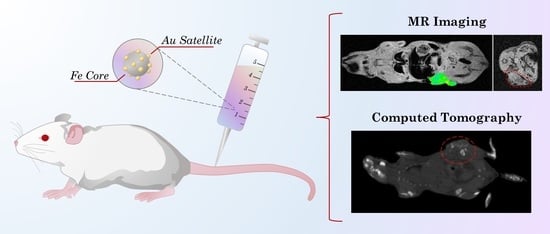
Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Cell Lines
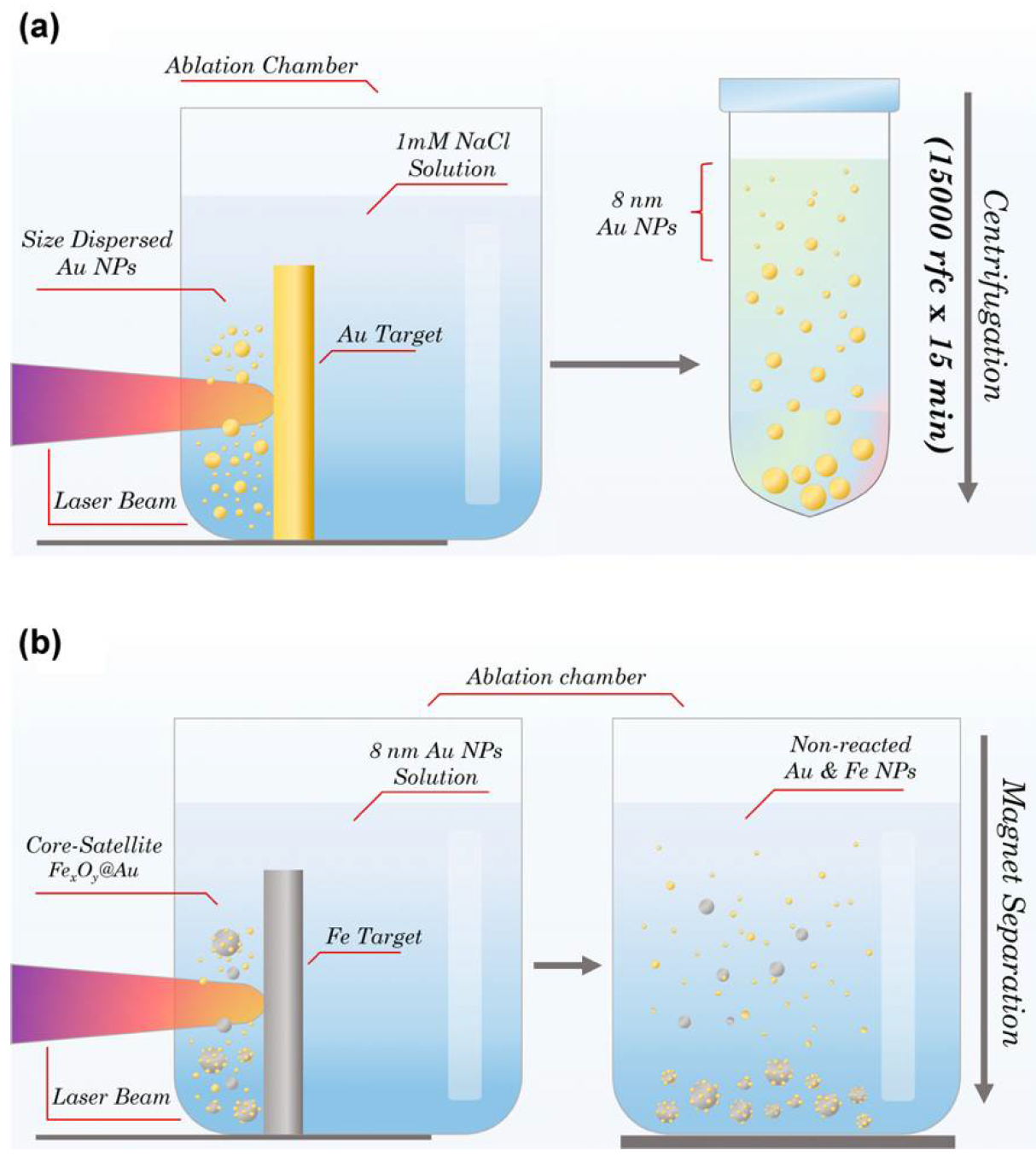
2.2. Synthesis of Fe-Au Nanoparticles
2.3. Nanoparticle Characterization
2.4. Study of Photothermal Properties
2.5. In Vitro Studies
2.6. Animals
2.7. Pharmacokinetics Study
2.8. Magnetic Resonance Imaging
2.9. Computed Tomography
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Nanoparticle Synthesis and Characterization
3.2. Photothermal Properties of Fe-Au@PAA Nanoparticles
3.3. In Vitro Studies of Fe-Au@PAA Biocompatibility and Toxicity after the Photothermal Treatment
3.4. Pharmacokinetics of Fe-Au@PAA Nanoparticles
3.5. Magnetic Resonance Imaging
3.6. Computed Tomography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sizikov, A.A.; Kharlamova, M.V.; Nikitin, M.P.; Nikitin, P.I.; Kolychev, E.L. Nonviral Locally Injected Magnetic Vectors for In vivo Gene Delivery: A Review of Studies on Magnetofection. Nanomaterials (Basel) 2021, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargazi, S.; Hajinezhad, M.R.; Rahdar, A.; Mukhtar, M.; Karamzadeh-Jahromi, M.; Almasi-Kashi, M.; Alikhanzadeh-Arani, S.; Barani, M.; Baino, F. CoNi alloy nanoparticles for cancer theranostics: Synthesis, physical characterization, in vitro and in vivo studies. Appl. Phys. A 2021, 127. [Google Scholar] [CrossRef]
- Arkaban, H.; Khajeh Ebrahimi, A.; Yarahmadi, A.; Zarrintaj, P.; Barani, M. Development of a multifunctional system based on CoFe2O4@polyacrylic acid NPs conjugated to folic acid and loaded with doxorubicin for cancer theranostics. Nanotechnology 2021, 32. [Google Scholar] [CrossRef]
- van de Walle, A.; Kolosnjaj-Tabi, J.; Lalatonne, Y.; Wilhelm, C. Ever-Evolving Identity of Magnetic Nanoparticles within Human Cells: The Interplay of Endosomal Confinement, Degradation, Storage, and Neocrystallization. Acc. Chem. Res. 2020, 53, 2212–2224. [Google Scholar] [CrossRef]
- Briley-Saebo, K.; Bjørnerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res. 2004, 316, 315–323. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Alphandéry, E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef]
- Ivanov, V.; Lizunova, A.; Rodionova, O.; Kostrov, A.; Kornyushin, D.; Aybush, A.; Golodyayeva, A.; Efimov, A.; Nadtochenko, V. Aerosol Dry Printing for SERS and Photoluminescence-Active Gold Nanostructures Preparation for Detection of Traces in Dye Mixtures. Nanomaterials (Basel) 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-plasmonic Au-Fe alloy nanoparticles designed for multimodal SERS-MRI-CT imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef]
- Terentyuk, G.S.; Maslyakova, G.N.; Suleymanova, L.V.; Khlebtsov, N.G.; Khlebtsov, B.N.; Akchurin, G.G.; Maksimova, I.L.; Tuchin, V.V. Laser-induced tissue hyperthermia mediated by gold nanoparticles: Toward cancer phototherapy. J. Biomed. Opt. 2009, 14, 21016. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Chang, Z.; Tan, J.; Wang, X.; Zhang, P.; Lin, S.; Liu, J.; Li, A. Superoxide Radical-Mediated Self-Synthesized Au/MoO3-x Hybrids with Enhanced Peroxidase-like Activity and Photothermal Effect for Anti-MRSA Therapy. ACS Appl. Mater. Interfaces 2022, 14, 13025–13037. [Google Scholar] [CrossRef] [PubMed]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef]
- Xu, C.; Tung, G.A.; Sun, S. Size and Concentration Effect of Gold Nanoparticles on X-ray Attenuation As Measured on Computed Tomography. Chem. Mater. 2008, 20, 4167–4169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caro, C.; Gámez, F.; Quaresma, P.; Páez-Muñoz, J.M.; Domínguez, A.; Pearson, J.R.; Pernía Leal, M.; Beltrán, A.M.; Fernandez-Afonso, Y.; de La Fuente, J.M.; et al. Fe3O4-Au Core-Shell Nanoparticles as a Multimodal Platform for In vivo Imaging and Focused Photothermal Therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Xu, D.; Han, Y.; Lv, X.; Chen, Z.; Zhou, T.; Ren, L.; Zhou, X. Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 545–549. [Google Scholar] [CrossRef]
- Wang, W.; Hao, C.; Sun, M.; Xu, L.; Xu, C.; Kuang, H. Spiky Fe 3 O 4 @Au Supraparticles for Multimodal In vivo Imaging. Adv. Funct. Mater. 2018, 28, 1800310. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Femtosecond laser ablation in aqueous solutions: A novel method to synthesize non-toxic metal colloids with controllable size. J. Phys.: Conf. Ser. 2007, 59, 354–359. [Google Scholar] [CrossRef]
- Baati, T.; Al-Kattan, A.; Esteve, M.-A.; Njim, L.; Ryabchikov, Y.; Chaspoul, F.; Hammami, M.; Sentis, M.; Kabashin, A.V.; Braguer, D. Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: In vivo assessment of safety and biodistribution. Sci. Rep. 2016, 6, 25400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [Green Version]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111717. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Mashkovich, E.A.; Lipey, N.A.; Popov, A.A.; Shipunova, V.O.; Griaznova, O.Y.; Deryabin, M.S.; Kurin, V.V.; Nikitin, P.I.; Kabashin, A.V.; et al. Direct photoacoustic measurement of silicon nanoparticle degradation promoted by a polymer coating. Chem. Eng. J. 2022, 430, 132860. [Google Scholar] [CrossRef]
- Popov, A.A.; Swiatkowska-Warkocka, Z.; Marszalek, M.; Tselikov, G.; Zelepukin, I.V.; Al-Kattan, A.; Deyev, S.M.; Klimentov, S.M.; Itina, T.E.; Kabashin, A.V. Laser-Ablative Synthesis of Ultrapure Magneto-Plasmonic Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials (Basel) 2022, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, P.I.; Vetoshko, P.M.; Ksenevich, T.I. New type of biosensor based on magnetic nanoparticle detection. J. Magn. Magn. Mater. 2007, 311, 445–449. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Yuryev, M.V.; Mirkasymov, A.B.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Fast processes of nanoparticle blood clearance: Comprehensive study. J. Control. Release 2020, 326, 181–191. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; an imprint of Elsevier; Tekade, R.K., Ed.; Academic Press: London, UK; San Diego, CA, USA, 2019; ISBN 9780128179093. [Google Scholar]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chan, R., Gupta, N., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 43–58. ISBN 9780081005576. [Google Scholar]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [Green Version]
- Kura, H.; Takahashi, M.; Ogawa, T. Synthesis of Monodisperse Iron Nanoparticles with a High Saturation Magnetization Using an Fe(CO) x −Oleylamine Reacted Precursor. J. Phys. Chem. C Nanomater. Interfaces 2010, 114, 5835–5838. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Kenwat, R.; Maiti, S.; Paliwal, R. Nanotheranostics for Cancer Therapy and Detection: State of the Art. Curr. Pharm. Des. 2020, 26, 5503–5517. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of Gold Nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, Q.; Paulus, Y.M. Innovations in Retinal Laser Technology. OPJ 2018, 8, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Huang, H.; Yu, B.; Qiu, K.; Huang, J.; Wang, S.; Jiang, L.; Gasser, G.; Ji, L.; et al. Unexpected high photothemal conversion efficiency of gold nanospheres upon grafting with two-photon luminescent ruthenium(II) complexes: A way towards cancer therapy? Biomaterials 2015, 63, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-W.; Ko, H.; Huang, W.-S.; Chiu, Y.-C.; Yang, L.-X.; Chia, Z.-C.; Chin, Y.-C.; Chen, Y.-J.; Tsai, Y.-T.; Hsu, C.-W.; et al. Tannic acid-induced interfacial ligand-to-metal charge transfer and the phase transformation of Fe3O4 nanoparticles for the photothermal bacteria destruction. Chem. Eng. J. 2022, 428, 131237. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Christou, E.; Pearson, J.R.; Beltrán, A.M.; Fernández-Afonso, Y.; Gutiérrez, L.; de La Fuente, J.M.; Gámez, F.; García-Martín, M.L.; Caro, C. Iron-Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy. Pharmaceutics 2022, 14, 636. [Google Scholar] [CrossRef]
- Cameron, R.B.; Hou, D. Intraoperative hyperthermic chemotherapy perfusion for malignant pleural mesothelioma: An in vitro evaluation. J. Thorac. Cardiovasc. Surg. 2013, 145, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buch, K.; Peters, T.; Nawroth, T.; Sänger, M.; Schmidberger, H.; Langguth, P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay--a comparative study. Radiat. Oncol. 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Pan, X.; Xie, F.; Lu, Y.; Zou, H.; Yin, C.; Zhang, Y.; Gao, J. Codelivery of doxorubicin and elacridar to target both liver cancer cells and stem cells by polylactide-co-glycolide/d-alpha-tocopherol polyethylene glycol 1000 succinate nanoparticles. Int. J. Nanomedicine 2018, 13, 6855–6870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [Green Version]
- Aires, A.; Cabrera, D.; Alonso-Pardo, L.C.; Cortajarena, A.L.; Teran, F.J. Elucidation of the Physicochemical Properties Ruling the Colloidal Stability of Iron Oxide Nanoparticles under Physiological Conditions. ChemNanoMat 2017, 3, 183–189. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Lee, S.; Han, H.; Koo, H.; Na, J.H.; Yoon, H.Y.; Lee, K.E.; Lee, H.; Kim, H.; Kwon, I.C.; Kim, K. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J. Control. Release 2017, 263, 68–78. [Google Scholar] [CrossRef]
- Cai, H.; Li, K.; Shen, M.; Wen, S.; Luo, Y.; Peng, C.; Zhang, G.; Shi, X. Facile assembly of Fe3O4@Au nanocomposite particles for dual mode magnetic resonance and computed tomography imaging applications. J. Mater. Chem. 2012, 22, 15110. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, B.; Xing, D. Bio-modified Fe 3 O 4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J. Mater. Chem. 2012, 22, 470–477. [Google Scholar] [CrossRef]
- Storm, L.; Israel, H.I. Photon cross sections from 1 keV to 100 MeV for elements Z=1 to Z=100. At. Data Nucl. Data Tables 1970, 7, 565–681. [Google Scholar] [CrossRef]
- Dou, Y.; Li, X.; Yang, W.; Guo, Y.; Wu, M.; Liu, Y.; Li, X.; Zhang, X.; Chang, J. PB@Au Core-Satellite Multifunctional Nanotheranostics for Magnetic Resonance and Computed Tomography Imaging in vivo and Synergetic Photothermal and Radiosensitive Therapy. ACS Appl. Mater. Interfaces 2017, 9, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, T.; Motiei, M.; Romman, Z.; Popovtzer, A.; Popovtzer, R. Targeted gold nanoparticles enable molecular CT imaging of cancer: An in vivo study. Int. J. Nanomedicine 2011, 6, 2859–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Facile one-pot synthesis of Fe3O4@Au composite nanoparticles for dual-mode MR/CT imaging applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.M.; Chernov, V.I.; Deyev, S.M. Targeted nuclear medicine. Seek and destroy. Russian Chem. Rev. 2022, 91. [Google Scholar] [CrossRef]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, e1804105. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-D.; Huang, L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. J. Control. Release 2010, 145, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Mirkasymov, A.B.; Zelepukin, I.V.; Nikitin, P.I.; Nikitin, M.P.; Deyev, S.M. in vivo blockade of mononuclear phagocyte system with solid nanoparticles: Efficiency and affecting factors. J. Control. Release 2021, 330, 111–118. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griaznova, O.Y.; Belyaev, I.B.; Sogomonyan, A.S.; Zelepukin, I.V.; Tikhonowski, G.V.; Popov, A.A.; Komlev, A.S.; Nikitin, P.I.; Gorin, D.A.; Kabashin, A.V.; et al. Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics 2022, 14, 994. https://doi.org/10.3390/pharmaceutics14050994
Griaznova OY, Belyaev IB, Sogomonyan AS, Zelepukin IV, Tikhonowski GV, Popov AA, Komlev AS, Nikitin PI, Gorin DA, Kabashin AV, et al. Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics. 2022; 14(5):994. https://doi.org/10.3390/pharmaceutics14050994
Chicago/Turabian StyleGriaznova, Olga Yu., Iaroslav B. Belyaev, Anna S. Sogomonyan, Ivan V. Zelepukin, Gleb V. Tikhonowski, Anton A. Popov, Aleksei S. Komlev, Petr I. Nikitin, Dmitry A. Gorin, Andrei V. Kabashin, and et al. 2022. "Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy" Pharmaceutics 14, no. 5: 994. https://doi.org/10.3390/pharmaceutics14050994
APA StyleGriaznova, O. Y., Belyaev, I. B., Sogomonyan, A. S., Zelepukin, I. V., Tikhonowski, G. V., Popov, A. A., Komlev, A. S., Nikitin, P. I., Gorin, D. A., Kabashin, A. V., & Deyev, S. M. (2022). Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics, 14(5), 994. https://doi.org/10.3390/pharmaceutics14050994