailoring Lipid Nanoconstructs for the Oral Delivery of Paliperidone: Formulation, Optimization and in Vitro Evaluation. Chem. Phys. Lipids 2021, 234, 105005. [Google Scholar] [CrossRef]
- Al-Mohizea, A.M. Influence of Intestinal Efflux Pumps on the Absorption and Transport of Furosemide. Saudi Pharm. J. 2010, 18, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Manca, M.L.; Manconi, M.; Nacher, A.; Carbone, C.; Valenti, D.; MacCioni, A.M.; Sinico, C.; Fadda, A.M. Development of Novel Diolein-Niosomes for Cutaneous Delivery of Tretinoin: Influence of Formulation and in Vitro Assessment. Int. J. Pharm. 2014, 477, 176–186. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Curcumin-Loaded Lipid Nanocarrier for Improving Bioavailability, Stability and Cytotoxicity against Malignant Glioma Cells. Drug Deliv. 2016, 23, 214–229. [Google Scholar] [CrossRef]
- Ji, C.; Shin, J.A.; Hong, S.T.; Lee, K.T. In Vitro Study for Lipolysis of Soybean Oil, Pomegranate Oil, and Their Blended and Interesterified Oils under a PH-Stat Model and a Simulated Model of Small Intestinal Digestion. Nutrients 2019, 11, 678. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Shakeel, F.; Singh, S.K.; Alsarra, I.A.; Faruk, A.; Alanazi, F.K.; Peter Christoper, G.V. Solidified SNEDDS for the Oral Delivery of Rifampicin: Evaluation, Proof of Concept, in Vivo Kinetics, and in Silico GastroPlusTM Simulation. Int. J. Pharm. 2019, 566, 203–217. [Google Scholar] [CrossRef]
- Guo, S.; Shi, Y.; Liang, Y.; Liu, L.; Sun, K.; Li, Y. Relationship and Improvement Strategies between Drug Nanocarrier Characteristics and Hemocompatibility: What Can We Learn from the Literature. Asian J. Pharm. Sci. 2021, 16, 551–576. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Arshad, R.; Razzaq, S.; Nousheen, K.; Kiani, M.H.; Shahnaz, G. Formulation and Evaluation of Hyaluronic Acid-Based Mucoadhesive Self Nanoemulsifying Drug Delivery System (SNEDDS) of Tamoxifen for Targeting Breast Cancer. Int. J. Biol. Macromol. 2020, 152, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, A.; Cavalli, R.; Bocca, C.; Gabriel, L.; Rosa Gasco, M. Cellular Uptake and Cytotoxicity of Solid Lipid Nanospheres (SLN) Incorporating Doxorubicin or Paclitaxel. Int. J. Pharm. 2000, 210, 61–67. [Google Scholar] [CrossRef]
- Isnaini, I.; Permatasari, N.; Mintaroem, K.; Prihartini, B.; Widodo, M.A. Oxidants-Antioxidants Profile in the Breast Cancer Cell Line MCF-7. Asian Pac. J. Cancer Prev. 2018, 19, 3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badia, E.; Morena, M.; Lauret, C.; Boulahtouf, A.; Boulle, N.; Cavaillès, V.; Balaguer, P.; Cristol, J.P. Effect of Tamoxifen and Fulvestrant Long-Term Treatments on ROS Production and (pro/Anti)-Oxidant Enzymes MRNA Levels in a MCF-7-Derived Breast Cancer Cell Line. Breast Cancer 2016, 23, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Metallothionein and Superoxide Dismutase-Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3253. [Google Scholar] [CrossRef] [Green Version]
- Alarifi, S. Assessment of MCF-7 Cells as an in Vitro Model System for Evaluation of Chemical Oxidative Stressors. Afr. J. Biotechnol. 2011, 10, 3872–3879. [Google Scholar] [CrossRef]
- Bandivadeka, M.M.; Pancholi, S.S.; Kaul-Ghanekar, R.; Choudhari, A.; Koppikar, S. Self-Microemulsifying Smaller Molecular Volume Oil (Capmul MCM) Using Non-Ionic Surfactants: A Delivery System for Poorly Water-Soluble Drug. Drug Dev. Ind. Pharm. 2012, 38, 883–892. [Google Scholar] [CrossRef]
- Solanki, S.S.; Sarkar, B.; Dhanwani, R.K. Microemulsion Drug Delivery System: For Bioavailability Enhancement of Ampelopsin. ISRN Pharm. 2012, 2012, 108164. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-Nanoemulsifying Drug Delivery System (SNEDDS) of the Poorly Water-Soluble Grapefruit Flavonoid Naringenin: Design, Characterization, in Vitro and in Vivo Evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Shah, M.; Agrawal, Y.K.; Garala, K.; Ramkishan, A. Solid Lipid Nanoparticles of a Water Soluble Drug, Ciprofloxacin Hydrochloride. Indian J. Pharm. Sci. 2012, 74, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Alghananim, A.; Özalp, Y.; Mesut, B.; Serakinci, N.; Özsoy, Y.; Güngör, S. A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-Snedds) of Deferasirox for Improved Solubility: Optimization, Characterization, and in Vitro Cytotoxicity Studies. Pharmaceuticals 2020, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Ashhar, M.U.; Kumar, S.; Ali, J.; Baboota, S. CCRD Based Development of Bromocriptine and Glutathione Nanoemulsion Tailored Ultrasonically for the Combined Anti-Parkinson Effect. Chem. Phys. Lipids 2021, 235, 105035. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.; Parikh, A.; Chaudhuri, A.; Ali, J.; Baboota, S.; Garg, S. Lipid-Based Combinational Drug Delivery Systems: Maximizing the Performance of Cocktail Therapy. Nanocarr. Deliv. Comb. Drugs 2021, 259–305. [Google Scholar] [CrossRef]
- Dangre, P.; Gilhotra, R.; Dhole, S. Formulation and Statistical Optimization of Self-Microemulsifying Drug Delivery System of Eprosartan Mesylate for Improvement of Oral Bioavailability. Drug Deliv. Transl. Res. 2016, 6, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, P.; Pokharkar, V. Risk Assessment and QbD Based Optimization of an Eprosartan Mesylate Nanosuspension: In-Vitro Characterization, PAMPA and in-Vivo Assessment. Int. J. Pharm. 2019, 567, 118415. [Google Scholar] [CrossRef]
- Yan, J.; Yao, Y.; Yan, S.; Gao, R.; Lu, W.; He, W. Chiral Protein Supraparticles for Tumor Suppression and Synergistic Immunotherapy: An Enabling Strategy for Bioactive Supramolecular Chirality Construction. Nano Lett. 2020, 20, 5844–5852. [Google Scholar] [CrossRef]
- Severino, P.; Pinho, S.C.; Souto, E.B.; Santana, M.H. Polymorphism, Crystallinity and Hydrophilic-Lipophilic Balance of Stearic Acid and Stearic Acid-Capric/Caprylic Triglyceride Matrices for Production of Stable Nanoparticles. Colloids Surf. B Biointerfaces 2011, 86, 125–130. [Google Scholar] [CrossRef]
- Kamel, A.O.; Mahmoud, A.A. Enhancement of Human Oral Bioavailability and in Vitro Antitumor Activity of Rosuvastatin via Spray Dried Self-Nanoemulsifying Drug Delivery System. J. Biomed. Nanotechnol. 2013, 9, 26–39. [Google Scholar] [CrossRef]
- Ujilestari, T.; Martien, R.; Ariyadi, B.; Dono, N.D. Zuprizal Self-Nanoemulsifying Drug Delivery System (SNEDDS) of Amomum Compactum Essential Oil: Design, Formulation, and Characterization. J. Appl. Pharm. Sci. 2018, 8, 14–21. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Morales-Hernández, N.; Lobato-Calleros, C.; Vernon-Carter, E.J. Mesquite Gum/Chitosan Insoluble Complexes: Relationship between the Water State and Viscoelastic Properties. J. Dispers. Sci. Technol. 2019, 40, 1345–1352. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Alias, D.; Yunus, R.; Chong, G.H.; Che Abdullah, C.A. Single Step Encapsulation Process of Tamoxifen in Biodegradable Polymer Using Supercritical Anti-Solvent (SAS) Process. Powder Technol. 2017, 309, 89–94. [Google Scholar] [CrossRef]
- Neerati, P.; Palle, S. Resveratrol Nanoparticle Pretreatment Improved the Oral Bioavailability of Bromocriptine: Involvement of Liver and Intestinal CYP3A Enzyme Inhibition. J. Nat. Sci. Biol. Med. 2019, 10, 209–216. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges for Delivery of Resveratrol: In Vitro Characterisation, Stability, Cytotoxicity and Permeation Study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Sreeharsha, N.; Hiremath, J.G.; Chilukuri, S.; Aitha, R.K.; Al-Dhubiab, B.E.; Venugopala, K.N.; Alzahrani, A.M.; Meravanige, G. An Approach to Enhance Dissolution Rate of Tamoxifen Citrate. Biomed. Res. Int. 2019, 2019, 2161348. [Google Scholar] [CrossRef]
- Patel, P.; Ahir, K.; Patel, V.; Manani, L.; Patel, C. Drug-Excipient Compatibility Studies: First Step for Dosage Form Development. Pharma Innov. J. 2015, 4, 14–20. [Google Scholar]
- Khade, S.; Pore, Y. Formulation and Evaluation of Neusilin® US2 Adsorbed Amorphous Solid Self-Microemulsifying Delivery System of Atorvastatin Calcium. Asian J. Pharm. Clin. Res. 2016, 9, 93–100. [Google Scholar]
- Choi, J.S.; Choi, B.C.; Kang, K.W. Effect of Resveratrol on the Pharmacokinetics of Oral and Intravenous Nicardipine in Rats: Possible Role of P-Glycoprotein Inhibition by Resveratrol. Pharmazie 2009, 64, 49–52. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Cheng, H.; Zhang, M.; Kou, Y.; Guan, J.; Liu, Q.; Gao, M.; Wang, X.; Mao, S. Modulating Intestinal Mucus Barrier for Nanoparticles Penetration by Surfactants. Asian J. Pharm. Sci. 2019, 14, 543–551. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.K.; Verma, P.R.P. Fabrication of Lipidic Nanocarriers of Loratadine for Facilitated Intestinal Permeation Using Multivariate Design Approach. Drug Dev. Ind. Pharm. 2016, 42, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, S.K.; Anantharaman, N.V.; Subramanian, S.; Sivasubramanian, S.; Madhan, B. Paclitaxel/Epigallocatechin Gallate Coloaded Liposome: A Synergistic Delivery to Control the Invasiveness of MDA-MB-231 Breast Cancer Cells. Colloids Surfaces B Biointerfaces 2015, 125, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pruchnik, H.; Włoch, A.; Bonarska-Kujawa, D.; Kleszczyńska, H. An In Vitro Study of the Effect of Cytotoxic Triorganotin Dimethylaminophenylazobenzoate Complexes on Red Blood Cells. J. Membr. Biol. 2018, 251, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtartavan, S.; Karimi, M.; Karimian, K.; Azarpira, N.; Khatami, M.; Heli, H. Evaluation of a Self-Nanoemulsifying Docetaxel Delivery System. Biomed. Pharmacother. 2019, 109, 2427–2433. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative Cellular Uptake, Localization and Cytotoxicity of Curcumin in Normal and Tumor Cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bae, E.J.; Lee, M.K. Enhanced Anticancer Activity and Intracellular Uptake of Paclitaxel-Containing Solid Lipid Nanoparticles in Multidrug-Resistant Breast Cancer Cells. Int. J. Nanomed. 2018, 13, 7549–7563. [Google Scholar] [CrossRef] [Green Version]
- Gundimeda, U.; Chen, Z.H.; Gopalakrishna, R. Tamoxifen Modulates Protein Kinase C via Oxidative Stress in Estrogen Receptor-Negative Breast Cancer Cells. J. Biol. Chem. 1996, 271, 13504–13514. [Google Scholar] [CrossRef] [Green Version]
- Bekele, R.T.; Venkatraman, G.; Liu, R.Z.; Tang, X.; Mi, S.; Benesch, M.G.K.; MacKey, J.R.; Godbout, R.; Curtis, J.M.; McMullen, T.P.W.; et al. Oxidative Stress Contributes to the Tamoxifen-Induced Killing of Breast Cancer Cells: Implications for Tamoxifen Therapy and Resistance. Sci. Rep. 2016, 6, 21164. [Google Scholar] [CrossRef] [Green Version]
- Shibata, T.; Kokubu, A.; Gotoh, M.; Ojima, H.; Ohta, T.; Yamamoto, M.; Hirohashi, S. Genetic Alteration of Keap1 Confers Constitutive Nrf2 Activation and Resistance to Chemotherapy in Gallbladder Cancer. Gastroenterology 2008, 135, 1358–1368. [Google Scholar] [CrossRef]
- Singh, A.K.; Chaurasiya, A.; Singh, M.; Upadhyay, S.C.; Mukherjee, R.; Khar, R.K. Exemestane Loaded Self-Microemulsifying Drug Delivery System (SMEDDS): Development and Optimization. AAPS PharmSciTech 2008, 9, 628–634. [Google Scholar] [CrossRef]
- Cho, S.K.; Pedram, A.; Levin, E.R.; Kwon, Y.J. Acid-Degradable Core-Shell Nanoparticles for Reversed Tamoxifen-Resistance in Breast Cancer by Silencing Manganese Superoxide Dismutase (MnSOD). Biomaterials 2013, 34, 10228–10237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henidi, H.A.; Al-Abbasi, F.A.; El-Moselhy, M.A.; El-Bassossy, H.M.; Al-Abd, A.M.; Gil, G. Despite Blocking Doxorubicin-Induced Vascular Damage, Quercetin Ameliorates Its Antibreast Cancer Activity. Oxid. Med. Cell. Longev. 2020, 2020, 8157640. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patil, S.R.; Swarnakar, N.K.; Agrawal, A.K. Oral Delivery of Doxorubicin Using Novel Polyelectrolyte-Stabilized Liposomes (Layersomes). Mol. Pharm. 2012, 9, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Harivardhan Reddy, L.; Vivek, K.; Bakshi, N.; Murthy, R.S.R. Tamoxifen Citrate Loaded Solid Lipid Nanoparticles (SLNTM): Preparation, Characterization, in Vitro Drug Release, and Pharmacokinetic Evaluation. Pharm. Dev. Technol. 2006, 11, 167–177. [Google Scholar] [CrossRef]





| Factors | Level Used | ||||
|---|---|---|---|---|---|
| Independent Variables | Axial (−α) | Lower (−1) | Medium (0) | Upper (+1) | Axial (+α) |
| Oil (mL) = X1 | 0.358579 | 0.4 | 0.5 | 0.6 | 0.641421 |
| Smix (mL) = X2 | 1.75858 | 1.8 | 1.9 | 2.00 | 2.04142 |
| Dependent Variables | Constraints | ||||
| Droplet size (nm) = Y1 | Minimum | ||||
| PDI = Y2 | Minimum | ||||
| % Transmittance = Y3 | Maximum | ||||
| Dilutions | Formulations | Droplet Size (nm) | PDI | % Transmittance | Inference |
|---|---|---|---|---|---|
| 50 folds | s-SNEDDS | 87.98 ± 1.6 | 0.196 ± 0.21 | 97.41 ± 0.74 | No phase separation |
| 100 folds | s-SNEDDS | 95.71 ± 3.29 | 0.368 ± 0.05 | 94.37 ± 0.21 | No phase separation |
| 200 folds | s-SNEDDS | 95.75 ± 1.35 | 0.399 ± 0.47 | 94.57 ± 3.87 | No phase separation |
| Simulated Digestive Fluids | Droplet Size (nm) | PDI | % Transmittance | Inference |
|---|---|---|---|---|
| pH 1.2 | 72.78 ± 0.11 | 0.2 ± 0.003 | 91.62 ± 1.10 | No phase separation |
| pH 6.8 | 78.33 ± 0.40 | 0.149 ± 0.004 | 92.87 ±0.80 | No phase separation |
| After incubation period | ||||
| pH 1.2 | 82.94 ± 0.197 | 0.215 ± 0.002 | 90.307 ± 0.32 | No phase separation |
| pH 6.8 | 84.46 ± 0.525 | 0.185 ± 0.011 | 94.275 ± 0.04 | No phase separation |
| Pharmacokinetic Parameters | TAM Suspension | TAM-RES-Suspension | TAM-RES-s-SNEDDS | ||
|---|---|---|---|---|---|
| TAM | TAM | RES | TAM | RES | |
| Cmax (ng/mL) | 77.099 | 193.206 | 3933.471 | 321.480 | 8022.685 |
| Tmax (h) | 3 | 3 | 1 | 3 | 1 |
| AUC (ng/mL.h) | 1113.495 | 2290.534 | 18,926.140 | 3737.314 | 44,533.762 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastava, N.; Parikh, A.; Dewangan, R.P.; Biswas, L.; Verma, A.K.; Mittal, S.; Ali, J.; Garg, S.; Baboota, S. Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics 2022, 14, 1486. https://doi.org/10.3390/pharmaceutics14071486
Shrivastava N, Parikh A, Dewangan RP, Biswas L, Verma AK, Mittal S, Ali J, Garg S, Baboota S. Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics. 2022; 14(7):1486. https://doi.org/10.3390/pharmaceutics14071486
Chicago/Turabian StyleShrivastava, Nupur, Ankit Parikh, Rikeshwer Prasad Dewangan, Largee Biswas, Anita Kamra Verma, Saurabh Mittal, Javed Ali, Sanjay Garg, and Sanjula Baboota. 2022. "Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer" Pharmaceutics 14, no. 7: 1486. https://doi.org/10.3390/pharmaceutics14071486
APA StyleShrivastava, N., Parikh, A., Dewangan, R. P., Biswas, L., Verma, A. K., Mittal, S., Ali, J., Garg, S., & Baboota, S. (2022). Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics, 14(7), 1486. https://doi.org/10.3390/pharmaceutics14071486