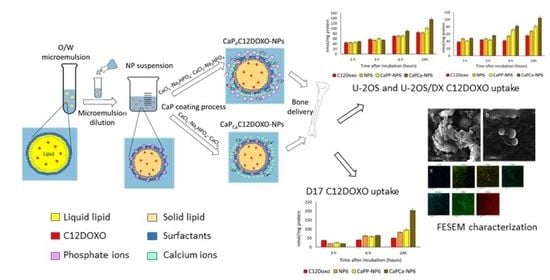
Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
C12DOXO Synthesis
2.2. Preparation of NPs and C12DOXO-Loaded NPs
2.3. Purification of NPs
2.4. CaP Coating
- NPs/C12DOXO NPs with calcium externally exposed (CaPCa-NPs/CaPCa-C12DOXO NPs) were obtained by adding layer by layer to the purified suspension 300 μL of 42 mM CaCl2, then 300 μL of 42 mM Na2HPO4, and finally 600 μL of 42 mM CaCl2
- NPs/C12DOXO NPs with phosphate externally exposed (CaPP-NPs and/CaPP-C12DOXO NPs) were obtained by adding layer by layer to the purified suspension 300 μL of 42 mM CaCl2, then 300 μL of 42 mM Na2HPO4, then 300 μL of 42 mM CaCl2, and finally 300 μL of 42 mM Na2HPO4
2.5. Characterization of NPs
2.5.1. Particle Size and Zeta Potential Measurements
2.5.2. C12DOXO Entrapment Efficiency
2.5.3. FESEM Characterization
2.5.4. In Vitro Release Study
2.6. In Vitro Cell Studies
2.6.1. Human Osteosarcoma U-2OS Cells
2.6.2. Canine Osteosarcoma D17 Cells
2.6.3. Cytotoxicity Assay
2.6.4. C12DOXO Cellular Uptake
2.6.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of NPs
3.1.1. Particle Size, Zeta Potential, and C12DOXO Entrapment Efficiency Measurements
3.1.2. FESEM Characterization
3.2. In Vitro Release Study
3.3. In Vitro Cell Studies
3.3.1. Human Osteosarcoma U-2OS Cells
3.3.2. Canine Osteosarcoma D17 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Odore, R.; Re, G. Expression and functionality of TRPV1 receptor in human MCF-7 and canine CF.41 cells. Vet. Comp. Oncol. 2015, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine osteosarcoma: A naturally occurring disease to inform pediatric oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.; Dunning, M.D.; De Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ro, J.Y. The 2020 WHO classification of tumors of bone: An updated review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef]
- Loukopoulos, P.; Robinson, W.F. Clinicopathological relevance of tumour grading in canine osteosarcoma. J. Comp. Pathol. 2007, 136, 65–73. [Google Scholar] [CrossRef]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk factors for development of canine and human osteosarcoma: A comparative review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Anfinsen, K.P.; Grotmol, T.; Bruland, O.S.; Jonasdottir, T.J. Breed-specific incidence rates of canine primary bone tumors—A population based survey of dogs in Norway. Can. J. Vet. Res. 2011, 75, 209–215. [Google Scholar]
- Longhi, A.; Pasini, A.; Cicognani, A.; Baronio, F.; Pellacan, A.; Baldini, N.; Bacci, G. Height as a risk factor for osteosarcoma. J. Pediatr. Hematol. Oncol. 2005, 27, 314–318. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Choong, P.F.M.; Dunstan, D.E. Osteosarcoma treatment: State of the art. Cancer Metastasis Rev. 2009, 28, 247–263. [Google Scholar] [CrossRef]
- Reddy, K.I.A.; Wafa, H.; Gaston, C.L.; Grimer, R.J.; Abudu, A.T.; Jeys, L.M.; Carter, S.R.; Tillman, R.M. Does amputation ofer any survival beneft over limb salvage in osteosarcoma patients with poor chemonecrosis and close margins? Bone Jt. J. 2015, 97, 115–120. [Google Scholar] [CrossRef]
- Ferrari, S.; Serra, M. An update on chemotherapy for osteosarcoma. Expert Opin. Pharmacother. 2015, 16, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone diseases: Current approach and future perspectives in drug delivery systems for bone targeted therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.K.; Wood, G.A.; Viloria-Petit, A.M. Recent advances in the discovery of biomarkers for canine osteosarcoma. Front. Vet. Sci. 2021, 8, 734965. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L.; Fidel, J. P-glycoprotein mediated drug interactions in animals and humans with cancer. J. Vet. Intern. Med. 2015, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Małek, A.; Taciak, B.; Sobczak, K.; Grzelak, A.; Wójcik, M.; Mieczkowski, J.; Lechowski, R.; Zabielska-Koczywąs, K.A. Enhanced cytotoxic effect of doxorubicin conjugated to glutathione-stabilized gold nanoparticles in canine osteosarcoma—in vitro studies. Molecules 2021, 26, 3487. [Google Scholar] [CrossRef]
- Zandvliet, M.; Teske, E. Mechanisms of drug resistance in veterinary oncology—A review with an emphasis on canine lymphoma. Vet. Sci. 2015, 2, 150–184. [Google Scholar] [CrossRef] [Green Version]
- Marconato, L.; Melacarne, A.; Aralla, M.; Sabattini, S.; Tiraboschi, L.; Ferrari, V.; Zeira, O.; Balboni, A.; Faroni, E.; Guerra, D.; et al. A target animal effectiveness study on adjuvant peptide-based vaccination in dogs with non-metastatic appendicular osteosarcoma undergoing amputation and chemotherapy. Cancers 2022, 14, 1347. [Google Scholar] [CrossRef]
- Szewczyk, M.; Lechowski, R.; Zabielska, K. What do we know about canine osteosarcoma treatment? Review. Vet. Res. Commun. 2015, 39, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Q.; Bai, Q.; Jiang, W. Advances of smart nano-drug delivery systems in osteosarcoma treatment. J. Mater. Chem. B 2021, 9, 5439–5450. [Google Scholar] [CrossRef]
- Huang, X.; Wu, W.; Yang, W.; Qing, X.; Shao, Z. Surface engineering of nanoparticles with ligands for targeted delivery to osteosarcoma. Colloid Surf. B 2020, 190, 110891. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Lin, Z.; Li, M.; Hu, X.; Zhang, Y.; Peng, M.; Xia, H.; Han, G. Enhancing osteosarcoma killing and CT imaging using ultrahigh drug loading and NIR-responsive bismuth sulfide@mesoporous silica nanoparticles. Adv. Healthc. Mater. 2018, 7, 1800602. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Raposo, L.R.; Roma-Rodrigues, C.; Jesus, J.; Martins, L.M.D.R.S.; Pombeiro, A.J.; Baptista, P.V.; Fernandes, A.R. Targeting canine mammary tumours via gold nanoparticles functionalized with promising Co(II) and Zn(II) compounds. Vet. Comp. Oncol. 2017, 15, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Fantoni, L.; Casotti, C.; Riganti, C.; Serra, M. Drug resistance in osteosarcoma: Emerging biomarkers, therapeutic targets and treatment strategies. Cancers 2021, 13, 2878. [Google Scholar] [CrossRef]
- Khan, M.A.; Wu, V.M.; Ghosh, S.; Uskokovic, V. Gene delivery using calcium phosphate nanoparticles: Optimization of the transfection process and the effects of citrate and poly(l-lysine) as additives. J. Colloid Interface Sci. 2016, 471, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Victor, S.P.; Paul, W.; Sharma, C.P. Calcium phosphate nanoplatforms for drug delivery and theranostic applications. In Drug Delivery Nanosystems for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–179. [Google Scholar]
- Schmidt, H.T.; Gray, B.L.; Wingert, P.A.; Ostafin, A.E. Assembly of aqueous-cored calcium phosphate nanoparticles for drug delivery. Chem. Mater. 2004, 16, 4942–4947. [Google Scholar] [CrossRef]
- Yeo, C.H.; Zein, S.H.S.; Ahmad, A.L.; McPhail, D.S. Comparison of DOPA and DPPA liposome templates for the synthesis of calcium phosphate nanoshells. Ceram. Int. 2012, 38, 561–570. [Google Scholar] [CrossRef]
- Rivero Berti, I.; Dell’ Arciprete, M.L.; Dittler, M.L.; Miñan, A.; Fernández Lorenzo de Mele, M.; Gonzalez, M. Delivery of fluorophores by calcium phosphate-coated nanoliposomes and interaction with Staphylococcus aureus biofilms. Colloids Surf. Biointerfaces 2016, 142, 214–222. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Sapino, S.; Chindamo, G.; Chirio, D.; Manzoli, M.; Peira, E.; Riganti, C.; Gallarate, M. Calcium phosphate-coated lipid nanoparticles as a potential tool in bone diseases therapy. Nanomaterials 2021, 11, 2983. [Google Scholar] [CrossRef] [PubMed]
- Peira, E.; Chirio, D.; Battaglia, L.; Barge, A.; Chegaev, K.; Gigliotti, C.L.; Ferrara, B.; Dianzani, C.; Gallarate, M. Solid lipid nanoparticles carrying lipophilic derivatives of doxorubicin: Preparation, characterization, and in vitro cytotoxicity studies. J. Microencapsul. 2016, 33, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of solid lipid nanoparticles by cold dilution of microemulsions: Curcumin loading, preliminary in vitro studies, and biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotta, M.; Peira, E.; Carlotti, M.E.; Gallarate, M. Deformable liposomes for dermal administration of methotrexate. Int. J. Pharm. 2004, 270, 119–125. [Google Scholar] [CrossRef]
- Serra, M.; Scotlandi, K.; Manara, M.C.; Maurici, D.; Lollini, P.L.; De Giovanni, C.; Toffoli, G.; Baldini, N. Establishment and characterization of multidrug-resistant human osteosarcoma cell lines. Anticancer Res. 1993, 13, 323–329. [Google Scholar]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Racca, S.; Abbadessa, G.; Piccione, F.; Re, G. Transient receptor potential vanilloid 1 expression and functionality in MCF-7 cells: A preliminary investigation. J. Breast Cancer 2014, 17, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Barraud, L.; Merle, P.; Soma, E.; Lefrançois, L.; Guerret, S.; Chevallier, M.; Vitvitski, L. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J. Hepatol. 2005, 42, 736–743. [Google Scholar] [CrossRef]
- Duggan, S.T.; Keating, G.M. Pegylated liposomal doxorubicin. Drugs 2011, 71, 2531–2558. [Google Scholar] [CrossRef]
- Petschauer, J.S.; Madden, A.J.; Kirschbrown, W.P.; Song, G.; Zamboni, W.C. The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents. Nanomedicine 2015, 10, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Buondonno, I.; Gazzano, E.; Tavanti, E.; Chegaev, K.; Kopecka, J.; Fanelli, M.; Rolando, B.; Fruttero, R.; Gasco, A.; Hattinger, C.; et al. Endoplasmic reticulum-targeting doxorubicin: A new tool effective against doxorubicin-resistant osteosarcoma. Cell. Mol. Life Sci. 2019, 76, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]








| Ingredients (mg) | µE0/ NP0 | µE2/ NP2 | µE4/ NP4 | µE6/ NP6 | ||
|---|---|---|---|---|---|---|
| Nanoparticles | Microemulsions | Trilaurin | 60 | 60 | 60 | 60 |
| s-EA | 200 | 200 | 200 | 200 | ||
| Epikuron®200 | 170 | 170 | 170 | 170 | ||
| Cremophor®RH6 | 50 | 35 | 35 | 35 | ||
| Propylene glycol | 100 | 90 | 90 | 90 | ||
| s-Water | 700 | 700 | 700 | 700 | ||
| C12DOXO | - | 2 | 4 | 6 | ||
| Dilution water (mL) | 5 | 5 | 5 | 5 |
| Mean Diameter (nm) PDI | Zeta Potential (mV) | C12DOXO %EE | |
|---|---|---|---|
| NP0 | 209.7 ± 2.8 0.128 | −23.5 ± 1.4 | - |
| CaPCa-NP0 | 232.2 ± 4.0 0.145 | −1.6 ± 0.2 | - |
| CaPP-NP0 | 257.5 ± 2.8 0.165 | −19.6 ± 3.7 | - |
| NP2 | 253.4 ± 3.4 0.130 | −21.2 ± 1.0 | 83.2 ± 2.4 |
| CaPCa-NP2 | 290.2 ± 3.5 0.147 | −6.8 ± 0.8 | 70.0 ± 3.2 |
| CaPP-NP2 | 293.8 ± 4.2 0.176 | −18.7 ± 3.1 | 61.2 ± 4.1 |
| NP4 | 268.5 ± 2.1 0.153 | −14.9 ± 2.0 | 84.7 ± 3.8 |
| CaPCa-NP4 | 285.2 ± 2.6 0.188 | −4.2 ± 1.0 | 73.0 ± 2.5 |
| CaPP-NP4 | 311.2 ± 1.6 0.195 | −13.5 ± 3.9 | 62.6 ± 2.4 |
| NP6 | 282.7 ± 3.2 0.154 | +12.7 ± 1.5 | 87.2 ± 2.9 |
| CaPCa-NP6 | 311.8 ± 6.3 0.163 | +1.6 ± 2.0 | 74.2 ± 2.4 |
| CaPP-NP6 | 322.7 ± 8.5 0.186 | −3.9 ± 0.9 | 71.5 ± 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirio, D.; Sapino, S.; Chindamo, G.; Peira, E.; Vercelli, C.; Riganti, C.; Manzoli, M.; Gambino, G.; Re, G.; Gallarate, M. Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics 2022, 14, 1362. https://doi.org/10.3390/pharmaceutics14071362
Chirio D, Sapino S, Chindamo G, Peira E, Vercelli C, Riganti C, Manzoli M, Gambino G, Re G, Gallarate M. Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics. 2022; 14(7):1362. https://doi.org/10.3390/pharmaceutics14071362
Chicago/Turabian StyleChirio, Daniela, Simona Sapino, Giulia Chindamo, Elena Peira, Cristina Vercelli, Chiara Riganti, Maela Manzoli, Graziana Gambino, Giovanni Re, and Marina Gallarate. 2022. "Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy" Pharmaceutics 14, no. 7: 1362. https://doi.org/10.3390/pharmaceutics14071362
APA StyleChirio, D., Sapino, S., Chindamo, G., Peira, E., Vercelli, C., Riganti, C., Manzoli, M., Gambino, G., Re, G., & Gallarate, M. (2022). Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics, 14(7), 1362. https://doi.org/10.3390/pharmaceutics14071362