Bioactivity of Novel Pyrazole-Thiazolines Scaffolds against Trypanosoma cruzi: Computational Approaches and 3D Spheroid Model on Drug Discovery for Chagas Disease
Abstract
:1. Introduction
2. Materials and Methods
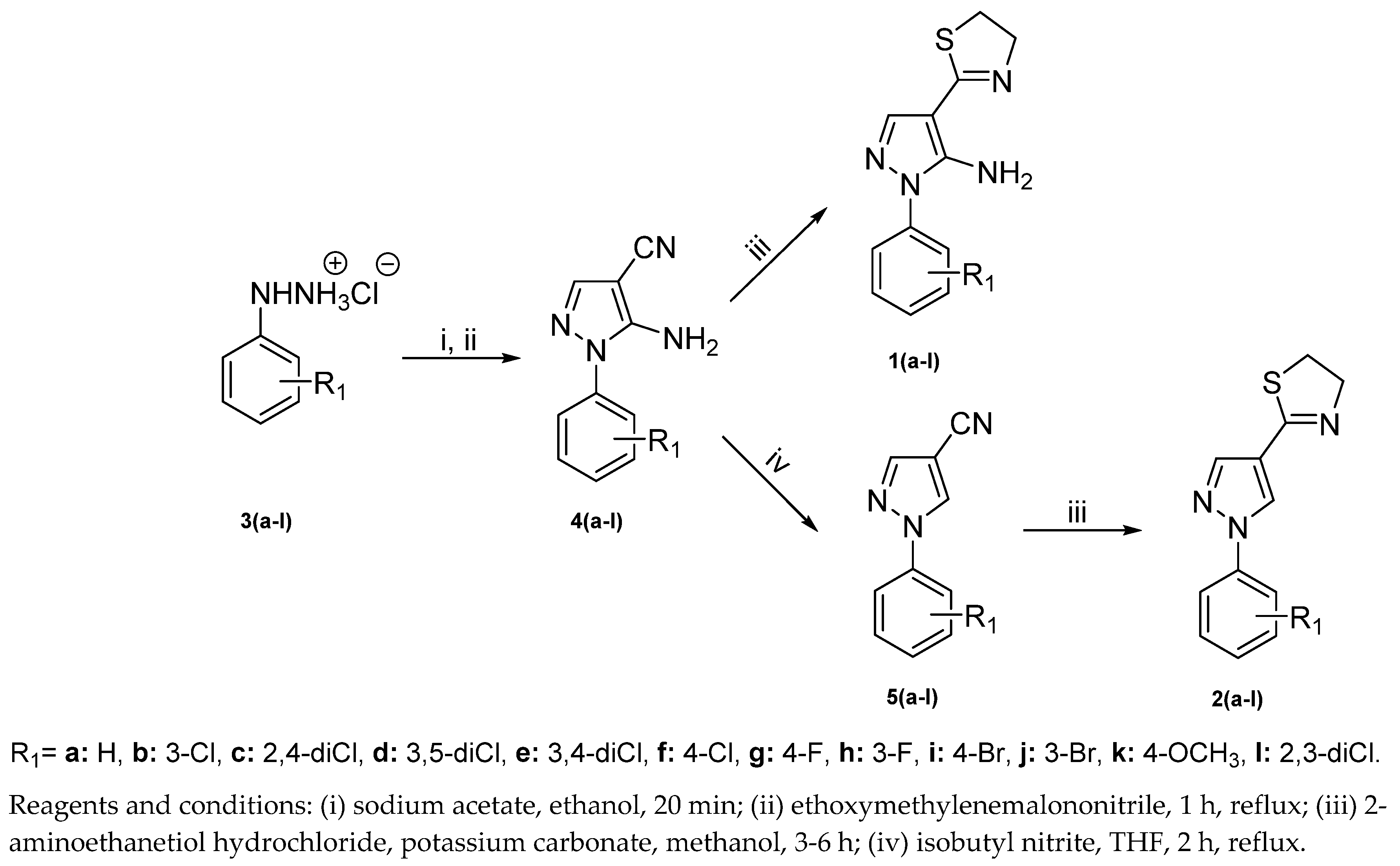
2.1. Chemistry
- General procedure for synthesis of 2-(5-amino-1-aryl-1H-pyrazole-4-yl)-4,5-dihydrothiazoles1(a-l)and 2-(1-aryl-1H-pyrazole-4-yl)-4,5-dihydrothiazoles2(a-l)
- 2-(5-amino-1-phenyl-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1a)
- 2-(5-amino-1-(3-chlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1b)
- 2-(5-amino-1-(2,4-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1c)
- 2-(5-amino-1-(3,5-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1d)
- 2-(5-amino-1-(3,4-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1e)
- 2-(5-amino-1-(4-chlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1f)
- 2-(5-amino-1-(4-fluorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1g)
- 2-(5-amino-1-(3-fluorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1h)
- 2-(5-amino-1-(4-bromophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1i)
- 2-(5-amino-1-(3-bromophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1j)
- 2-(5-amino-1-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1k)
- 2-(5-amino-1-(2,3-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(1l)
- 2-(1-phenyl-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2a)
- 2-(1-(3-chlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2b)
- 2-(1-(2,4-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2c)
- 2-(1-(3,5-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2d)
- 2-(1-(3,4-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2e)
- 2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2f)
- 2-(1-(4-fluorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2g)
- 2-(1-(3-fluorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2h)
- 2-(1-(4-bromophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2i)
- 2-(1-(3-bromophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2j)
- 2-(1-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2k)
- 2-(1-(2,3-dichlorophenyl)-1H-pyrazol-4-yl)-4,5-dihydrothiazole(2l)
2.2. Cell Culture
2.3. Parasite
2.4. Cytotoxicity In Vitro Assay
2.5. Antiparasitic Assay
2.6. Reversibility Assays
2.7. 3D Culture Model
2.8. In Vitro Combination Therapy
2.9. Measurement of Reactive Oxygen Species (ROS) Levels
2.10. In Silico Analysis
2.11. Proteinase Activity in Solution
3. Results and Discussion
3.1. Chemistry
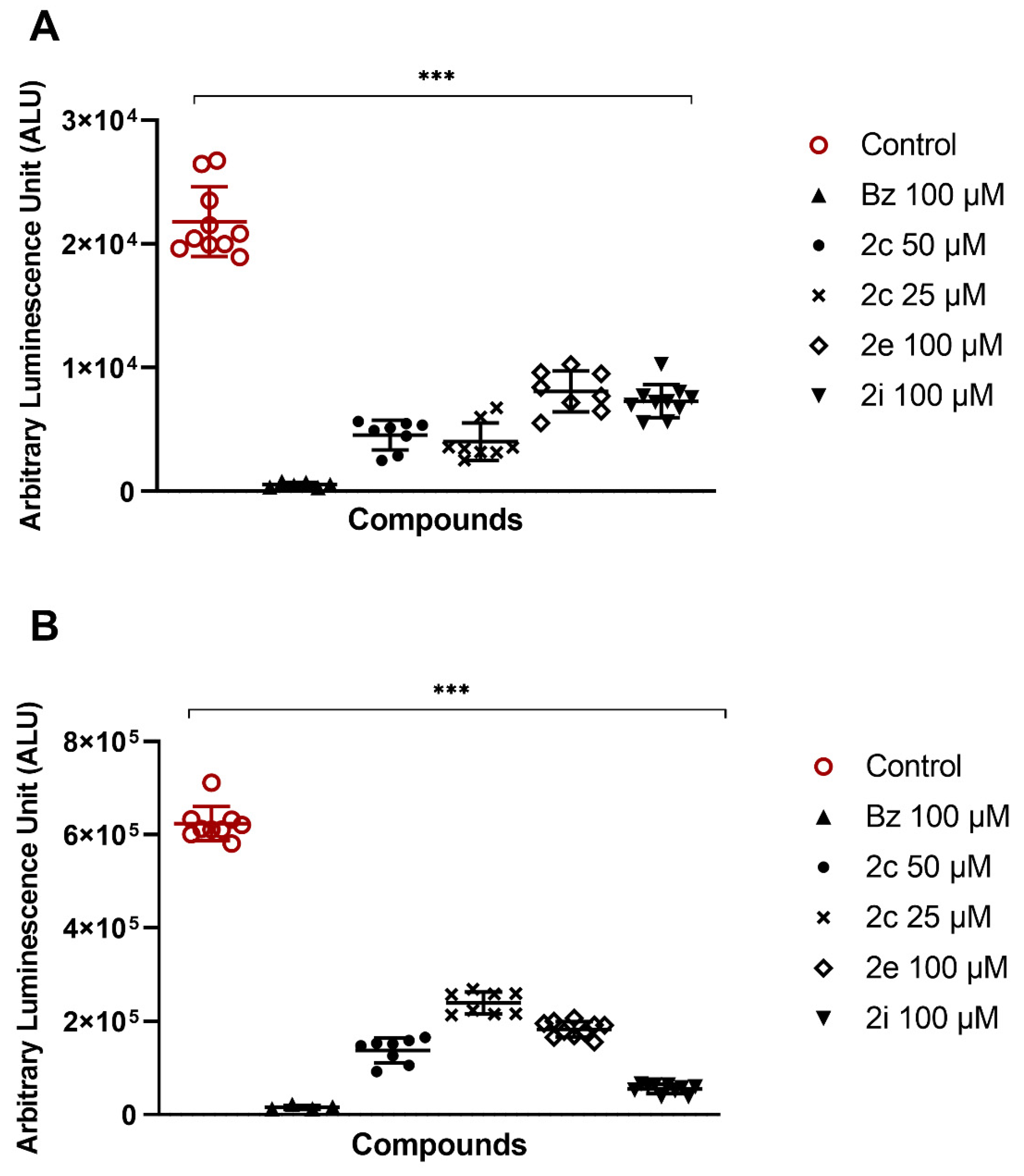
3.2. Toxicity and Phenotypic Screening of Pyrazole-Thiazoline Derivatives
3.3. Structure–Activity Relationship Analysis
3.4. Physicochemical and ADMET Properties
3.5. Drug Efficacy on 3D Culture Model
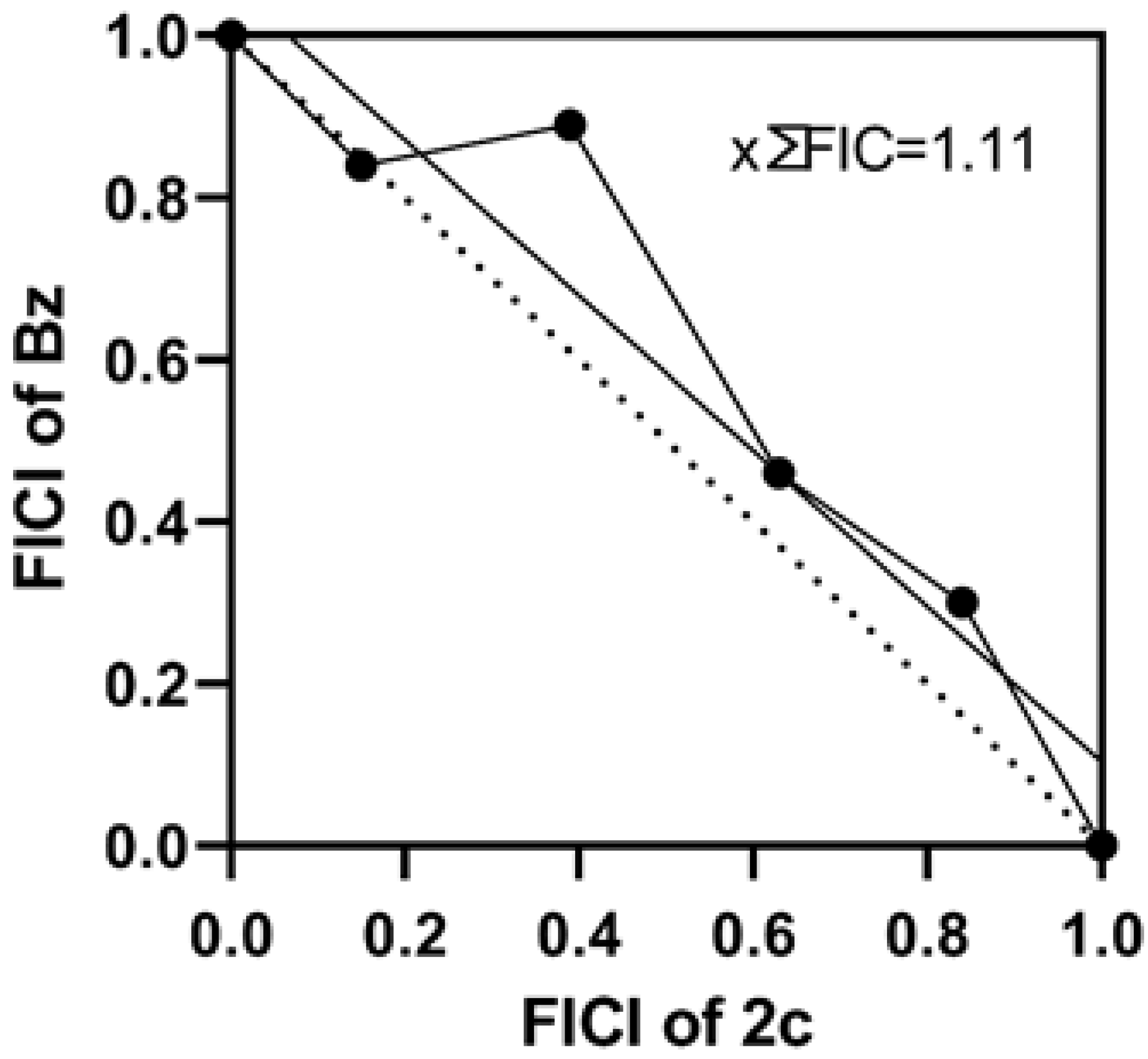
3.6. Potential of Pyrazole-Thiazoline Derivatives to Prevent Parasite Resurgence and the Effect in Combination Therapy
3.7. Drug Target and Mechanism of Action
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Chagas Disease (American Trypanosomiasis). Available online: http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed on 3 February 2022).
- Bocchi, E.A. Heart failure in South America. Curr. Cardiol. Rev. 2013, 9, 147–156. [Google Scholar] [CrossRef]
- Avaria, A.; Ventura-Garcia, L.; Sanmartino, M.; Van der Laat, C. Population movements, borders, and Chagas disease. Mem. Inst. Oswaldo Cruz 2021, 116, e210151. [Google Scholar]
- Arias, A.R.; Monroy, C.; Guhl, F.; Sosa-Estani, S.; Santos, W.S.; Abad-Franch, F. Chagas disease control-surveillance in the Americas: The multinational initiatives and the practical impossibility of interrupting vector-borne Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz 2021, 116, e210130. [Google Scholar]
- Yasuda, M.A.S. Emerging and reemerging forms of Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz 2021, 116, e210033. [Google Scholar]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Chadalawada, S.; Sillau, S.; Archuleta, S.; Mundo, W.; Bandali, M.; Parra-Henao, G.; Rodriguez-Morales, A.J.; Villamil-Gomez, W.E.; Suárez, J.A.; Shapiro, L.; et al. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of Chagas disease: A systematic review and meta-analysis. JAMA Netw. Open. 2020, 3, e2015072. [Google Scholar] [CrossRef]
- Malone, C.J.; Nevis, I.; Fernández, E.; Sanchez, A. A Rapid Review on the Efficacy and Safety of Pharmacological Treatments for Chagas Disease. Trop. Med. Infect. Dis. 2021, 12, 128. [Google Scholar] [CrossRef]
- Bern, C. Antitrypanosomal therapy for chronic Chagas’ disease. N. Engl. J. Med. 2011, 364, 2527–2534. [Google Scholar] [CrossRef]
- Nunes, M.C.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L. Council on Chagas Disease of the Interamerican Society of Cardiology. Chagas disease: An overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013, 62, 767–776. [Google Scholar] [CrossRef]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, I.; Prat, J.G.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, A.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): A phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- DNDi. Drugs for Neglected Diseases Initiative. Fexinidazole for Chagas. Available online: https://dndi.org/research-development/portfolio/fexinidazole-chagas/ (accessed on 3 February 2022).
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Elguero, J.; Silva, A.M.S. Current progress on antioxidants incorporating the pyrazole core. Eur. J. Med. Chem. 2018, 156, 394–429. [Google Scholar] [CrossRef]
- Yang, Z.; Li, P.; Gan, X. Novel Pyrazole-Hydrazone Derivatives Containing an Isoxazole Moiety: Design, Synthesis, and Antiviral Activity. Molecules 2018, 23, 1798. [Google Scholar] [CrossRef] [Green Version]
- Alnufaie, R.; Raj, K.C.H.; Alsup, N.; Whitt, J.; Andrew Chambers, S.; Gilmore, D.; Alam, M.A. Synthesis and antimicrobial studies of Coumarin-substituted pyrazole derivatives as potent anti-Staphylococcus aureus agents. Molecules 2020, 25, 2758. [Google Scholar] [CrossRef] [PubMed]
- Ashourpour, M.; Mostafavi Hosseini, F.; Amini, M.; Saeedian Moghadam, E.; Kazerouni, F.; Arman, S.Y.; Shahsavari, Z. Pyrazole Derivatives Induce Apoptosis via ROS Generation in the Triple Negative Breast Cancer Cells, MDA-MB. Asian Pac. J. Cancer Prev. 2021, 22, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Nasralla, S.N.; El-Agroudy, E.J.; Hamouda, N.; El-Fattah, A.A.; Bekhit, S.A.; Amagase, K.; Ibrahim, T.M. Investigation of the anti-inflammatory and analgesic activities of promising pyrazole derivative. Eur. J. Pharm. Sci. 2022, 168, 106080. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.J.V.; Jacomini, A.P.; Gonçalves, D.S.; Pianoski, K.E.; Poletto, J.; Lazarin-Bidóia, D.; Volpato, H.; Nakamura, C.V.; Rosa, F.A. Discovery of 1,3,4,5-tetrasubstituted pyrazoles as anti-trypanosomatid agents: Identification of alterations in flagellar structure of L. amazonensis. Bioorg. Chem. 2021, 114, 105082. [Google Scholar] [CrossRef]
- Camargo, J.N.A.; Pianoski, K.E.; Dos Santos, M.G.; Lazarin-Bidóia, D.; Volpato, H.; Moura, S.; Nakamura, C.V.; Rosa, F.A. Antiparasitic behavior of trifluoromethylated pyrazole 2-amino-1,3,4-thiadiazole hybrids and their analogues: Synthesis and structure-activity relationship. Front. Pharmacol. 2020, 11, 591570. [Google Scholar] [CrossRef]
- Reviriego, F.; Olmo, F.; Navarro, P.; Marín, C.; Ramírez-Macías, I.; García-España, E.; Albelda, M.T.; Gutiérrez-Sánchez, R.; Sánchez-Moreno, M.; Arán, V.J. Simple dialkyl pyrazole-3,5-dicarboxylates show in vitro and in vivo activity against disease causing trypanosomatids. Parasitology 2017, 144, 1133–1143. [Google Scholar] [CrossRef]
- Varghese, S.; Rahmani, R.; Russell, S.; Deora, G.S.; Ferrins, L.; Toynton, A.; Jones, A.; Sykes, M.; Kessler, A.; Eufrásio, A.; et al. Discovery of potent N-ethylurea pyrazole derivatives as dual inhibitors of Trypanosoma brucei and Trypanosoma cruzi. ACS Med. Chem. Lett. 2019, 11, 278–285. [Google Scholar] [CrossRef]
- Monteiro, M.E.; Lechuga, G.; Lara, L.S.; Souto, B.A.; Viganó, M.G.; Bourguignon, S.C.; Calvet, C.M.; Oliveira, F.O.R., Jr.; Alves, C.R.; Souza-Silva, F.; et al. Synthesis, structure-activity relationship and trypanocidal activity of pyrazole-imidazoline and new pyrazole-tetrahydropyrimidine hybrids as promising chemotherapeutic agents for Chagas disease. Eur. J. Med. Chem. 2019, 182, 111610–111623. [Google Scholar] [CrossRef]
- Orlando, M.L.R.; Lechuga, G.C.; Lara, L.S.; Ferreira, B.S.; Pereira, C.N.; Silva, R.C.; Santos, M.S.; Pereira, M.C.S. Structural optimization and biological activity of pyrazole derivatives: Virtual computational analysis, recovery assay and 3D culture model as potential predictive tools of effectiveness against Trypanosoma cruzi. Molecules 2021, 26, 6742. [Google Scholar] [CrossRef]
- Santos, M.S.; Oliveira, M.L.; Bernardino, A.M.; de Léo, R.M.; Amaral, V.F.; de Carvalho, F.T.; Leon, L.L.; Canto-Cavalheiro, M.M. Synthesis and antileishmanial evaluation of 1-aryl-4-(4,5-dihydro-1H-imidazol-2-yl)-1H-pyrazole derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 7451–7454. [Google Scholar] [CrossRef]
- Santos, M.S.; Bernardino, A.M.R.; Pinheiro, L.C.S.; Canto-Cavalheiro, M.M.; Leon, L.L. An efficient synthesis of new 5-(1-Aryl-1Hpyrazole-4-yl)-1H-tetrazoles from 1-Aryl-1H-pyrazole-4-carbonitriles via [3 + 2] cycloaddition reaction. J. Heterocycl. Chem. 2012, 49, 1425–1428. [Google Scholar] [CrossRef]
- Henriques, C.; Castro, D.P.; Gomes, L.H.F.; Garcia, E.S.; de Souza, W. Bioluminescent imaging of Trypanosoma cruzi infection in Rhodnius prolixus. Parasites Vectors 2012, 5, 214–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques, C.; Henriques-Pons, A.; Meuser-Batista, M.; Ribeiro, A.S.; de Souza, W. In vivo imaging of mice infected with bioluminescent Trypanosoma cruzi unveils novel sites of infection. Parasites Vectors 2014, 7, 89–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, L.S.; Lechuga, G.C.; Moreira, C.D.S.; Santos, T.B.; Ferreira, V.F.; da Rocha, D.R.; Pereira, M.C.S. Optimization of 1,4-naphthoquinone hit compound: A computational, phenotypic, and in vivo screening against Trypanosoma cruzi. Molecules 2021, 26, 423. [Google Scholar] [CrossRef]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef] [Green Version]
- Cardoso-Santos, C.; Fiuza, L.F.A.; Silva, C.F.; Mazzeti, A.L.; Girão, R.D.; Oliveira, G.M.; Batista, D.G.J.; Moreira, O.C.; Gomes, N.L.S.; Maes, L.; et al. 7-Aryl-7-deazapurine 3’-deoxyribonucleoside derivative as a novel lead for Chagas’ disease therapy: In vitro and in vivo pharmacology. JAC Antimicrob. Resist. 2021, 3, 168–177. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; Alves, D.R.; Miranda-Sapla, M.M.; de Morais, S.M.; Assolini, J.P.; da Silva Bortoleti, B.T.; Gonçalves, M.D.; Cataneo, A.; Kian, D.; Madeira, T.B.; et al. Caryocar coriaceum extracts exert leishmanicidal effect acting in promastigote forms by apoptosis-like mechanism and intracellular amastigotes by Nrf2/HO-1/ferritin dependent response and iron depletion: Leishmanicidal effect of Caryocar coriaceum leaf exracts. Biomed. Pharmacother. 2018, 98, 662–672. [Google Scholar]
- Sander, T.; Freyss, J.; von Korff, M.; DataWarrior, R.C. An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015, 43, W200–W207. [Google Scholar] [CrossRef]
- Sumaryada, T.; Astarina, A.S.; Ambarsari, L. Molecular Docking and physicochemical analysis of the active compounds of Soursop (Annonamuricata Linn) for an anti-breast cancer agent. Biointerface Res. Appl. Chem. 2021, 11, 11380–11389. [Google Scholar]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- MacLean, L.M.; Thomas, J.; Lewis, M.D.; Cotillo, I.; Gray, D.W.; Rycker, M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug Discovery. PLoS Negl. Trop. Dis. 2018, 12, e0006612. [Google Scholar] [CrossRef]
- Sánchez-Moreno, M.; Marín, C.; Navarro, P.; Lamarque, L.; García-España, E.; Miranda, C.; Huertas, O.; Olmo, F.; Gómez-Contreras, F.; Pitarch, J.; et al. In vitro and in vivo trypanosomicidal activity of pyrazole-containing macrocyclic and macrobicyclic polyamines: Their action on acute and chronic phases of Chagas disease. J. Med. Chem. 2012, 55, 4231–4243. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.S.; Wilkinson, S.R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012, 56, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Goijman, S.G.; Stoppani, A.O. Effects of nitroheterocyclic drugs on macromolecule synthesis and degradation in Trypanosoma cruzi. Biochem. Pharmacol. 1985, 34, 1331–1336. [Google Scholar] [CrossRef]
- Kasai, H.; Iwamoto-Tanaka, N.; Fukada, S. DNA modifications by the mutagen glyoxal: Adduction to G and C, deamination of C and GC and GA cross-linking. Carcinogenesis 1998, 19, 1459–1465. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.A.; Awad, S.M.; Said, A.M.; Mahgoub, S.; Taha, H.; Ahmed, N.M. Design, synthesis, molecular modelling and biological evaluation of novel 3-(2-naphthyl)-1-phenyl-1H-pyrazole derivatives as potent antioxidants and 15-Lipoxygenase inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 847–863. [Google Scholar] [CrossRef]









| Compounds | Trypanocidal Activity (Mean ± SD μM) | Cytotoxicity (Mean ± SD μM) | |||||
|---|---|---|---|---|---|---|---|
| Trypomastigotes | Intracellular Amastigotes | Vero Cells | |||||
| IC50 | IC90 | SI | IC50 | IC90 | SI | CC50 | |
| 1a | 66.4 ± 2.7 | >100 | 3.7 | 80.7 ± 3.9 | >100 | 3.1 | 248.4 ± 22.0 |
| 1b | 96.9 ± 3.1 | >100 | >5.2 | 95.7 ± 4.8 | >100 | 5.2 | >500 |
| 1c | >100 | >100 | <5 | >100 | >100 | <5 | >50 |
| 1d | >100 | >100 | >4.1 | 63.2 ± 5.4 | >100 | 6.6 | 415.5 ± 34.6 |
| 1e | >100 | >100 | <5 | >100 | >100 | <5 | >500 |
| 1f | 88.3 ± 7.1 | >100 | <5 | >100 | >100 | <5 | >500 |
| 1g | >100 | >100 | <5 | 96.3 ± 2.7 | >100 | 5.6 | 483.0 ± 16.6 |
| 1h | >100 | >100 | <5 | >100 | >100 | <5 | >500 |
| 1i | 49.3 ± 4.7 | >100 | >10.1 | >100 | >100 | <5 | >500 |
| 1j | 52.3 ±3.4 | >100 | >9.5 | 26.6 ±3.1 | >100 | >18.7 | >500 |
| 1k | 12.5 ± 3.5 | 93.4 ± 5.5 | >39.9 | 79.1 ± 4.4 | 97.3 ± 4.7 | >6.3 | >500 |
| 1l | >100 | >100 | <5 | 11.1 ± 2.0 | >100 | >44.9 | >500 |
| 2a | 1.1 ± 0.3 | 10.4 ± 1.1 | 166.8 | 10.7 ± 2.2 | 89.0 ± 2.6 | 17.4 | 187.3 ± 12.7 |
| 2b | 0.8 ± 0.3 | 8.8 ± 1.3 | 100.2 | 8.3 ± 1.9 | 31.9 ± 1.3 | 9.4 | 78.3 ± 7.7 |
| 2c | 0.4 ± 0.02 | 2.8 ± 0.6 | 269.9 | 1.4 ± 0.4 | 16.9 ± 2.5 | 76.2 | 106.7 ± 11.4 |
| 2d | >100 | >100 | <5 | >100 | >100 | <5 | >500 |
| 2e | 1.9 ± 0.4 | 6.9 ± 2.0 | >254 | 7.84 ± 1.0 | 30.4 ± 3.7 | >62.9 | >500 |
| 2f | 0.6 ± 0.3 | 13.3 ± 2.3 | 143.6 | 7.6 ± 2.5 | 33.5 ± 4.4 | 12.0 | 91.7 ± 13.1 |
| 2g | 7.9 ± 1.0 | 30.1 ± 2.8 | 7.3 | 14.0 ± 1.8 | 98.0 ± 3.4 | 4.18 | 58.6 ± 8.11 |
| 2h | 9.8 ± 1.5 | 36.3 ± 3.2 | 3.8 | 9.1 ± 1.7 | 76.3 ± 6.1 | 4.19 | 37.9 ± 4.8 |
| 2i | 2.1 ± 0.9 | 13.3 ± 2.8 | 101.2 | 6.8 ± 1.1 | 27.1 ± 3.6 | 31.8 | 217.2 ± 15.2 |
| 2j | 9.1 ± 0.9 | 48.2 ± 4.1 | 7.11 | 8.1 ± 1.3 | 31.1 ± 3.1 | 7.9 | 64.5 ± 7.3 |
| 2k | 3.1 ± 0.3 | 36.1 ± 2.6 | 36.1 | 22.4 ± 2.8 | 53.0 ± 9.8 | 4.9 | 110.1 ± 9.4 |
| 2l | 25.8 ± 1.9 | >100 | 3.44 | 25.3 ± 2.4 | 75.5 ± 8.3 | 3.5 | 88.7 ± 9.3 |
| Bz | 19.6 ± 2.3 | 95.9 ± 4.7 | >18.9 | 3.3 ± 1.1 | 13.7 ± 4.2 | >216 | >500 |
 General Structure | |||
|---|---|---|---|
| Compounds | R1 | R2 | Intracellular Amastigotes pIC50 |
| 1a | H | NH2 | 4.09 |
| 1b | 3-Cl | NH2 | 4.01 |
| 1c | 2,4-diCl | NH2 | <4 |
| 1d | 3,5-diCl | NH2 | 4.19 |
| 1e | 3,4-diCl | NH2 | <4 |
| 1f | 4-Cl | NH2 | <4 |
| 1g | 4-F | NH2 | 4.01 |
| 1h | 3-F | NH2 | <4 |
| 1i | 4-Br | NH2 | <4 |
| 1j | 3-Br | NH2 | 4.57 |
| 1k | 4-OCH3 | NH2 | 4.10 |
| 1l | 2,3-diCl | NH2 | 4.95 |
| 2a | H | H | 4.97 |
| 2b | 3-Cl | H | 5.08 |
| 2c | 2,4-diCl | H | 5.85 |
| 2d | 3,5-diCl | H | <4 |
| 2e | 3,4-diCl | H | 5.10 |
| 2f | 4-Cl | H | 5.11 |
| 2g | 4-F | H | 4.85 |
| 2h | 3-F | H | 5.04 |
| 2i | 4-Br | H | 5.16 |
| 2j | 3-Br | H | 5.09 |
| 2k | 4-OCH3 | H | 4.64 |
| 2l | 2,3-diCl | H | 4.59 |
| Bz | - | - | 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, L.d.S.; Lechuga, G.C.; Orlando, L.M.R.; Ferreira, B.S.; Souto, B.A.; dos Santos, M.S.; Pereira, M.C.d.S. Bioactivity of Novel Pyrazole-Thiazolines Scaffolds against Trypanosoma cruzi: Computational Approaches and 3D Spheroid Model on Drug Discovery for Chagas Disease. Pharmaceutics 2022, 14, 995. https://doi.org/10.3390/pharmaceutics14050995
Lara LdS, Lechuga GC, Orlando LMR, Ferreira BS, Souto BA, dos Santos MS, Pereira MCdS. Bioactivity of Novel Pyrazole-Thiazolines Scaffolds against Trypanosoma cruzi: Computational Approaches and 3D Spheroid Model on Drug Discovery for Chagas Disease. Pharmaceutics. 2022; 14(5):995. https://doi.org/10.3390/pharmaceutics14050995
Chicago/Turabian StyleLara, Leonardo da Silva, Guilherme Curty Lechuga, Lorraine Martins Rocha Orlando, Byanca Silva Ferreira, Bernardo Araújo Souto, Maurício Silva dos Santos, and Mirian Claudia de Souza Pereira. 2022. "Bioactivity of Novel Pyrazole-Thiazolines Scaffolds against Trypanosoma cruzi: Computational Approaches and 3D Spheroid Model on Drug Discovery for Chagas Disease" Pharmaceutics 14, no. 5: 995. https://doi.org/10.3390/pharmaceutics14050995
APA StyleLara, L. d. S., Lechuga, G. C., Orlando, L. M. R., Ferreira, B. S., Souto, B. A., dos Santos, M. S., & Pereira, M. C. d. S. (2022). Bioactivity of Novel Pyrazole-Thiazolines Scaffolds against Trypanosoma cruzi: Computational Approaches and 3D Spheroid Model on Drug Discovery for Chagas Disease. Pharmaceutics, 14(5), 995. https://doi.org/10.3390/pharmaceutics14050995








