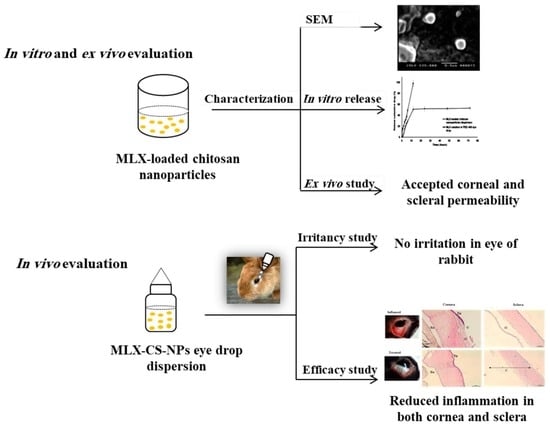
Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Study of MLX
2.3. Preparation of MLX-CS-NPs
2.4. In Vitro Characterization of the Prepared MLX-CS-NPs
2.4.1. Entrapment Efficiency (EE%)
2.4.2. Evaluation of the Average Particle Size, Zeta Potential, and Morphology
2.4.3. pH Determination
2.4.4. Viscosity Measurements
2.4.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4.6. In Vitro Release Studies and Kinetic Analysis of the Release Data
2.4.7. Ex Vivo Ocular Permeation Study
Permeation Data Analysis
2.5. Preparation of MLX-CS-NPs Eye Drop Dispersion for the In Vivo Studies
2.6. In Vivo Studies
2.6.1. Eye Irritancy Evaluation
- Group I:
- Blank CS/PEG 400 eye drop solution (drug-free).
- Group II:
- MLX-eye drop solution.
- Group III:
- MLX-CS-NPs eye drop dispersion.
2.6.2. Anti-Inflammatory Activity Study
- Group I:
- Blank CS/PEG 400 eye drop solution (drug-free)
- Group II:
- MLX-eye drop solution.
- Group III:
- MLX-CS-NPs eye drop dispersion.
2.6.3. Histopathological Examination
2.7. Statistical Analyses
3. Results and Discussion
3.1. Solubility Study of MLX
3.2. Preparation and Characterization of MLX-CS-NPs
3.2.1. Morphology, Entrapment Efficiency, Average Particle Size, and Zeta Potential Measurements
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.3. In Vitro Release and Kinetic Studies
3.2.4. Ex Vivo Corneal and Scleral Permeability
3.3. In Vivo Studies
3.3.1. Eye Irritancy Assessment
3.3.2. In Vivo Anti-Inflammatory Study
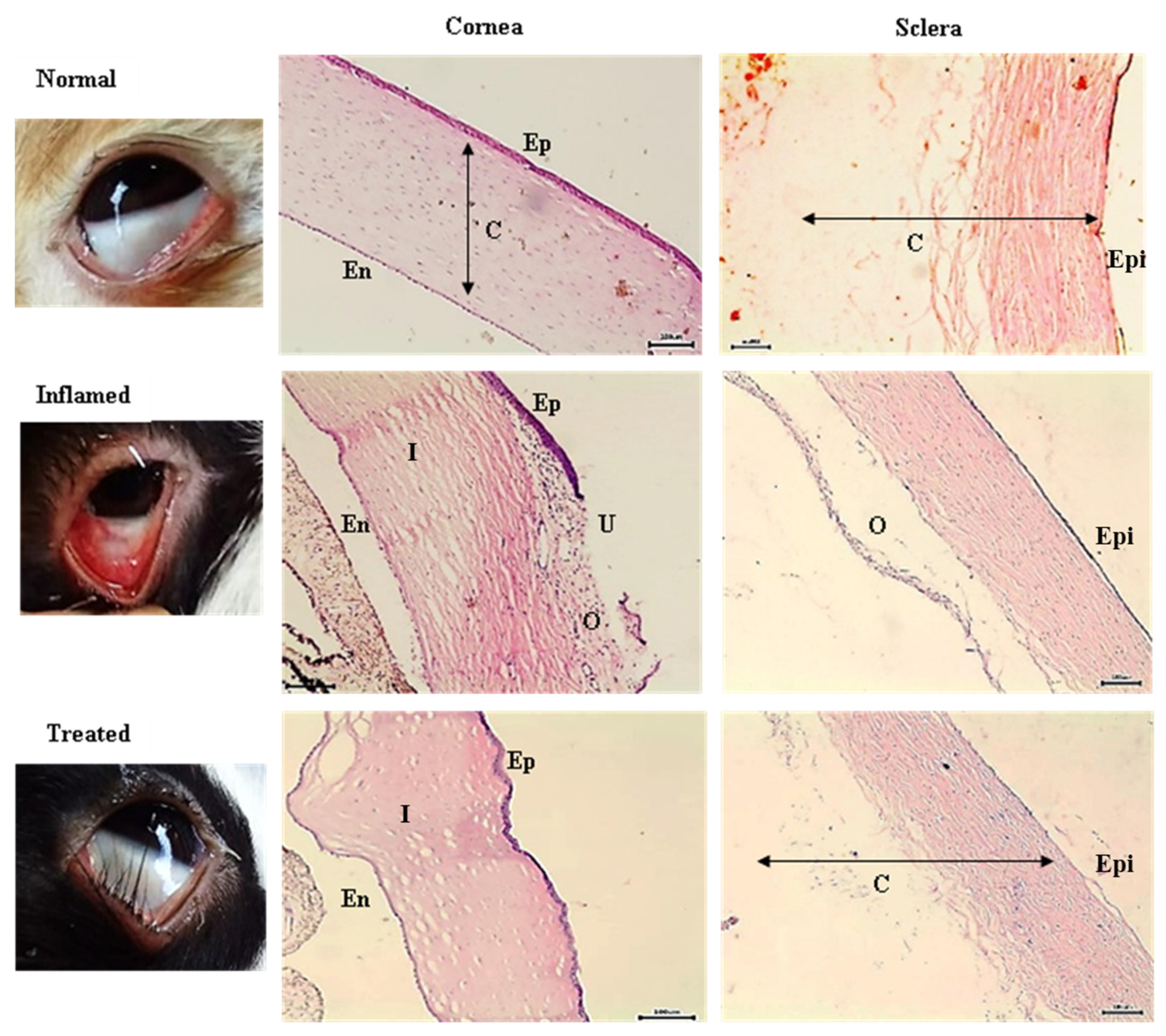
3.3.3. Pathohistological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagi Glass, L.R.; Freitag, S.K. Orbital inflammation: Corticosteroids first. Surv. Ophthalmol. 2016, 61, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.F.; Abdalla, A.M.; Guda, T.K.; Gabr, K.E.; Royall, P.G.; Alqurshi, A. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int. J. Nanomed. 2019, 14, 3679–3689. [Google Scholar] [CrossRef] [PubMed]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Kadam, R.S.; Lee, V.H. Recent advances in ophthalmic drug delivery. Ther. Deliv. 2010, 1, 435–456. [Google Scholar] [CrossRef]
- Wisher, D. Martindale: The Complete Drug Reference. J. Med. Libr. Assoc. JMLA 2012, 100, 75–76. [Google Scholar] [CrossRef]
- Distel, M.; Mueller, C.; Bluhmki, E.; Fries, J. Safety of meloxicam: A global analysis of clinical trials. Br. J. Rheumatol. 1996, 35 (Suppl. S1), 68–77. [Google Scholar] [CrossRef]
- Sindi, A.M.; Hosny, K.M.; Alharbi, W.S. Lyophilized Composite Loaded with Meloxicam-Peppermint oil Nanoemulsion for Periodontal Pain. Polymers 2021, 13, 2317. [Google Scholar] [CrossRef]
- Cruz, R.; Quintana-Hau, J.D.; González, J.R.; Tornero-Montaño, R.; Baiza-Durán, L.M.; Vega, L. Effects of an ophthalmic formulation of meloxicam on COX-2 expression, PGE2 release, and cytokine expression in a model of acute ocular inflammation. Br. J. Ophthalmol. 2008, 92, 120–125. [Google Scholar] [CrossRef]
- Zhang, W.; Zu, D.; Chen, J.; Peng, J.; Liu, Y.; Zhang, H.; Li, S.; Pan, W. Bovine serum albumin-meloxicam nanoaggregates laden contact lenses for ophthalmic drug delivery in the treatment of postcataract endophthalmitis. Int. J. Pharm. 2014, 475, 25–34. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, M.T.-Y.; Ng, A.H.C.; Wong, T.T.; Mehta, J.S. Nanotechnology for the Treatment of Allergic Conjunctival Diseases. Pharmaceuticals 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, D.A.; Lohiya, G.; Katti, D.S. Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kang, C.; Fang, F. Biometric Measurement of Anterior Segment: A Review. Sensors 2020, 20, 4285. [Google Scholar] [CrossRef] [PubMed]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.C.; Chang, S.F.; Liu, C.Y.; Kao, W.W.; Huang, C.H.; Liaw, J. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J. Gene Med. 2007, 9, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef]
- Moya-Ortega, M.D.; Alves, T.F.; Alvarez-Lorenzo, C.; Concheiro, A.; Stefánsson, E.; Thorsteinsdóttir, M.; Loftsson, T. Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int. J. Pharm. 2013, 441, 507–515. [Google Scholar] [CrossRef]
- Jóhannesson, G.; Moya-Ortega, M.D.; Ásgrímsdóttir, G.M.; Lund, S.H.; Thorsteinsdóttir, M.; Loftsson, T.; Stefánsson, E. Kinetics of γ-cyclodextrin nanoparticle suspension eye drops in tear fluid. Acta Ophthalmol. 2014, 92, 550–556. [Google Scholar] [CrossRef]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef]
- Hassan, E.E.; Gallo, J.M. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef]
- Alonso, M.J.; Sánchez, A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2003, 55, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Abd-Elgawad, A.H.; Soliman, O.A.; Jablonski, M.M. Stability and Ocular Pharmacokinetics of Celecoxib-Loaded Nanoparticles Topical Ophthalmic Formulations. J. Pharm. Sci. 2016, 105, 3691–3701. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-J.; Kim, J.-G.; Kim, J.-Y.; Kim, S.; Lee, H.; Hyun, C.-G. Anti-inflammatory effect of chitosan oligosaccharides in RAW 264.7 cells. Cent. Eur. J. Biol. 2010, 5, 95–102. [Google Scholar] [CrossRef]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Chandra Hembram, K.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314. [Google Scholar] [CrossRef]
- López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698. [Google Scholar] [CrossRef]
- Pund, S.; Rasve, G.; Borade, G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 195–201. [Google Scholar] [CrossRef]
- Gómez-Segura, L.; Parra, A.; Calpena-Campmany, A.C.; Gimeno, Á.; Gómez de Aranda, I.; Boix-Montañes, A. Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues. Nanomaterials 2020, 10, 355. [Google Scholar] [CrossRef]
- Enríquez de Salamanca, A.; Diebold, Y.; Calonge, M.; García-Vazquez, C.; Callejo, S.; Vila, A.; Alonso, M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef]
- OECD. Test No. 405: Acute Eye Irritation/Corrosion; OECD: Paris, France, 2021. [Google Scholar] [CrossRef]
- Krishnatreyya, H.; Hazarika, H.; Saha, A.; Chattopadhyay, P. Capsaicin, the primary constituent of pepper sprays and its pharmacological effects on mammalian ocular tissues. Eur. J. Pharmacol. 2018, 819, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.G.; Garcia de la Rubia, P.; Gallar, J.; Belmonte, C. Reduction of capsaicin-induced ocular pain and neurogenic inflammation by calcium antagonists. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3329–3335. [Google Scholar]
- Habib, F.S.; El-mahdy, M.M.; Abdel-Hafez, A.M.M.; Maher, S. Microemulsion for ocular delivery: Ocular irritancy test and in vivo studies of anti-inflammatory action. J. Drug Deliv. Sci. Technol. 2012, 22, 541–544. [Google Scholar] [CrossRef]
- Wilhelmus, K.R. The Draize eye test. Surv. Ophthalmol. 2001, 45, 493–515. [Google Scholar] [CrossRef]
- El-Maradny, H.A.; Mortada, S.A.; Kamel, O.A.; Hikal, A.H. Characterization of ternary complexes of meloxicam-HPbetaCD and PVP or L-arginine prepared by the spray-drying technique. Acta Pharm. 2008, 58, 455–466. [Google Scholar] [CrossRef]
- Al-Nima, A.; Alkotaji, M.; Ahlam, A.K. Preparation and evaluation of meloxicam solid dispersions by solvent evaporation method. Int. Res. J. Pharm. 2014, 5, 838–845. [Google Scholar] [CrossRef]
- Nalluri, B.N.; Chowdary, K.P.R.; Murthy, K.V.R.; Satyanarayana, V.; Hayman, A.R.; Becket, G. Inclusion Complexation and Dissolution Properties of Nimesulideand Meloxicam–hydroxypropyl-β-cyclodextrin Binary Systems. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 103–110. [Google Scholar] [CrossRef]
- Obaidat, R.; Altaani, B.; Al-quraan, H. Effect of selected polymers on dissolution and stabilization of amorphous form of meloxicam. Int. J. Pharm. Pharm. Sci. 2017, 9, 33. [Google Scholar] [CrossRef][Green Version]
- Babu, P.R.S.; Subrahmanyam, C.V.S.; Thimmasetty, J.; Manavalan, R.; Valliappan, K. Solubility of Meloxicam in Mixed Solvent Systems. Ethiop. Pharm. J. 2007, 25, 23–28. [Google Scholar] [CrossRef]
- Seedher, N.; Bhatia, S. Solubility enhancement of Cox-2 inhibitors using various solvent systems. AAPS PharmSciTech 2003, 4, E33. [Google Scholar] [CrossRef]
- Babu, P.R.; Subrahmanyam, C.V.; Thimmasetty, J.; Manavalan, R.; Valliappan, K. Extended Hansen’s solubility approach: Meloxicam in individual solvents. Pak. J. Pharm. Sci. 2007, 20, 311–316. [Google Scholar] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.A.; Hamidi, M.; Mohammadi-Samani, S. Preparation, optimization, and in-vitro/in-vivo/ex-vivo characterization of chitosan-heparin nanoparticles: Drug-induced gelation. J. Pharm. Pharmacol. 2013, 65, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Rezaei Mokarram, A.; Alonso, M.J. Preparation and evaluation of chitosan nanoparticles containing Diphtheria toxoid as new carriers for nasal vaccine delivery in mice. Arch. Razi Inst. 2006, 61, 13–25. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, 1st ed.; Nimesh, S., Chandra, R., Gupta, Eds.; Woodhead Publishing: Sawston, UK, 2017; Volume 1, pp. 44–58. [Google Scholar]
- Badawi, A.A.; El-Laithy, H.M.; El Qidra, R.K.; El Mofty, H.; El dally, M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch. Pharm. Res. 2008, 31, 1040–1049. [Google Scholar] [CrossRef]
- Büyük, N.İ.; Arayici, P.P.; Derman, S.; Mustafaeva, Z.; Yücel, S. An Optimization Study for Chitosan Nanoparticles: Synthesis and Characterization. Celal Bayar Univ. J. Sci. 2020, 16, 119–127. [Google Scholar]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen Biodegradable Nanoparticles: One Step Closer towards a Better Ocular Interaction Study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, Y.; Gao, F.; Yuan, H.; Lan, M.; Lou, K.; Wang, W. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 2013, 446, 191–198. [Google Scholar] [CrossRef]
- Attia Shafie, M.A.; Mohammed Fayek, H. Formulation and evaluation of betamethasone sodium phosphate loaded nanoparticles for ophthalmic delivery. J. Clin. Exp. Ophthalmol. 2013, 4, 2. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef] [PubMed]





| System Code | Polymers | |||||
|---|---|---|---|---|---|---|
| PG (% w/v) | HPβ-CD (% w/v) | PVP (% w/v) | HPβ-CD (10% w/v): PF-127 (5% w/v) | TPP (% w/v) | PEG 400 (%) | |
| A1 | 10 | ------ | ------ | ------ | ------ | ------ |
| A2 | 20 | ------ | ------ | ------ | ------ | ------ |
| A3 | 100 | ------ | ------ | ------ | ------ | ------ |
| A4 | ------ | 1 | ------ | ------ | ------ | ------ |
| A5 | ------ | 2.5 | ------ | ------ | ------ | ------ |
| A6 | ------ | 5 | ------ | ------ | ------ | ------ |
| A7 | ------ | 10 | ------ | ------ | ------ | ------ |
| A8 | ------ | ------ | ------ | 9:1 | ------ | ------ |
| A9 | ------ | ------ | ------ | 8:2 | ------ | ------ |
| A10 | ------ | ------ | ------ | 7:3 | ------ | ------ |
| A11 | ------ | ------ | ------ | 6:4 | ------ | ------ |
| A12 | ------ | ------ | ------ | 5:5 | ------ | ------ |
| A13 | ------ | ------ | ------ | 4:6 | ------ | ------ |
| A14 | ------ | ------ | ------ | 3:7 | ------ | ------ |
| A15 | ------ | ------ | ------ | 2:8 | ------ | ------ |
| A16 | ------ | ------ | ------ | 1:9 | ------ | ------ |
| A17 | ------ | ------ | ------ | ------ | 0.1 | ------ |
| A18 | ------ | ------ | ------ | ------ | 0.25 | ------ |
| A19 | ------ | 1 | 1 | ------ | ------ | ------ |
| A20 | ------ | 2 | 1 | ------ | ------ | ------ |
| A21 | ------ | 3 | 1 | ------ | ------ | ------ |
| A22 | ------ | 4 | 1 | ------ | ------ | ------ |
| A23 | ------ | 5 | 1 | ------ | ------ | ------ |
| A24 | ------ | 6 | 1 | ------ | ------ | ------ |
| A25 | ------ | 7 | 1 | ------ | ------ | ------ |
| A26 | ------ | 8 | 1 | ------ | ------ | ------ |
| A27 | ------ | 9 | 1 | ------ | ------ | ------ |
| A28 | ------ | 10 | 1 | ------ | ------ | ------ |
| A29 | ------ | ------ | ------ | ------ | ------ | 100 |
| Formulation Number | Chitosan (% w/v) | Acetic Acid (% v/v) | MLX (mg) | 0.25% w/v TPP (mL) | PEG 400 (mL) |
|---|---|---|---|---|---|
| F1 | 0.5 | 1 | 1 | 1 | - |
| F2 | 0.5 | 1 | 1.5 | 1 | - |
| F3 | 0.5 | 1 | 3.7 | - | 1 |
| F4 | 0.25 | 0.5 | 1 | 1 | - |
| F5 | 0.25 | 0.5 | 1.5 | 1 | - |
| F6 | 0.25 | 0.5 | 3.7 | - | 1 |
| Score | Discomfort | Cornea | Conjunctiva | Discharge | Lids |
|---|---|---|---|---|---|
| 0 | No reaction | No changes | No changes | None | No edema |
| 1 | Blinking | Mild opacity |
| Mild, without wetted hair | Mild edema |
| 2 |
| Intense opacity |
| Intense, with wetted hair | Observed edema |
| Assessment | Score |
|---|---|
| Normal surface epithelium with intact microvilli and tight junctions | 0 |
| Some superficial cell sloughing and pitting with reduced microvilli | 1 |
| Denuded superficial cells with intact underlying cells | 2 |
| Partial loss of wing cells in the middle epithelial layer | 3 |
| Loss of outermost epithelial cells exposing the basal epithelial cells | 4 |
| Systems | Apparent MLX Solubility at 37 °C (mg/mL) | Comments |
|---|---|---|
| A1 (10% PG) | 0.027 | Increase MLX solubility with increasing PG concentration |
| A2 (20% PG) | 0.05 | |
| A3 (100% PG) | 0.28 | |
| A4 (1% HP-β-CD) | 0.022 | Increase MLX solubility with increasing HP-β-CD concentration |
| A5 (2.5% HP-β-CD) | 0.048 | |
| A6 (5% HP-β-CD) | 0.095 | |
| A7 (10% HP-β-CD) | 0.18 | |
| A8 (10% HP-β-CD:5% PF-127) (9:1) | 0.15 | Decrease MLX solubility with decreasing the amount of HP-β-CD |
| A9 (10% HP-β-CD:5% PF-127) (8:2) | 0.089 | |
| A10 (10% HP-β-CD:5% PF-127) (7:3) | 0.082 | |
| A11 (10% HP-β-CD:5% PF-127) (6:4) | 0.076 | |
| A12 (10% HP-β-CD:5% PF-127) (5:5) | 0.072 | |
| A13 (10% HP-β-CD:5% PF-127) (4:6) | 0.068 | |
| A14 (10% HP-β-CD:5% PF-127) (3:7) | 0.062 | |
| A15 (10% HP-β-CD:5% PF-127) (2:8) | 0.058 | |
| A16 (10% HP-β-CD:5% PF-127) (1:9) | 0.055 | |
| A17 (0.1%TPP) | 1.3 | Increase MLX solubility with increasing TPP concentration |
| A18 (0.25% TPP) | 1.9 | |
| A19 (1% HP-β-CD + 1% PVP) | 0.026 | Increase MLX solubility by increasing the percent of HP-β-CD in the presence of 1% PVP |
| A20 (2% HP-β-CD + 1% PVP) | 0.041 | |
| A21 (3% HP-β-CD + 1% PVP) | 0.052 | |
| A22 (4% HP-β-CD + 1% PVP) | 0.072 | |
| A23 (5% HP-β-CD + 1% PVP) | 0.098 | |
| A24 (6% HP-β-CD + 1% PVP) | 0.11 | |
| A25 (7% HP-β-CD + 1% PVP) | 0.14 | |
| A26 (8% HP-β-CD + 1% PVP) | 0.16 | |
| A27 (9% HP-β-CD + 1% PVP) | 0.19 | |
| A28 (10% HP-β-CD + 1% PVP) | 0.23 | |
| A29 (PEG 400) | 3.8 | Highest MLX solubility |
| Formulation No. | Average Particle Size (nm) | PDI | Zeta Potential (mV) | EE (%) | pH |
|---|---|---|---|---|---|
| F1 | 335 ± 23 | 0.41± 0.0 | 49.2 ± 1.0 | 72 ± 4.5 | 5.6 ± 0.1 |
| F2 | 597 ± 14 | 0.36 ± 0.1 | 44.4 ± 2.8 | 75 ± 2.0 | 6.2 ± 0.2 |
| F3 | 195 ± 30 | 0.42 ± 0.0 | 28.2 ± 1.1 | 96 ± 1.5 | 5.6 ± 0.1 |
| F4 | 266 ± 24 | 0.34 ± 0.1 | 57.0 ± 1.1 | 71 ± 2.0 | 5.5 ± 0.1 |
| F5 | 493 ± 36 | 0.46 ± 0.0 | 55.9 ± 1.1 | 70 ± 2.5 | 6.3 ± 0.1 |
| F6 | 242 ± 35 | 0.51 ± 0.0 | 17.3 ± 0.5 | 91 ± 2.0 | 5.5 ± 0.2 |
| Formulations | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas |
|---|---|---|---|---|
| R2 | R2 | R2 | N | |
| MLX-CS-NPs dispersion | 0.75 | 0.78 | 0.87 | 0.69 |
| MLX solution in PEG 400 | 0.99 | 0.91 | 0.94 | 0.96 |
| Formulation | Permeation Parameters | |||
|---|---|---|---|---|
| Cornea | Sclera | |||
| J (µg·cm−2·h−1) | P (cm·h−1) | J (µg·cm−2·h−1) | P (cm·h−1) | |
| MLX-CS-NPs dispersion | 29.9 | 0.0199 | 23.7 | 0.0158 |
| MLX solution in PEG 400 | 95.1 | 0.0634 | 36.8 | 0.0246 |
| Experiment Groups | The Average Score of Eye Inflammation | ||
|---|---|---|---|
| Before the Start of Treatment | After One Day of Treatment | After Three Days of Treatment | |
| Group I: Blank CS/PEG 400 eye drop solution | 8 ± 0.3 | 6.3 ± 0.7 | 2.7 ± 0.5 |
| Group II: MLX-eye drop solution | 10 ± 0.8 | 7.3 ± 0.8 | 5.0 ± 0.7 |
| Group III: MLX-CS-NPs eye drop dispersion. | 10 ± 1.0 | 4.0 ± 0.5 | 0.3 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, H.B.; Attia Shafie, M.A.; Mekkawy, A.I. Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model. Pharmaceutics 2022, 14, 893. https://doi.org/10.3390/pharmaceutics14050893
Mohamed HB, Attia Shafie MA, Mekkawy AI. Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model. Pharmaceutics. 2022; 14(5):893. https://doi.org/10.3390/pharmaceutics14050893
Chicago/Turabian StyleMohamed, Hebatallah B., Mohamed Ali Attia Shafie, and Aml I. Mekkawy. 2022. "Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model" Pharmaceutics 14, no. 5: 893. https://doi.org/10.3390/pharmaceutics14050893
APA StyleMohamed, H. B., Attia Shafie, M. A., & Mekkawy, A. I. (2022). Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model. Pharmaceutics, 14(5), 893. https://doi.org/10.3390/pharmaceutics14050893