Nanotheranostics for Image-Guided Cancer Treatment
Abstract
:1. Introduction
2. Strategies of Constructing Nanotheranostics
2.1. Nanoparticle Composition, Size and Shape
2.1.1. Liposomes
2.1.2. Polymeric Nanoparticles
2.1.3. Metallic and Inorganic NPs
2.2. Targeting Moieties
2.3. Imaging Labels for Nanotheranostics
2.3.1. Radiolabels
2.3.2. Magnetic Resonance Imaging Labels
2.3.3. Ultrasound Labels
2.3.4. Optoacoustic Labels
2.3.5. Computed Tomography Labels
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA A Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.K.; Johnston, M.C.; Scott, C.J. Nanomedicine in Pancreatic Cancer: Current Status and Future Opportunities for Overcoming Therapy Resistance. Cancers 2021, 13, 6175. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Lacal, P.M.; Graziani, G. Antibody-drug conjugates: Resurgent anticancer agents with multi-targeted therapeutic potential. Pharmacol. Ther. 2022, 236, 108106. [Google Scholar] [CrossRef] [PubMed]
- Damiano, M.G.; Mutharasan, R.K.; Tripathy, S.; McMahon, K.M.; Thaxton, C.S. Templated high density lipoprotein nanoparticles as potential therapies and for molecular delivery. Adv. Drug Deliv. Rev. 2013, 65, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-K.; Strauss, R.; Richter, M.; Yun, C.-O.; Lieber, A. Strategies to Increase Drug Penetration in Solid Tumors. Front. Oncol. 2013, 3, 193. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Endo, Y.; Shen, Y.; Rotstein, D.; Dokmanovic, M.; Mohan, N.; Mukhopadhyay, P.; Gao, B.; Pacher, P.; Wu, W.J. Ado-Trastuzumab Emtansine Targets Hepatocytes Via Human Epidermal Growth Factor Receptor 2 to Induce Hepatotoxicity. Mol. Cancer Ther. 2015, 15, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Frickenstein, A.; Hagood, J.; Britten, C.; Abbott, B.; McNally, M.; Vopat, C.; Patterson, E.; MacCuaig, W.; Jain, A.; Walters, K.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef]
- Zuazu-Jausoro, I.; Borrell, M.; Urrutia, T.; Oliver, A.; Montserrat, I.; Mateo, J.; Ribera, L.; Fontcuberta, J. Detection of thrombin-antithrombin complexes in hypercoagulability conditions. Analysis of 182 cases. Sangre 1990, 35, 375–379. [Google Scholar]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.-J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate Gold Cluster Molecules with Core Diameters from 1.5 to 5.2 nm: Core and Monolayer Properties as a Function of Core Size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.-Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming Limitations in Nanoparticle Drug Delivery: Triggered, Intravascular Release to Improve Drug Penetration into Tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shin, M.C.; David, A.E.; Zhou, J.; Lee, K.; He, H.; Yang, V.C. Long-Circulating Heparin-Functionalized Magnetic Nanoparticles for Potential Application as a Protein Drug Delivery Platform. Mol. Pharm. 2013, 10, 3892–3902. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, F.; Gupta, S.; Li, C. Interventional Nanotheranostics of Pancreatic Ductal Adenocarcinoma. Theranostics 2016, 6, 1393–1402. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zang, Y.; Lu, Y.; Han, J.; Xiong, Q.; Xiong, J. Photothermal Effect and Multi-Modality Imaging of Up-Conversion Nanomaterial Doped with Gold Nanoparticles. Int. J. Mol. Sci. 2022, 23, 1382. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Bao, G. Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Curr. Opin. Biomed. Eng. 2021, 20, 100330. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, K.; Esendagli, G.; Gürbüz, M.U.; Tülü, M.; Çalış, S. Effective targeting of gemcitabine to pancreatic cancer through PEG-cored Flt-1 antibody-conjugated dendrimers. Int. J. Pharm. 2017, 517, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, S.L.; Ouyang, Z.; Song, C.; Rodrigues, J.; Shen, M.; Shi, X. Dendrimer-Based Nanogels for Cancer Nanomedicine Applications. Bioconjugate Chem. 2021, 33, 87–96. [Google Scholar] [CrossRef]
- Liu, X.; Situ, A.; Kang, Y.; Villabroza, K.R.; Liao, Y.; Chang, C.H.; Donahue, T.; Nel, A.E.; Meng, H. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Jin, C.; Yang, D.; Jiang, Y.; Li, J.; Di, Y.; Hu, J.; Wang, C.; Ni, Q.; Fu, D. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer 2011, 47, 1873–1882. [Google Scholar] [CrossRef]
- Rosenberger, I.; Strauss, A.; Dobiasch, S.; Weis, C.; Szanyi, S.; Gil-Iceta, L.; Alonso, E.; Esparza, M.G.; Vallejo, V.G.; Szczupak, B.; et al. Targeted diagnostic magnetic nanoparticles for medical imaging of pancreatic cancer. J. Control Release 2015, 214, 76–84. [Google Scholar] [CrossRef]
- Nigam, P.; Waghmode, S.; Louis, M.; Wangnoo, S.; Chavan, P.; Sarkar, D. Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J. Mater. Chem. B 2014, 2, 3190–3195. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.; Confeld, M.; Borowicz, P.; Wang, T.; Mallik, S.; Quadir, M. PEG-b-poly(carbonate)-derived nanocarrier platform with pH-responsive properties for pancreatic cancer combination therapy. Colloids Surf. B Biointerfaces 2019, 174, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Qian, W.P.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satpathy, M.; Nie, S.; Mao, H.; Yang, L. Theranostic Nanoparticles with Controlled Release of Gemcitabine for Targeted Therapy and MRI of Pancreatic Cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.P.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.-R.; Hasan, A.; Ertsey, R.; et al. Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Wang, M.; Liu, K.; Hoover, A.R.; Li, M.; Towner, R.A.; Mukherjee, P.; Zhou, F.; Qu, J.; et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater 2022, 138, 453–462. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, M.; Afzal, A.; Yang, T.; Gai, Y.; Raza, S.M.; Khan, M.W.; Cheng, Y.; Ma, X.; Xiang, G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics 2018, 10, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, W.T.; Bao, A.; Sou, K.; Li, S.; Goins, B. Radiolabeled liposomes as drug delivery nanotheranostics. In Drug Delivery Applications of Noninvasive Imaging Validation from Biodistribution to Sites of Action; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 252–267. [Google Scholar]
- Petersen, A.L.; Hansen, A.E.; Gabizon, A.; Andresen, T.L. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Meng, X.; Lu, H.; Chang, H.; Dong, H.; Zhang, X. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials 2018, 185, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2020, 156, 105576. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Kouchak, M.; Ramezani, Z.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A.; Rezaei, M. New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: Strong implication for enhanced potency and safety. Life Sci. 2019, 227, 39–50. [Google Scholar] [CrossRef]
- Thomas, A.; Samykutty, A.; Gomez-Gutierrez, J.G.; Yin, W.; Egger, M.E.; McNally, M.; Chuong, P.; MacCUAIG, W.M.; Albeituni, S.; Zeiderman, M.; et al. Actively Targeted Nanodelivery of Echinomycin Induces Autophagy-Mediated Death in Chemoresistant Pancreatic Cancer In Vivo. Cancers 2020, 12, 2279. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.; Karavas, E.; Bikiaris, D.N. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, P.; Zhang, P.; Yi, X.; Xiao, C.; Chen, X. Polypeptides–Drug Conjugates for Anticancer Therapy. Adv. Health Mater. 2021, 10, 2001974. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varani, M.; Campagna, G.; Bentivoglio, V.; Serafinelli, M.; Martini, M.L.; Galli, F.; Signore, A. Synthesis and Biodistribution of 99mTc-Labeled PLGA Nanoparticles by Microfluidic Technique. Pharmaceutics 2021, 13, 1769. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhao, T.; Wang, C.; Nham, K.; Xiong, Y.; Gao, X.; Wang, Y.; Hao, G.; Ge, W.P.; Sun, X.; et al. PET imaging of occult tumours by temporal integration of tumour-acidosis signals from pH-sensitive (64)Cu-labelled polymers. Nat. Biomed. Eng. 2020, 4, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Aso, E.; Martinsson, I.; Appelhans, D.; Effenberg, C.; Benseny-Cases, N.; Cladera, J.; Gouras, G.; Ferrer, I.; Klementieva, O. Poly(propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kesharwani, P. Dendrimer as a promising nanocarrier for the delivery of doxorubicin as an anticancer therapeutics. J. Biomater. Sci. Polym. Ed. 2021, 32, 1882–1909. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Boinapally, S.; Banerjee, S.R.; Azad, B.B.; Foss, C.A.; Shen, C.; Lisok, A.; Wharram, B.; Nimmagadda, S.; Pomper, M.G. Evaluation of PSMA-Targeted PAMAM Dendrimer Nanoparticles in a Murine Model of Prostate Cancer. Mol. Pharm. 2019, 16, 2590–2604. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Chu, L.; Wang, Y.; Duan, Y.; Feng, L.; Yang, C.; Wang, L.; Kong, D. Novel peptide–dendrimer conjugates as drug carriers for targeting nonsmall cell lung cancer. Int. J. Nanomed. 2011, 6, 59. [Google Scholar]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Toljic, D.; Angelovski, G. Translating a Low-Molecular-Weight MRI Probe Sensitive to Amino Acid Neurotransmitters into a PAMAM Dendrimer Conjugate: The Impact of Conjugation. ChemNanoMat 2019, 5, 1456–1460. [Google Scholar] [CrossRef]
- Almasi, T.; Gholipour, N.; Akhlaghi, M.; Kheirabadi, A.M.; Mazidi, S.M.; Hosseini, S.H.; Geramifar, P.; Beiki, D.; Rostampour, N.; Gahrouei, D.S. Development of Ga-68 radiolabeled DOTA functionalized and acetylated PAMAM dendrimer-coated iron oxide nanoparticles as PET/MR dual-modal imaging agent. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 1077–1089. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, L.; Zhu, J.; Li, Y.; Song, N.; Xing, Y.; Qiao, W.; Huang, H.; Zhao, J. 131I-labeled polyethylenimine-entrapped gold nanoparticles for targeted tumor SPECT/CT imaging and radionuclide therapy. Int. J. Nanomed. 2019, 14, 4367–4381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreffler, J.W.; Pullan, J.E.; Dailey, K.M.; Mallik, S.; Brooks, A.E. Overcoming Hurdles in Nanoparticle Clinical Translation: The Influence of Experimental Design and Surface Modification. Int. J. Mol. Sci. 2019, 20, 6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.K.; Bhatt, T.; Agrawal, M.; Saha, R.N.; Saraf, S.; Saraf, S.; Alexander, A. Application of chitosan modified nanocarriers in breast cancer. Int. J. Biol. Macromol. 2022, 194, 521–538. [Google Scholar] [CrossRef]
- Sampath, M.; Pichaimani, A.; Kumpati, P.; Sengottuvelan, B. The remarkable role of emulsifier and chitosan, dextran and PEG as capping agents in the enhanced delivery of curcumin by nanoparticles in breast cancer cells. Int. J. Biol. Macromol. 2020, 162, 748–761. [Google Scholar] [CrossRef]
- Wang, F.H.; Bae, K.; Huang, Z.W.; Xue, J.M. Two-photon graphene quantum dot modified Gd2O3 nanocomposites as a dual-mode MRI contrast agent and cell labelling agent. Nanoscale 2018, 10, 5642–5649. [Google Scholar] [CrossRef]
- Tanaka, S.; Lin, J.; Kaneti, Y.V.; Yusa, S.-I.; Jikihara, Y.; Nakayama, T.; Zakaria, M.B.; Alshehri, A.A.; You, J.; Hossain, S.A.; et al. Gold nanoparticles supported on mesoporous iron oxide for enhanced CO oxidation reaction. Nanoscale 2018, 10, 4779–4785. [Google Scholar] [CrossRef]
- Motiei, M.; Dreifuss, T.; Sadan, T.; Omer, N.; Blumenfeld-Katzir, T.; Fragogeorgi, E.; Loudos, G.; Popovtzer, R.; Ben-Eliezer, N. Trimodal nanoparticle contrast agent for ct, mri and spect imaging: Synthesis and characterization of radiolabeled core/shell iron oxide@ gold nanoparticles. Chem. Lett. 2019, 48, 291–294. [Google Scholar] [CrossRef]
- Atukorale, P.U.; Covarrubias, G.; Bauer, L.; Karathanasis, E. Vascular targeting of nanoparticles for molecular imaging of diseased endothelium. Adv. Drug Deliv. Rev. 2016, 113, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molema, G. Tumor vasculature directed drug targeting: Applying new technologies and knowledge to the development of clinically relevant therapies. Pharm. Res. 2002, 19, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Martin, J.; Snuderl, M.; Mpekris, F.; Jain, S.R.; Jain, R.K. Coevolution of Solid Stress and Interstitial Fluid Pressure in Tumors during Progression: Implications for Vascular Collapse. Cancer Res. 2013, 73, 3833–3841. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- MacCuaig, W.M.; Fouts, B.L.; McNally, M.W.; Grizzle, W.E.; Chuong, P.; Samykutty, A.; Mukherjee, P.; Li, M.; Jasinski, J.B.; Behkam, B.; et al. Active Targeting Significantly Outperforms Nanoparticle Size in Facilitating Tumor-Specific Uptake in Orthotopic Pancreatic Cancer. ACS Appl. Mater. Interfaces 2021, 13, 49614–49630. [Google Scholar] [CrossRef]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2018, 234, 4959–4969. [Google Scholar] [CrossRef]
- Herting, C.J.; Karpovsky, I.; Lesinski, G.B. The tumor microenvironment in pancreatic ductal adenocarcinoma: Current perspectives and future directions. Cancer Metastasis Rev. 2021, 40, 675–689. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Jiang, S.-H.; Hu, L.-P.; Huang, P.-Q.; Wang, X.; Li, J.; Zhang, X.-L.; Nie, H.-Z.; Zhang, Z.-G. Targeting the tumor microenvironment for pancreatic ductal adenocarcinoma therapy. Chin. Clin. Oncol. 2019, 8, 18. [Google Scholar] [CrossRef]
- Man, F.; Lammers, T.; De Rosales, R.T.M. Imaging Nanomedicine-Based Drug Delivery: A Review of Clinical Studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Cuaron, J.; Hirsch, J.; Medich, D.; Rosenstein, B.; Martel, C.; Hirsch, A. A Proposed Methodology to Select Radioisotopes for Use in Radionuclide Therapy. Am. J. Neuroradiol. 2009, 30, 1824–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipowska, M.; Klenc, J.; Taylor, A.T.; Marzilli, L.G. fac-99mTc/Re-tricarbonyl complexes with tridentate aminocarboxyphosphonate ligands: Suitability of the phosphonate group in chelate ligand design of new imaging agents. Inorg. Chim. Acta 2018, 486, 529–537. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Jiang, D.; Ehlerding, E.B.; Huang, P.; Cai, W. Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications. Acc. Chem. Res. 2018, 51, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Good, S.; Walter, M.A.; Waser, B.; Wang, X.; Müller-Brand, J.; Béhé, M.P.; Reubi, J.-C.; Maecke, H.R. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur. J. Pediatr. 2008, 35, 1868–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.J.; Sohn, R.; Lu, Z.H.; Arbeit, J.M.; Lapi, S.E. Detection of Rapalog-Mediated Therapeutic Response in Renal Cancer Xenografts Using 64Cu-bevacizumab ImmunoPET. PLoS ONE 2013, 8, e58949. [Google Scholar] [CrossRef]
- Shokeen, M.; Anderson, C.J. Molecular Imaging of Cancer with Copper-64 Radiopharmaceuticals and Positron Emission Tomography (PET). Acc. Chem. Res. 2009, 42, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hong, H.; Engle, J.W.; Bean, J.; Yang, Y.; Leigh, B.R.; Barnhart, T.E.; Cai, W. Positron Emission Tomography Imaging of CD105 Expression with a 64Cu-Labeled Monoclonal Antibody: NOTA Is Superior to DOTA. PLoS ONE 2011, 6, e28005. [Google Scholar] [CrossRef] [Green Version]
- Rauscher, A.; Frindel, M.; Rajerison, H.; Gouard, S.; Maurel, C.; Barbet, J.; Faivre-Chauvet, A.; Mougin-Degraef, M. Improvement of the Targeting of Radiolabeled and Functionalized Liposomes with a Two-Step System Using a Bispecific Monoclonal Antibody (Anti-CEA x Anti-DTPA-In). Front. Med. 2015, 2, 83. [Google Scholar] [CrossRef] [Green Version]
- Borràs, J.; Mesa, V.; Suades, J.; Barnadas-Rodriguez, R. Direct Synthesis of Rhenium and Technetium-99m Metallosurfactants by a Transmetallation Reaction of Lipophilic Groups: Potential Applications in the Radiolabeling of Liposomes. Langmuir 2020, 36, 1993–2002. [Google Scholar] [CrossRef]
- Aranda-Lara, L.; Morales-Avila, E.; Luna-Gutiérrez, M.A.; Olivé-Alvarez, E.; Isaac-Olivé, K. Radiolabeled liposomes and lipoproteins as lipidic nanoparticles for imaging and therapy. Chem. Phys. Lipids 2020, 230, 104934. [Google Scholar] [CrossRef] [PubMed]
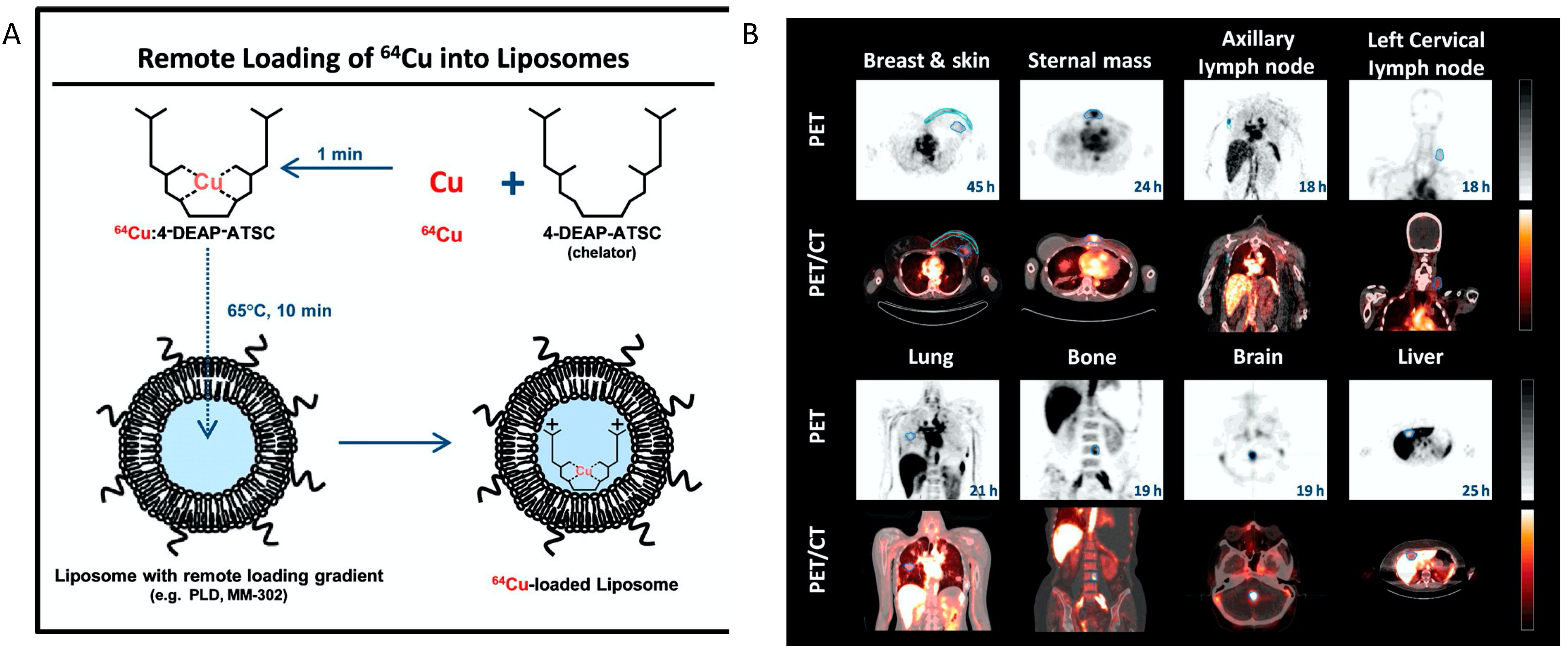
- Petersen, A.L.; Binderup, T.; Rasmussen, P.; Henriksen, J.R.; Elema, D.R.; Kjaer, A.; Andresen, T.L. 64Cu loaded liposomes as positron emission tomography imaging agents. Biomaterials 2011, 32, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Engudar, G.; Schaarup-Jensen, H.; Fliedner, F.P.; Hansen, A.E.; Kempen, P.; Jølck, R.I.; Kjaer, A.; Andresen, T.L.; Clausen, M.H.; Jensen, A.; et al. Remote loading of liposomes with a 124I-radioiodinated compound and their in vivo evaluation by PET/CT in a murine tumor model. Theranostics 2018, 8, 5828–5841. [Google Scholar] [CrossRef]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, E.C.; Shaffer, T.M.; Grimm, J. Nanoparticles and radiotracers: Advances toward radionanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 872–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Xu, C.; Yang, K.; Goel, S.; Valdovinos, H.; Luo, H.; Ehlerding, E.B.; England, C.G.; Cheng, L.; Chen, F.; et al. Chelator-Free Radiolabeling of Nanographene: Breaking the Stereotype of Chelation. Angew. Chem. Int. Ed. 2017, 56, 2889–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, M.A.; Shaffer, T.M.; Harmsen, S.; Tschaharganeh, D.-F.; Huang, C.-H.; Lowe, S.W.; Drain, C.M.; Kircher, M.F. Chelator-Free Radiolabeling of SERRS Nanoparticles for Whole-Body PET and Intraoperative Raman Imaging. Theranostics 2017, 7, 3068–3077. [Google Scholar] [CrossRef]
- Tang, T.; Wei, Y.; Yang, Q.; Yang, Y.; Sailor, M.J.; Pang, H.-B. Rapid chelator-free radiolabeling of quantum dots for in vivo imaging. Nanoscale 2019, 11, 22248–22254. [Google Scholar] [CrossRef]
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I.; et al. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naive, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 352. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Zheng, J.; Gaddy, D.; Orcutt, K.D.; Leonard, S.; Geretti, E.; Hesterman, J.; Harwell, C.; Hoppin, J.; Jaffray, D.A.; et al. A gradient-loadable 64Cu-chelator for quantifying tumor deposition kinetics of nanoliposomal therapeutics by positron emission tomography. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 155–165. [Google Scholar] [CrossRef]
- Jentzen, W.; Verschure, F.; van Zon, A.; van de Kolk, R.; Wierts, R.; Schmitz, J.; Bockisch, A.; Binse, I. 124I PET Assessment of Response of Bone Metastases to Initial Radioiodine Treatment of Differentiated Thyroid Cancer. J. Nucl. Med. 2016, 57, 1499–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopci, E.; Chiti, A.; Castellani, M.R.; Pepe, G.; Antunovic, L.; Fanti, S.; Bombardieri, E. Matched pairs dosimetry: 124I/131I metaiodobenzylguanidine and 124I/131I and 86Y/90Y antibodies. Eur. J. Pediatr. 2011, 38, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Marsh, I.R.; Grudzinski, J.J.; Baiu, D.C.; Besemer, A.; Hernandez, R.; Jeffery, J.J.; Weichert, J.P.; Otto, M.; Bednarz, B.P. Preclinical Pharmacokinetics and Dosimetry Studies of 124I/131I-CLR1404 for Treatment of Pediatric Solid Tumors in Murine Xenograft Models. J. Nucl. Med. 2019, 60, 1414–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, S.; Ferreira, C.A.; Dogra, P.; Yu, B.; Kutyreff, C.J.; Siamof, C.M.; Engle, J.W.; Barnhart, T.E.; Cristini, V.; Wang, Z.; et al. Size-Optimized Ultrasmall Porous Silica Nanoparticles Depict Vasculature-Based Differential Targeting in Triple Negative Breast Cancer. Small 2019, 15, e1903747. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Goel, S.; Ehlerding, E.B.; Rosenkrans, Z.T.; Jiang, D.; Sun, T.; Aluicio-Sarduy, E.; Engle, J.W.; Ni, D.; Cai, W. Ultrasmall Porous Silica Nanoparticles with Enhanced Pharmacokinetics for Cancer Theranostics. Nano Lett. 2021, 21, 4692–4699. [Google Scholar] [CrossRef]
- Imlimthan, S.; Khng, Y.C.; Keinänen, O.; Zhang, W.; Airaksinen, A.J.; Kostiainen, M.A.; Zeglis, B.M.; Santos, H.A.; Sarparanta, M. A Theranostic Cellulose Nanocrystal-Based Drug Delivery System with Enhanced Retention in Pulmonary Metastasis of Melanoma. Small 2021, 17, e2007705. [Google Scholar] [CrossRef]
- Gaikwad, G.; Rohra, N.; Kumar, C.; Jadhav, S.; Sarma, H.D.; Borade, L.; Chakraborty, S.; Bhagwat, S.; Dandekar, P.; Jain, R.; et al. A facile strategy for synthesis of a broad palette of intrinsically radiolabeled chitosan nanoparticles for potential use in cancer theranostics. J. Drug Deliv. Sci. Technol. 2021, 63, 102485. [Google Scholar] [CrossRef]
- Tweedle, M.F.; Wedeking, P.; Kumar, K. Biodistribution of Radiolabeled, Formulated Gadopentetate, Gadoteridol, Gadoterate, and Gadodiamide in Mice and Rats. Investig. Radiol. 1995, 30, 372–380. [Google Scholar] [CrossRef]
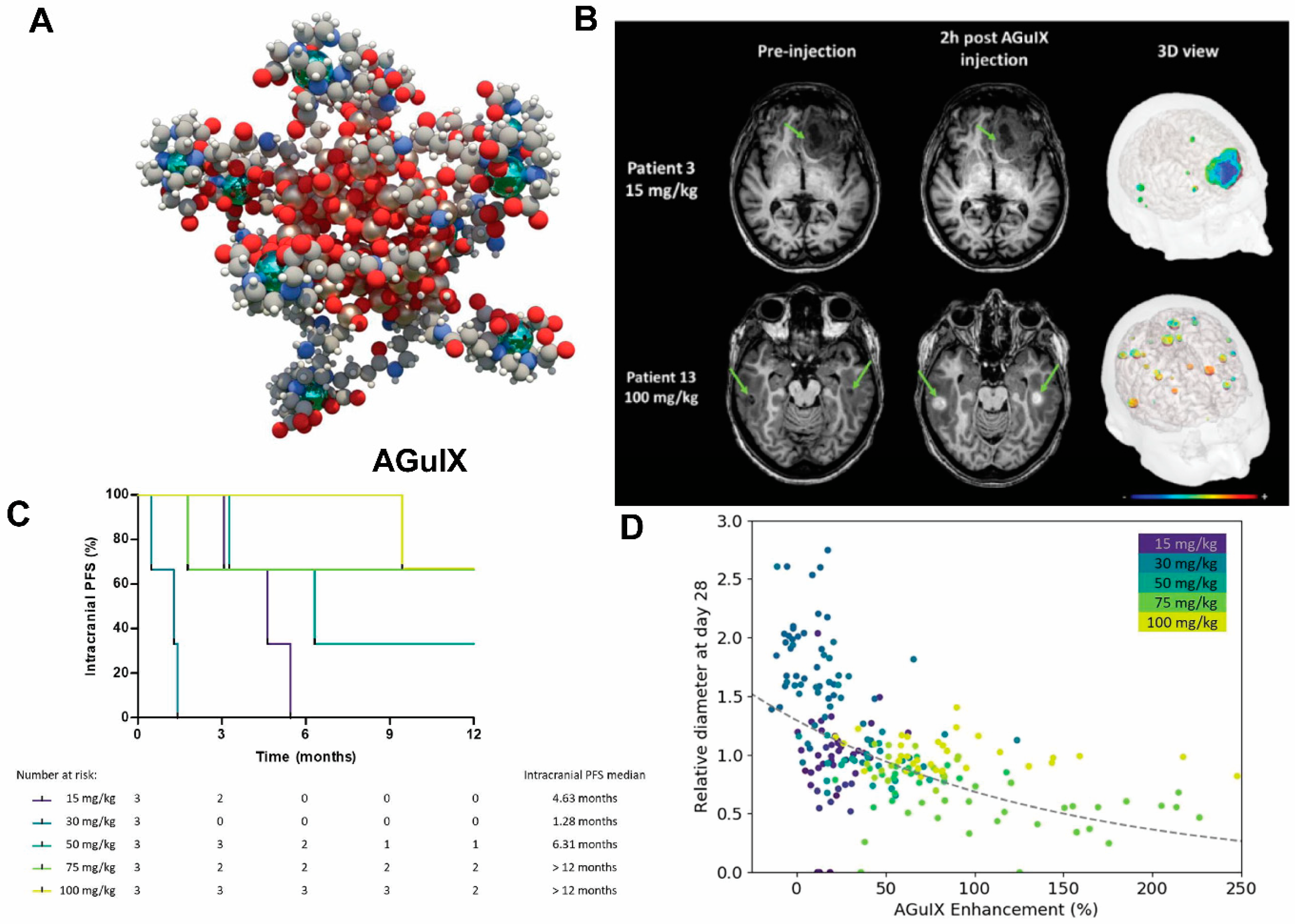
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX((R)) from bench to bedside-Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2019, 92, 20180365. [Google Scholar] [CrossRef]
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Crémillieux, Y.; Fries, P.; Coll, J.-L.; Rodriguez-Lafrasse, C.; et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014, 87, 20140134. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 2020, 6, eaay5279. [Google Scholar] [CrossRef] [PubMed]
- Gries, M.; Thomas, N.; Daouk, J.; Rocchi, P.; Choulier, L.; Jubréaux, J.; Pierson, J.; Reinhard, A.; Jouan-Hureaux, V.; Chateau, A.; et al. Multiscale Selectivity and in vivo Biodistribution of NRP-1-Targeted Theranostic AGuIX Nanoparticles for PDT of Glioblastoma. Int. J. Nanomed. 2020, 15, 8739–8758. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, Y.; Ding, D.; Lin, H.; Sun, W.; Wang, G.D.; Huang, W.; Zhang, W.; Lee, D.; Liu, G.; et al. Gadolinium-Encapsulated Graphene Carbon Nanotheranostics for Imaging-Guided Photodynamic Therapy. Adv. Mater. 2018, 30, e1802748. [Google Scholar] [CrossRef]
- Guan, M.; Zhou, Y.; Liu, S.; Chen, D.; Ge, J.; Deng, R.; Li, X.; Yu, T.; Xu, H.; Sun, D.; et al. Photo-triggered gadofullerene: Enhanced cancer therapy by combining tumor vascular disruption and stimulation of anti-tumor immune responses. Biomaterials 2019, 213, 119218. [Google Scholar] [CrossRef]
- Lu, Z.; Jia, W.; Deng, R.; Zhou, Y.; Li, X.; Yu, T.; Zhen, M.; Wang, C. Light-assisted gadofullerene nanoparticles disrupt tumor vasculatures for potent melanoma treatment. J. Mater. Chem. B 2020, 8, 2508–2518. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Roelle, S.; Chen, C.; Schiemann, W.P.; Lu, Z.-R. Targeted gadofullerene for sensitive magnetic resonance imaging and risk-stratification of breast cancer. Nat. Commun. 2017, 8, 692. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Zhang, G.; Wang, D.; Zhang, C.; Yang, C.; Bai, G.; Qian, J.; Chen, Q.; Zhang, Z.; Wu, Z.; et al. Nanostructure-enhanced water interaction to increase the dual-mode MR contrast performance of gadolinium-doped iron oxide nanoclusters. Chem. Eng. J. 2019, 360, 289–298. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Sanchez Duque, M.D.M.; Guari, Y.; Larionova, J.; Guérin, C.; Montero, J.-L.G.; Barragan-Montero, V.; et al. Water-Dispersible Sugar-Coated Iron Oxide Nanoparticles. An Evaluation of their Relaxometric and Magnetic Hyperthermia Properties. J. Am. Chem. Soc. 2011, 133, 10459–10472. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic Nanoparticle Clusters for Cancer Theranostics Combining Magnetic Resonance Imaging and Hyperthermia Treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Faraj, A.; Shaik, A.S.; Al Sayed, B. Preferential magnetic targeting of carbon nanotubes to cancer sites: Noninvasive tracking using MRI in a murine breast cancer model. Nanomedicine 2015, 10, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377. [Google Scholar] [CrossRef]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Préat, V. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Qi, X.; Li, S.; Liu, G.; Siddhanta, S.; Barman, I.; Song, X.; McMahon, M.T.; Bulte, J.W.M. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 2019, 18, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Xu, J.; Yadav, N.N.; Chan, K.W.; Luo, L.; McMahon, M.; Vogelstein, B.; Van Zijl, P.C.; Zhou, S.; et al. CEST theranostics: Label-free MR imaging of anticancer drugs. Oncotarget 2016, 7, 6369–6378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Li, Y.; Zhang, J.; Liu, J.; Chen, C.; Van Zijl, P.C.M.; Liu, G. Molecular imaging of deoxycytidine kinase activity using deoxycytidine-enhanced CEST MRI. Cancer Res. 2019, 79, 2775–2783. [Google Scholar] [CrossRef] [Green Version]
- Ngen, E.J.; Bar-Shir, A.; Jablonska, A.; Liu, G.; Song, X.; Ansari, R.; Bulte, J.W.M.; Janowski, M.; Pearl, M.; Walczak, P.; et al. Imaging the DNA Alkylator Melphalan by CEST MRI: An Advanced Approach to Theranostics. Mol. Pharm. 2016, 13, 3043–3053. [Google Scholar] [CrossRef]
- Han, Z.; Liu, G. CEST MRI trackable nanoparticle drug delivery systems. Biomed. Mater. 2021, 16, 024103. [Google Scholar] [CrossRef]
- Castelli, D.D.; Terreno, E.; Longo, D.; Aime, S. Nanoparticle-based chemical exchange saturation transfer (CEST) agents. NMR Biomed. 2013, 26, 839–849. [Google Scholar] [CrossRef]
- Zhou, L.-Q.; Li, P.; Cui, X.-W.; Dietrich, C.F. Ultrasound nanotheranostics in fighting cancer: Advances and prospects. Cancer Lett. 2019, 470, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Manzano, M.; Baeza, A. Controlled Release with Emphasis on Ultrasound-Induced Release. Enzymes 2018, 43, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Teng, R.; Liu, Y.; Li, J.; Yu, M. Drug Release from Gelsolin-Targeted Phase-Transition Nanoparticles Triggered by Low-Intensity Focused Ultrasound. Int. J. Nanomed. 2022, 17, 61–71. [Google Scholar] [CrossRef]
- Novoselova, M.V.; German, S.V.; Abakumova, T.O.; Perevoschikov, S.V.; Sergeeva, O.V.; Nesterchuk, M.V.; Efimova, O.I.; Petrov, K.S.; Chernyshev, V.S.; Zatsepin, T.S.; et al. Multifunctional nanostructured drug delivery carriers for cancer therapy: Multimodal imaging and ultrasound-induced drug release. Colloids Surf. B Biointerfaces 2021, 200, 111576. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Shi, D.; Roy, S.; Blum, N.T.; Chattaraj, R.; Cha, J.N.; Goodwin, A.P. Nanoparticle-Mediated Acoustic Cavitation Enables High Intensity Focused Ultrasound Ablation Without Tissue Heating. ACS Appl. Mater. Interfaces 2018, 10, 36786–36795. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrand, S.; Evertsson, M.; Jansson, T. Magnetomotive Ultrasound Imaging Systems: Basic Principles and First Applications. Ultrasound Med. Biol. 2020, 46, 2636–2650. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Caskey, C.F.; Ferrara, K.W. Ultrasound contrast microbubbles in imaging and therapy: Physical principles and engineering. Phys. Med. Biol. 2009, 54, R27–R57. [Google Scholar] [CrossRef] [PubMed]
- Bawiec, C.R.; Rosnitskiy, P.B.; Peek, A.T.; Maxwell, A.D.; Kreider, W.; ter Haar, G.R.; Sapozhnikov, O.A.; Khokhlova, V.A.; Khokhlova, T.D. Inertial Cavitation Behaviors Induced by Nonlinear Focused Ultrasound Pulses. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2884–2895. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J. Control Release 2020, 326, 75–90. [Google Scholar] [CrossRef]
- Hyvelin, J.-M.; Gaud, E.; Costa, M.; Helbert, A.; Bussat, P.; Bettinger, T.; Frinking, P. Characteristics and Echogenicity of Clinical Ultrasound Contrast Agents: An In Vitro and In Vivo Comparison Study. J. Ultrasound Med. 2017, 36, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, H.; Zheng, Y.; Ma, M.; Chen, Y.; Zhang, K.; Zeng, D.; Shi, J. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials 2012, 34, 2057–2068. [Google Scholar] [CrossRef]
- Li, J.; Ji, H.; Jing, Y.; Wang, S. pH- and acoustic-responsive platforms based on perfluoropentane-loaded protein nanoparticles for ovarian tumor-targeted ultrasound imaging and therapy. Nanoscale Res. Lett. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Min, H.-S.; Gil You, D.; Kim, K.; Kwon, I.C.; Rhim, T.; Lee, K.Y. Theranostic gas-generating nanoparticles for targeted ultrasound imaging and treatment of neuroblastoma. J. Control Release 2016, 223, 197–206. [Google Scholar] [CrossRef]
- Zhang, X.; Machuki, J.O.; Pan, W.; Cai, W.; Xi, Z.; Shen, F.; Zhang, L.; Yang, Y.; Gao, F.; Guan, M. Carbon Nitride Hollow Theranostic Nanoregulators Executing Laser-Activatable Water Splitting for Enhanced Ultrasound/Fluorescence Imaging and Cooperative Phototherapy. ACS Nano 2020, 14, 4045–4060. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, W.; Huang, Z.; Liu, S.; Guo, J.; Zhang, F.; Yuan, H.; Li, X.; Liu, F.; Liu, H. MOF-Derived Double-Layer Hollow Nanoparticles with Oxygen Generation Ability for Multimodal Imaging-Guided Sonodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 13557–13561. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, G.; Qin, Z.; Wang, X.; Zhao, G.; Ma, Q.; Zhu, L. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials 2017, 112, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Tang, Q.; Zhang, L.; Xu, M.; Sun, L.; Sun, S.; Zhang, J.; Wang, S.; Liang, X. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. ACS Nano 2021, 15, 11326–11340. [Google Scholar] [CrossRef] [PubMed]
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.E.; Grizzle, W.E. Current and emerging clinical applications of multispectral optoacoustic tomography (MSOT) in oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCuaig, W.M.; Jones, M.A.; Abeyakoon, O.; McNally, L.R. Development of Multispectral Optoacoustic Tomography as a Clinically Translatable Modality for Cancer Imaging. Radiol. Imaging Cancer 2020, 2, e200066. [Google Scholar] [CrossRef] [PubMed]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Laramie, M.D.; Fouts, B.L.; MacCuaig, W.M.; Buabeng, E.; Jones, M.A.; Mukherjee, P.; Behkam, B.; McNally, L.R.; Henary, M. Improved pentamethine cyanine nanosensors for optoacoustic imaging of pancreatic cancer. Sci. Rep. 2021, 11, 4366. [Google Scholar] [CrossRef] [PubMed]
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126. [Google Scholar] [CrossRef]
- Thomas, A.; Chiba, A.; Samykutty, A.; McNally, M.W.; McNally, L.R. Tumor specific cargo release in ex vivo patient samples and murine models of triple negative breast cancer by a pH-targeted nanoparticle: V3-RUBY. Cancer Res. 2020, 80, P3-06-04. [Google Scholar]
- Khanal, A.; Ullum, C.; Kimbrough, C.W.; Garbett, N.C.; Burlison, J.A.; McNally, M.W. Tumor targeted mesoporous silica-coated gold nanorods facilitate detection of pancreatic tumors using Multispectral optoacoustic tomography. Nano Res. 2015, 8, 3864–3877. [Google Scholar] [CrossRef]
- Xie, H.; Liu, M.; You, B.; Luo, G.; Chen, Y.; Liu, B.; Jiang, Z.; Chu, P.K.; Shao, J.; Yu, X.F. Biodegradable Bi2O2Se Quantum Dots for Photoacoustic Imaging-Guided Cancer Photothermal Therapy. Small 2020, 16, 1905208. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Li, Y.; Lu, Q.; Wang, X.; Li, J. Atomic-Level Nanorings (A-NRs) Therapeutic Agent for Photoacoustic Imaging and Photothermal/Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2019, 142, 1735–1739. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, X.; Liu, Y.; Chen, B.; Ding, X.; Zhao, N.; Xu, F. Controlled Synthesis and Surface Engineering of Janus Chitosan-Gold Nanoparticles for Photoacoustic Imaging-Guided Synergistic Gene/Photothermal Therapy. Small 2021, 17, 2006004. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Lin, L.; Liu, F.; Wang, Y.; Guo, Z.; Li, Y.; Tian, H.; Chen, X. Porphyrin-based covalent organic framework nanoparticles for photoacoustic imaging-guided photodynamic and photothermal combination cancer therapy. Biomaterials 2019, 223, 119459. [Google Scholar] [CrossRef]
- Rostami, A.; Sazgarnia, A. Gold nanoparticles as cancer theranostic agents. Nanomed. J. 2019, 6, 147–160. [Google Scholar] [CrossRef]
- Curry, T.; Kopelman, R.; Shilo, M.; Popovtzer, R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol. Imaging 2014, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, C.; Guo, W.; Zhao, X.; Liu, S.; Han, X. Multifunctional Bismuth Nanoparticles as Theranostic Agent for PA/CT Imaging and NIR Laser-Driven Photothermal Therapy. ACS Appl. Nano Mater. 2018, 1, 820–830. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.-Y.; Yu, C.; Sun, S.-K.; Yan, X.-P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726. [Google Scholar] [CrossRef] [PubMed]
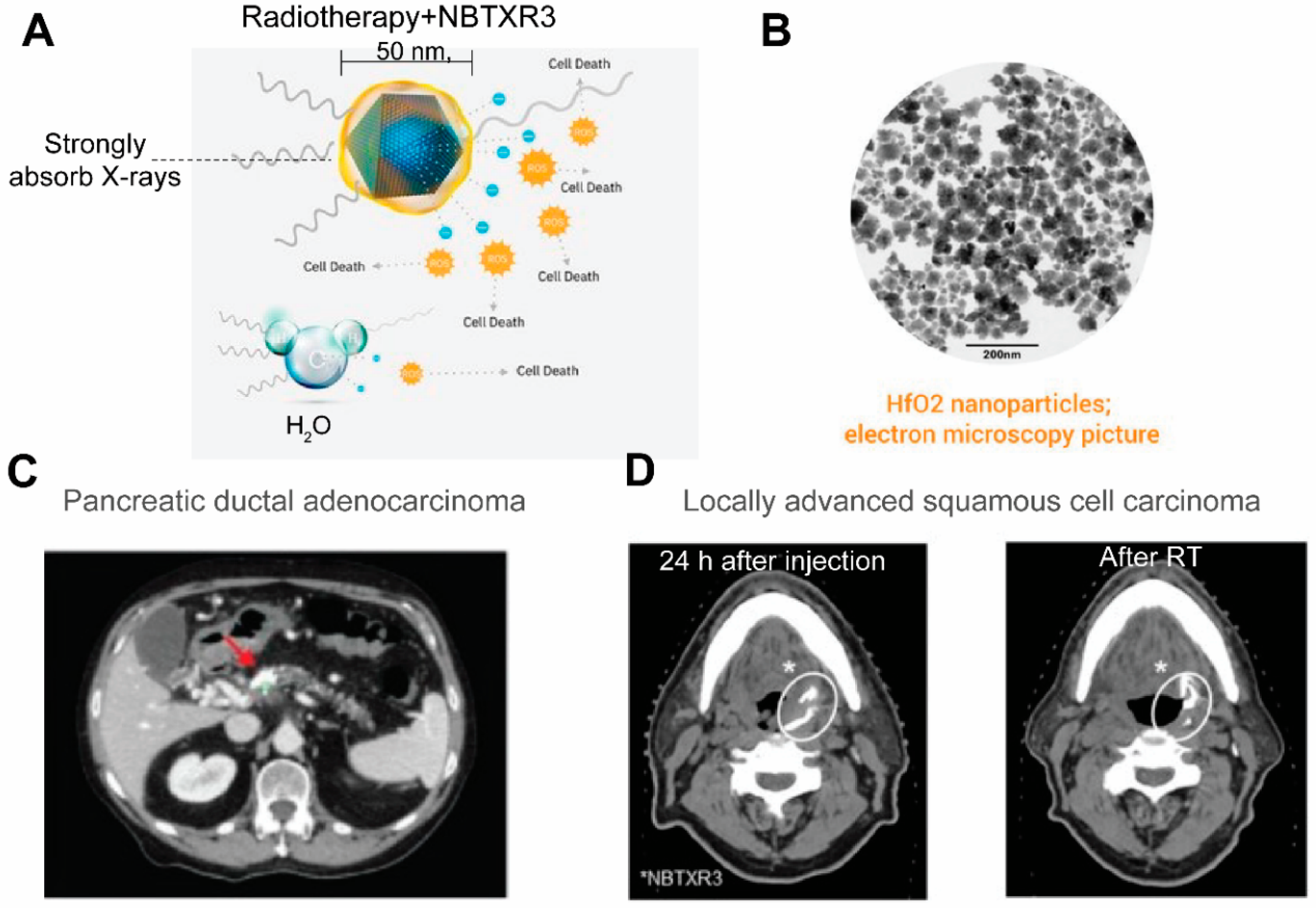
- Bagley, A.F.; Ludmir, E.B.; Maitra, A.; Minsky, B.D.; Smith, G.L.; Das, P.; Koong, A.C.; Holliday, E.B.; Taniguchi, C.M.; Katz, M.H.; et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: Report of first patient experience. Clin. Transl. Radiat. Oncol. 2022, 33, 66–69. [Google Scholar] [CrossRef]
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res. 2016, 23, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhou, I.Y.; Igarashi, T.; Longo, D.; Aime, S.; Sun, P.Z. A generalized ratiometric chemical exchange saturation transfer (CEST) MRI approach for mapping renal pH using iopamidol. Magn. Reson. Med. 2017, 79, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Airan, R.; Han, Z.; Xu, J.; Chan, K.W.Y.; Xu, Y.; Bulte, J.W.M.; van Zijl, P.C.M.; McMahon, M.T.; et al. CT and CEST MRI bimodal imaging of the intratumoral distribution of iodinated liposomes. Quant. Imaging Med. Surg. 2019, 9, 1579–1591. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Grueneisen, J.; Nagarajah, J.; Buchbender, C.; Hoffmann, O.; Schaarschmidt, B.M.; Poeppel, T.; Forsting, M.; Quick, H.H.; Umutlu, L.; Kinner, S. Positron Emission Tomography/Magnetic Resonance Imaging for Local Tumor Staging in Patients with Primary Breast Cancer: A Comparison with Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging. Invest. Radiol. 2015, 50, 505–513. [Google Scholar] [CrossRef]
- Vannier, M.W. CT clinical perspective: Challenges and the impact of future technology developments. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1909–1912. [Google Scholar]
- Vandenberghe, S.; Marsden, P.K. PET-MRI: A review of challenges and solutions in the development of integrated multimodality imaging. Phys. Med. Biol. 2015, 60, R115–R154. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhang, H.; Cai, J.; Chen, Y.; Ma, H.; Zhang, S.; Yi, J.B.; Liu, X.; Bay, B.-H.; Guo, Y.; et al. Structure–Relaxivity Mechanism of an Ultrasmall Ferrite Nanoparticle T1 MR Contrast Agent: The Impact of Dopants Controlled Crystalline Core and Surface Disordered Shell. Nano Lett. 2021, 21, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.L.; Abakumov, M.A.; Savintseva, I.V.; Ermakov, A.M.; Popova, N.R.; Ivanova, O.S.; Kolmanovich, D.D.; Baranchikov, A.E.; Ivanov, V.K. Biocompatible dextran-coated gadolinium-doped cerium oxide nanoparticles as MRI contrast agents with high T1 relaxivity and selective cytotoxicity to cancer cells. J. Mater. Chem. B 2021, 9, 6586–6599. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, J. Multimodal Molecular Imaging: Current Status and Future Directions. Contrast Media Mol. Imaging 2018, 2018, 1382183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Geest, T.; Laverman, P.; Gerrits, D.; Franssen, G.M.; Metselaar, J.M.; Storm, G.; Boerman, O.C. Comparison of three remote radiolabelling methods for long-circulating liposomes. J. Control Release 2015, 220 Pt A, 239–244. [Google Scholar] [CrossRef]
- Cho, M.H.; Shin, S.H.; Park, S.H.; Kadayakkara, D.K.; Kim, D.; Choi, Y. Targeted, Stimuli-Responsive, and Theranostic 19F Magnetic Resonance Imaging Probes. Bioconjug. Chem. 2019, 30, 2502–2518. [Google Scholar] [CrossRef]
- Zheng, B.; Yu, E.; Orendorff, R.; Lu, K.; Konkle, J.J.; Tay, Z.W.; Hensley, D.; Zhou, X.Y.; Chandrasekharan, P.; Saritas, E.U.; et al. Seeing SPIOs Directly In Vivo with Magnetic Particle Imaging. Mol. Imaging Biol. 2017, 19, 385–390. [Google Scholar] [CrossRef]
- Canetta, E. Current and Future Advancements of Raman Spectroscopy Techniques in Cancer Nanomedicine. Int. J. Mol. Sci. 2021, 22, 13141. [Google Scholar] [CrossRef]
- Han, Z.; Liu, S.; Pei, Y.; Ding, Z.; Li, Y.; Wang, X.; Zhan, D.; Xia, S.; Driedonks, T.; Witwer, K.W.; et al. Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J. Extracell. Vesicles 2021, 10, e12054. [Google Scholar] [CrossRef]
- Sun, X.; Hong, Y.; Gong, Y.; Zheng, S.; Xie, D. Bioengineered Ferritin Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 7023. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2019, 8, 552–568. [Google Scholar] [CrossRef] [PubMed]




| NP Type | Drugs Loaded | Clinical Stage | Reference | |
|---|---|---|---|---|
| Organic NPs | Liposome | Irinotecan, Doxorubicin, mRNAs | FDA-approved | [22] |
| Polymeric Nanoparticles | Paclitaxel, Gemcytabine, Doxorubicin, Platinum | FDA-approved | [23] | |
| Dendrimer | Camptothecin, Doxorubicin | Preclinical | [24,25] | |
| Metallic and inorganic NPs | Mesoporous Silica Nanoparticle (MSN) | Gemcitabine, Paclitaxel or Irinotecan | Preclinical | [25,26] |
| Carbon Dots (CDs) | Gemcitabine or Cyanine 7 | Preclinical | [25,27,28] | |
| Graphene Quantum Dots (CQDs) | Gemcitabine | Preclinical | [29] | |
| Gold Nanoparticles (AuNPs) | Gemcitabine and miR-21 inhibitor or Cetuximab | Clinical Phase I/II | [29,30,31,32] | |
| Iron Oxide Nanoparticles (IONPs) | Gemcitabine or Doxorubicin or Imiquimod | FDA-approved | [33,34,35,36,37] |
| Imaging Labels | Therapeutic Properties | |
|---|---|---|
| Nuclear Imaging | Radioactive Isotopes | Radiotherapy |
| MRI | Lanthanide metal ions, including Gd2+, Mn2+ | Radiosensitizing |
| Iron oxide nanoparticles | Photodynamic therapy | |
| Magnetic hyperthermia treatment | ||
| Labile protons | Magnetic targeting | |
| Ultrasound | Phase-transition material Calcium carbonate | US-triggered release Physical shock High-intensity focused ultrasound therapy |
| Optical or optoacoustic | NIR dyes | Photothermal therapy |
| Metallic and inorganic NPs, e.g., gold nanorod, quantum dots | Photodynamic therapy |
| Drug Name | Composition | Imaging Label (Modality) | Therapeutic Agent (Mechanism) | NCT | Phase(s) | Cancer Type |
|---|---|---|---|---|---|---|
| [64Cu]MM-302 | HER2-targeted 64Cu-labeled liposome containing doxorubicin | 64Cu (PET) | Doxorubicin (chemotherapy) | NCT01304797 | I | Breast cancer |
| NCT02213744 | II | |||||
| [89Zr]-Df-CriPec® | 89Zr labeled micellar docetaxel conjugate | 89Zr (PET) | Docetaxel (chemotherapy) | NCT03712423 | I | Solid Tumor |
| AGuIX® | Polysiloxane matrix nanoparticles with Gd chelates | Gd (MRI) | Gd (radiosensitizer) | NCT02820454 | I | Multiple brain metastases |
| NCT03818386 | II | |||||
| NCT04899908 | II | |||||
| NCT03308604 | I | Locally advanced cervical cancer | ||||
| NCT04881032 | I/II | Newly Diagnosed Glioblastoma | ||||
| NBTXR3 | Hafnium oxide nanoparticles | Hafnium oxide (CT) | Hafnium oxide (radioenhancer) | NCT01433068 | I | Soft tissue sarcoma |
| NCT02379845 | II/III | |||||
| NCT02805894 | I/II | Prostate adenocarcinoma | ||||
| NCT04505267 | I | Non-small cell lung cancer | ||||
| NCT04834349 | II | Head and neck squamous cell cancer (inoperable or recurrent) | ||||
| NCT04484909 | I | Pancreatic cancer | ||||
| NCT04615013 | I | Esophageal adenocarcinoma | ||||
| NCT04862455 | II | Head and neck squamous cancer (recurrent or metastatic) | ||||
| NCT05039632 | II | |||||
| NCT04892173 | III | Locally advanced squamous cell carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennahy, I.S.; Han, Z.; MacCuaig, W.M.; Chalfant, H.M.; Condacse, A.; Hagood, J.M.; Claros-Sorto, J.C.; Razaq, W.; Holter-Chakrabarty, J.; Squires, R.; et al. Nanotheranostics for Image-Guided Cancer Treatment. Pharmaceutics 2022, 14, 917. https://doi.org/10.3390/pharmaceutics14050917
Dennahy IS, Han Z, MacCuaig WM, Chalfant HM, Condacse A, Hagood JM, Claros-Sorto JC, Razaq W, Holter-Chakrabarty J, Squires R, et al. Nanotheranostics for Image-Guided Cancer Treatment. Pharmaceutics. 2022; 14(5):917. https://doi.org/10.3390/pharmaceutics14050917
Chicago/Turabian StyleDennahy, Isabel S., Zheng Han, William M. MacCuaig, Hunter M. Chalfant, Anna Condacse, Jordan M. Hagood, Juan C. Claros-Sorto, Wajeeha Razaq, Jennifer Holter-Chakrabarty, Ronald Squires, and et al. 2022. "Nanotheranostics for Image-Guided Cancer Treatment" Pharmaceutics 14, no. 5: 917. https://doi.org/10.3390/pharmaceutics14050917
APA StyleDennahy, I. S., Han, Z., MacCuaig, W. M., Chalfant, H. M., Condacse, A., Hagood, J. M., Claros-Sorto, J. C., Razaq, W., Holter-Chakrabarty, J., Squires, R., Edil, B. H., Jain, A., & McNally, L. R. (2022). Nanotheranostics for Image-Guided Cancer Treatment. Pharmaceutics, 14(5), 917. https://doi.org/10.3390/pharmaceutics14050917







