Activated Carbon for Drug Delivery from Composite Biomaterials: The Effect of Grinding on Sirolimus Binding and Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of 3H-SRL
2.2. Preparation of AC
2.3. Characterization of the Activated Carbon
2.3.1. Dynamic Light Scattering
2.3.2. SEM Analysis
2.3.3. X-ray Photoelectron Spectroscopy
2.4. SRL Adsorption and Release Kinetics
2.5. Adsorption Isotherms of SRL on Different Fractions of AC
3. Results and Discussion
3.1. Characterization of the Activated Carbon
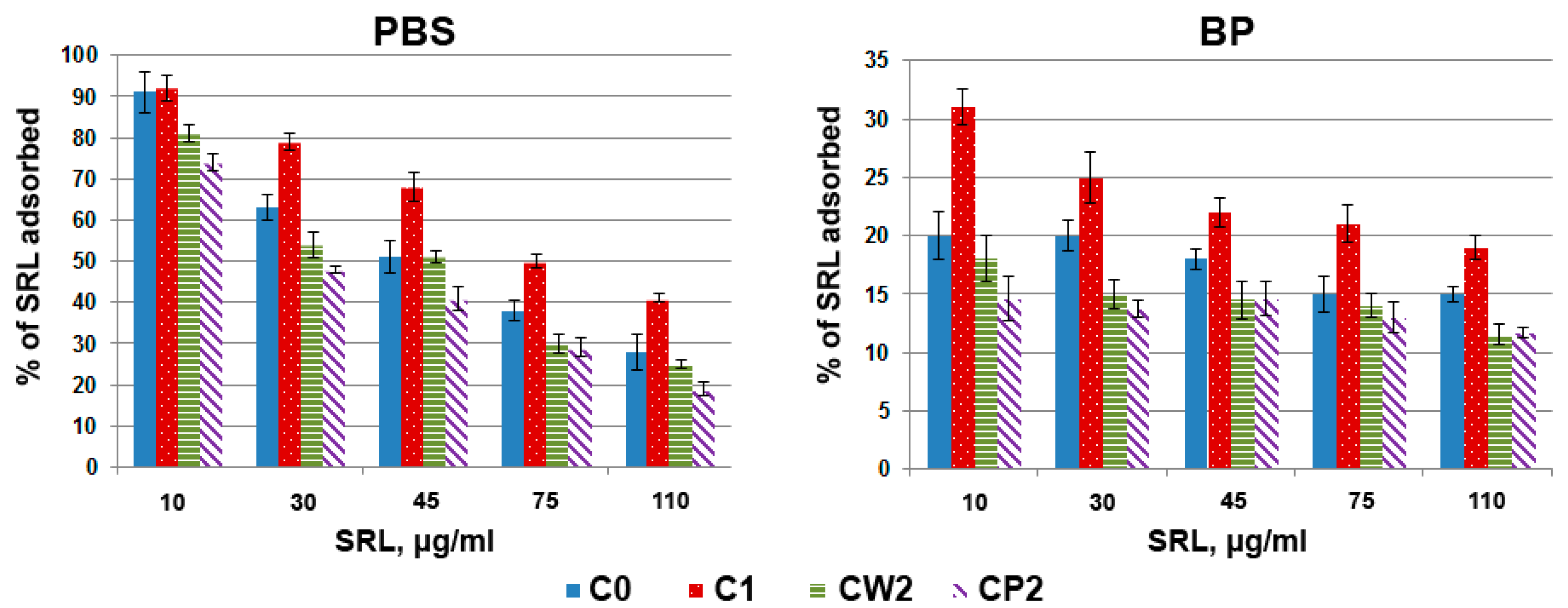
3.2. Study of SRL Adsorption
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
3.3. SRL Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Olson, K.R. Activated charcoal for acute poisoning: One toxicologist’s journey. J. Med. Toxicol. 2010, 6, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juurlink, D.N. Activated charcoal for acute overdose: A reappraisal. Br. J. Clin. Pharmacol. 2016, 81, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, M.I.; Alonso-Morales, N.; Baeza, J.A.; Rodríguez, J.J.; Gilarranz, M.A. High load drug release systems based on carbon porous nanocapsule carriers. Ibuprofen case study. J. Mater. Chem. B 2020, 8, 5293–5304. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.K.; Bashirelahi, N.; Reynolds, M.A. Charcoal and charcoal-based dentifrices: A literature review. J. Am. Dent. Assoc. 2017, 148, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.; Fayne, R.; Burroway, B. Charcoal: An ancient material with a new face. Clin. Dermatol. 2020, 38, 262–264. [Google Scholar] [CrossRef]
- Silberman, J.; Galuska, M.A.; Taylor, A. Activated Charcoal. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Iguchi, S.; Yamaguchi, N.; Takami, H.; Komatsu, T.; Ookubo, H.; Sekii, H.; Inoue, K.; Okazaki, S.; Okai, I.; Maruyama, S.; et al. Higher efficacy of direct hemoperfusion using coated activated-charcoal column for disopyramide poisoning: A case report. Medicine 2017, 96, e8755. [Google Scholar] [CrossRef]
- Miriyala, N.; Ouyang, D.; Perrie, Y.; Lowry, D.; Kirby, D.J. Activated carbon as a carrier for amorphous drug delivery: Effect of drug characteristics and carrier wettability. Eur. J. Pharm. Biopharm. 2017, 115, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Goscianska, J.; Ejsmont, A.; Olejnik, A.; Ludowicz, D.; Stasiłowicz, A.; Cielecka-Piontek, J. Design of paracetamol delivery systems based on functionalized ordered mesoporous carbons. Materials 2020, 13, 4151. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chen, Y.C.; Lee, C.-H. Modified Activated Carbon for Copper Ion Removal from Aqueous Solution. Processes 2022, 10, 150. [Google Scholar] [CrossRef]
- JJandosov, J.; Alavijeh, M.; Sultakhan, S.; Baimenov, A.; Bernardo, M.; Sakipova, Z.; Azat, A.; Lyubchyk, S.; Zhylybayeva, N.; Naurzbayeva, G.; et al. Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac. Molecules 2022, 27, 2296. [Google Scholar] [CrossRef]
- Scheer, H.S.; Kaiser, M.; Zingg, U. Results of directly applied activated carbon cloth in chronic wounds: A preliminary study. J Wound Care. 2017, 26, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, F.; Bonnamy, S.; Rochet, N.; Drouet, C. Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System. Int. J. Mol. Sci. 2021, 22, 12247. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, K.A.; Stepanova, A.O.; Kvon, R.I.; Douglas, T.E.L.; Kuznetsov, N.A.; Chernonosova, V.S.; Zaporozhchenko, I.A.; Kharkova, M.V.; Romanova, I.V.; Karpenko, A.A.; et al. Electrospun Produced 3D Matrices for Covering of Vascular Stents: Paclitaxel Release Depending on Fiber Structure and Composition of the External Environment. Materials 2018, 11, 2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarkina, Z.K.; Chelobanov, B.P.; Chernonosova, V.S.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment. Materials 2020, 13, 2692. [Google Scholar] [CrossRef] [PubMed]
- Nazarkina, Z.K.; Chelobanov, B.P.; Kuznetsov, K.A.; Shutov, A.V.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting. Polymers 2021, 13, 1165. [Google Scholar] [CrossRef]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; John Wiley & Sons: New York, NY, USA, 1998; p. 560. [Google Scholar]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transpl. Proc. 2003, 35, 7S–14S. [Google Scholar] [CrossRef]
- Haeri, A.; Osouli, M.; Bayat, F.; Alavi, S.; Dadashzadeh, S. Nanomedicine approaches for sirolimus delivery: A review of pharmaceutical properties and preclinical studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Simamora, P.; Alvarez, J.M.; Yalkowsky, S.H. Solubilization of rapamycin. Int. J. Pharm. 2001, 213, 25–29. [Google Scholar] [CrossRef]
- Sidorov, V.N.; Polak, Y.V.; Laktionov, P.P.; Roshcke, V.V.; Kist, A.G. Method of Production of Tritium Labeled Organic Compounds and the Device for Its Implementation. US Patent 1823961 A3, 18 January 1991. [Google Scholar]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250. [Google Scholar] [CrossRef] [Green Version]
- Chernonosova, V.S.; Kvon, R.I.; Stepanova, A.O.; Larichev, Y.V.; Karpenko, A.A.; Chelobanov, B.P.; Kiseleva, E.V.; Laktionov, P.P. Human serum albumin in electrospun PCL fibers: Structure, release, and exposure on fiber surface. Polym. Adv. Technol. 2017, 28, 819–827. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Manjunatha, N.; Vijayan, R.; Khatwal, R.B.; Samanta, M.K. Determination and Estimation of Pharmacokinetic Profile of Caffeine in Form of Extract of Green Tea Leaves and its Analogy with Synthetic Form. Indian J. Pharm. Sci. 2011, 73, 649–655. [Google Scholar] [PubMed] [Green Version]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 5, 122383. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional role of polyvinylpyrrolidone in pharmaceutical formulations. AAPS PharmSciTech 2021, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption: A system of classification of solution adsorption isotherms. J. Chem. Soc. 1960, 3, 3973–3993. [Google Scholar] [CrossRef]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Ann. Clin. Biochem. 2016, 53, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, T., Jr. Ligand binding by albumin. In All about Albumin; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Poon, W.; Zhang, Y.N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]






| O/C | O */C | P/C | n/C | Zr/C | |
|---|---|---|---|---|---|
| C0 | 0.0500 | 0.0500 | 0.0038 | ||
| C1 | 0.0700 | 0.0700 | 0.0040 | 0.0030 | |
| CW2 | 0.1300 | 0.1000 | 0.0030 | 0.0060 | 0.0100 |
| CP2 | 0.1300 | 0.0800 | 0.0036 | 0.0160 | 0.0150 |
| Freundlich Isotherm (Low SRL Concentrations) | |||||||
| Adsorption in PBS | Adsorption in BP | ||||||
| AC Fraction | 1 /n | KF (mg/g (L/mg)1/n) | r2 | 1 /n | KF (mg/g (L/mg)1/n) | r2 | |
| C0 | 1.67 | 374 | 0.992 | 1.33 | 6.7 | 0.992 | |
| C1 | 1.55 | 440 | 0.980 | 1.18 | 12.2 | 0.868 | |
| CW2 | 1.39 | 192 | 0.978 | 1.48 | 1.0 | 0.992 | |
| CP2 | 1.41 | 129 | 0.976 | 1.20 | 1.2 | 0.988 | |
| Langmuir Isotherm (High SRL Concentrations) | |||||||
| Adsorption in PBS | Adsorption in BP | ||||||
| AC Fraction | qmax(exp) (mg/g) | qmax(calc) (mg/g) | KL (L/g) | r2 | qmax(calc) (mg/g) | KL (L/g) | r2 |
| C0 | 1850 | 2000 | 0.0308 | 0.965 | 2000 | 0.0028 | 0.989 |
| C1 | 2840 | 2500 | 0.0506 | 0.984 | 2500 | 0.0039 | 0.996 |
| CW2 | 1780 | 1666 | 0.0275 | 0.968 | 1213 | 0.0070 | 0.954 |
| CP2 | 1390 | 1566 | 0.0295 | 0.982 | 1111 | 0.0067 | 0.981 |
| Adsorption and Release in PBS | ||||
| Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |
| C0 | 0.528 | 0.534 | 0.706 | 0.883 |
| C1 | 0.776 | 0.783 | 0.918 | 0.981 |
| CW2 | 0.674 | 0.703 | 0.815 | 0.851 |
| CP2 | 0.501 | 0.585 | 0.748 | 0.877 |
| Adsorption in PBS and RELEASE in BP | ||||
| Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |
| C0 | 0.956 | 0.960 | 0.964 | 0.919 |
| C1 | 0.979 | 0.980 | 0.957 | 0.926 |
| CW2 | 0.777 | 0.806 | 0.887 | 0.859 |
| CP2 | 0.883 | 0.899 | 0.975 | 0.996 |
| Adsorption and Release in BP | ||||
| Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |
| C0 | 0.953 | 0.497 | 0.982 | 0.928 |
| C1 | 0.857 | 0.750 | 0.957 | 0.942 |
| CW2 | 0.931 | 0.668 | 0.971 | 0.944 |
| CP2 | 0.938 | 0.539 | 0.973 | 0.942 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarkina, Z.K.; Savostyanova, T.A.; Chelobanov, B.P.; Romanova, I.V.; Simonov, P.A.; Kvon, R.I.; Karpenko, A.A.; Laktionov, P.P. Activated Carbon for Drug Delivery from Composite Biomaterials: The Effect of Grinding on Sirolimus Binding and Release. Pharmaceutics 2022, 14, 1386. https://doi.org/10.3390/pharmaceutics14071386
Nazarkina ZK, Savostyanova TA, Chelobanov BP, Romanova IV, Simonov PA, Kvon RI, Karpenko AA, Laktionov PP. Activated Carbon for Drug Delivery from Composite Biomaterials: The Effect of Grinding on Sirolimus Binding and Release. Pharmaceutics. 2022; 14(7):1386. https://doi.org/10.3390/pharmaceutics14071386
Chicago/Turabian StyleNazarkina, Zhanna K., Tatyana A. Savostyanova, Boris P. Chelobanov, Irina V. Romanova, Pavel A. Simonov, Ren I. Kvon, Andrey A. Karpenko, and Pavel P. Laktionov. 2022. "Activated Carbon for Drug Delivery from Composite Biomaterials: The Effect of Grinding on Sirolimus Binding and Release" Pharmaceutics 14, no. 7: 1386. https://doi.org/10.3390/pharmaceutics14071386






