Preparation and Evaluation of Mucus-Penetrating Inhalable Microparticles of Tiotropium Bromide Containing Sodium Glycocholate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spray-Dried Tiotropium Microparticles and Physical Mixture
2.3. Physical and Chemical Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Particle Size Distribution via Laser Diffraction (PSD)
2.3.3. Powder X-ray Powder Diffraction (PXRD)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4. In Vitro Aerosol Dispersion Performance by Andersen Cascade Impactor (ACI)
2.5. Cell Culture
2.6. Transepithelial Electrical Resistance (TEER) Measurement
2.7. Cell Surface Staining for Mucus Detection
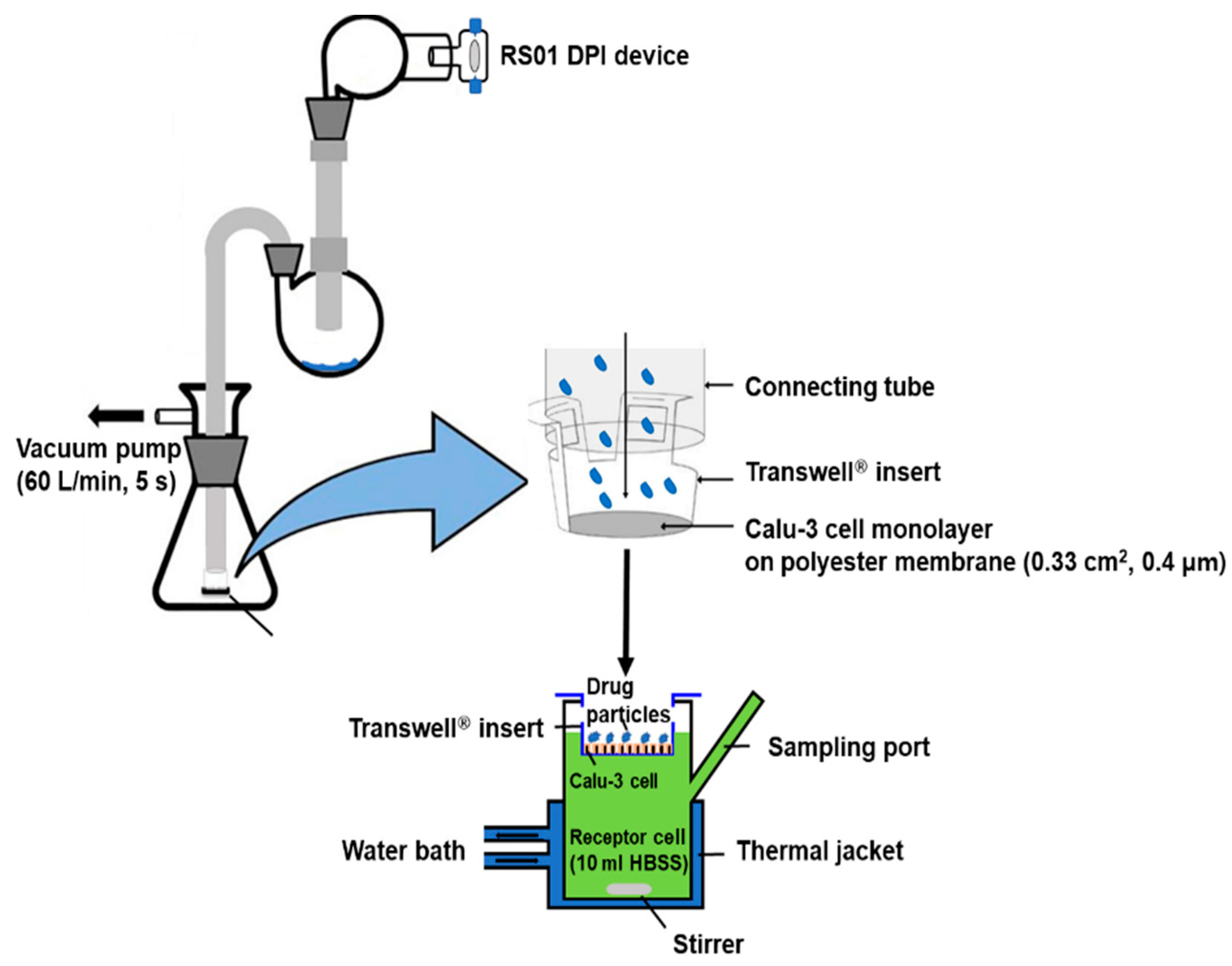
2.8. In Vitro Drug Diffusion Study Using the Calu-3 Cell
2.9. In Vivo Study Using Ovalbumin-Induced Asthma Rat Model and Treatment
2.9.1. Measurement of Bronchial Responsiveness
2.9.2. Histology of Lungs
3. Results and Discussion
3.1. Physical Characterization
3.1.1. Particle Morphology
3.1.2. PSD
3.1.3. XRD
3.1.4. DSC
3.1.5. FT-IR Spectroscopy
3.2. Andersen Cascade Impactor
3.3. TEER Measurements
3.4. Mucus Detection
3.5. In Vitro Drug Permeation Studies Using Calu-3 Cells
3.6. In Vivo Effects on Lung Function and Histological Changes in Asthmatic Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Sou, T.; Meeusen, E.N.; de Veer, M.; Morton, D.A.; Kaminskas, L.M.; McIntosh, M.P. New developments in dry powder pulmonary vaccine delivery. Trends Biotechnol. 2011, 29, 191–198. [Google Scholar] [CrossRef]
- Benita, S. Microencapsulation: Methods and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Conte, U.; Conti, B.; Giunchedi, P.; Maggi, L. Spray dried polylactide microsphere preparation: Influence of the technological parameters. Drug Dev. Ind. Pharm. 1994, 20, 235–258. [Google Scholar] [CrossRef]
- Blanco, M.; Bernardo, M.; Sastre, R.; Olmo, R.; Muniz, E.; Teijón, J. Preparation of bupivacaine-loaded poly (ε-caprolactone) microspheres by spray drying: Drug release studies and biocompatibility. Eur. J. Pharm. Biopharm. 2003, 55, 229–236. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chiang, C.-H.; Yeh, M.-K. Optimizing formulation factors in preparing chitosan microparticles by spray-drying method. J. Microencapsul. 2003, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Oneda, F.; Ré, M. The effect of formulation variables on the dissolution and physical properties of spray-dried microspheres containing organic salts. Powder Technol. 2003, 130, 377–384. [Google Scholar] [CrossRef]
- Oliveira, B.; Santana, M.; Ré, M.I. Spray-dried chitosan microspheres cross-linked with D, L-glyceraldehyde as a potential drug delivery system: Preparation and characterization. Braz. J. Chem. Eng. 2005, 22, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-W.; Li, X.; Vogt, F.G.; Hayes, D., Jr.; Zwischenberger, J.B.; Park, E.-S.; Mansour, H.M. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int. J. Pharm. 2013, 455, 374–392. [Google Scholar] [CrossRef]
- Wan, L.S.; Heng, P.W.; Chia, C.G. Spray drying as a process for microencapsulation and the effect of different coating polymers. Drug Dev. Ind. Pharm. 1992, 18, 997–1011. [Google Scholar] [CrossRef]
- Byron, P. Some future perspectives for unit dose inhalation aerosols. Drug Dev. Ind. Pharm. 1986, 12, 993–1015. [Google Scholar] [CrossRef]
- Timsina, M.; Martin, G.; Marriott, C.; Ganderton, D.; Yianneskis, M. Drug delivery to the respiratory tract using dry powder inhalers. Int. J. Pharm. 1994, 101, 1–13. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Hederer, B.; Glaab, T.; Schmidt, H.; Rutten-van Mölken, M.P.; Beeh, K.M.; Rabe, K.F.; Fabbri, L.M. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 364, 1093–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, G.A.; Jung, J.; Hanes, J.; Suk, J.S. The mucus barrier to inhaled gene therapy. Mol. Ther. 2016, 24, 2043–2053. [Google Scholar] [CrossRef] [Green Version]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.J. Pulmonary Delivery Nanomedicines Towards Circumventing Physiological Barriers: Strategies and Characterization Approaches. Adv. Drug Deliv. Rev. 2022, 2022, 114309. [Google Scholar] [CrossRef]
- Helmstädter, A. Endermatic, epidermatic, enepidermatic—The early history of penetration enhancers. Int. J. Pharm. 2011, 416, 12–15. [Google Scholar] [CrossRef]
- Murakami, T.; Sasaki, Y.; Yamajo, R.; Yata, N. Effect of bile salts on the rectal absorption of sodium ampicillin in rats. Chem. Pharm. Bull. 1984, 32, 1948–1955. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, D.; Illum, L. Absorption of peptides and proteins from the respiratory tract and the potential for development of locally administered vaccine. Crit. Rev. Ther. Drug Carr. Syst. 1990, 7, 35–97. [Google Scholar]
- Yamamoto, A.; Luo, A.; Dodda-Kashi, S.; Lee, V. The ocular route for systemic insulin delivery in the albino rabbit. J. Pharmacol. Exp. Ther. 1989, 249, 249–255. [Google Scholar]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-enhancing effects of bile salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Gebhardt, M.; Bian, S.; Kwon, K.A.; Shim, C.-K.; Chung, S.-J.; Kim, D.-D.J. Enhancing effect of surfactants on fexofenadine·HCl transport across the human nasal epithelial cell monolayer. Int. J. Pharm. 2007, 330, 23–31. [Google Scholar] [CrossRef]
- Hinchcliffe, M.; Illum, L.J. Intranasal insulin delivery and therapy. Adv. Drug Deliv. Rev. 1999, 35, 199–234. [Google Scholar] [CrossRef]
- Grainger, C.; Greenwell, L.; Martin, G.; Forbes, B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur. J. Pharm. Biopharm. 2009, 71, 318–324. [Google Scholar] [CrossRef]
- Bur, M.; Rothen-Rutishauser, B.; Huwer, H.; Lehr, C.-M. A novel cell compatible impingement system to study in vitro drug absorption from dry powder aerosol formulations. Eur. J. Pharm. Biopharm. 2009, 72, 350–357. [Google Scholar] [CrossRef]
- Hein, S.; Bur, M.; Schaefer, U.F.; Lehr, C.-M. A new Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) to evaluate pulmonary drug absorption for metered dose dry powder formulations. Eur. J. Pharm. Biopharm. 2011, 77, 132–138. [Google Scholar] [CrossRef]
- Haghi, M.; Young, P.M.; Traini, D.; Jaiswal, R.; Gong, J.; Bebawy, M. Time-and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev. Ind. Pharm. 2010, 36, 1207–1214. [Google Scholar] [CrossRef]
- Dong, F.; Wang, C.; Duan, J.; Zhang, W.; Xiang, D.; Li, M. Puerarin attenuates ovalbumin-induced lung inflammation and hemostatic unbalance in rat asthma model. Evid.-Based Complement. Altern. Med. 2014, 2014, 726740. [Google Scholar] [CrossRef] [Green Version]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Padrid, P.; Snook, S.; Finucane, T.; Shiue, P.; Cozzi, P.; Solway, J.; Leff, A.R. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am. J. Respir. Crit. Care Med. 1995, 151, 184–193. [Google Scholar] [CrossRef]
- Patel, B.; Gupta, N.; Ahsan, F.J. Aerosolized montelukast polymeric particles—an alternative to oral montelukast–alleviate symptoms of asthma in a rodent model. Pharm. Res. 2014, 31, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Ferdynand, M.S.; Nokhodchi, A.J. Co-spraying of carriers (mannitol-lactose) as a method to improve aerosolization performance of salbutamol sulfate dry powder inhaler. Drug Deliv. Transl. Res. 2020, 10, 1418–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A.J. Relationship between surface concentration of l-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.Y.; Chan, H.-K.J. Use of solid corrugated particles to enhance powder aerosol performance. Pharm. Res. 2001, 18, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miller, D.; Schmidt, C.P.J. Optimizing inventory’s contribution to profitability in a regulated utility: The Averch–Johnson effect. Int. J. Prod. Econ. 2016, 175, 132–141. [Google Scholar] [CrossRef]
- Lamy, B.; Serrano, D.R.; O’connell, P.; Couet, W.; Marchand, S.; Healy, A.M.; Tewes, F.J. Use of leucine to improve aerodynamic properties of ciprofloxacin-loaded maltose microparticles for inhalation. Eur. J. Pharm. Res. 2019, 1, 2–11. [Google Scholar] [CrossRef]
- Newman, S.; Anderson, P. Respiratory Drug Delivery: Essential Theory and Practice; Respiratory Drug Delivery Online: Richmond, VA, USA, 2009. [Google Scholar]
- Pritchard, J. The influence of lung deposition on clinical response. J. Aerosol Med. 2001, 14 (Suppl. 1), 19–26. [Google Scholar] [CrossRef]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.-H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A.J. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef]
- Cooney, D.; Kazantseva, M.; Hickey, A.J.J. Development of a size-dependent aerosol deposition model utilising human airway epithelial cells for evaluating aerosol drug delivery. Altern. Lab. Anim. 2004, 32, 581–590. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Fiegel, J.; Fuchs, S.; Abu-Dahab, R.; Schaefer, U.; Hanes, J.; Lehr, C.-M.J. Drug absorption by the respiratory mucosa: Cell culture models and particulate drug carriers. J. Aerosol Med. 2002, 15, 131–139. [Google Scholar] [CrossRef]
- Fiegel, J.; Ehrhardt, C.; Schaefer, U.F.; Lehr, C.-M.; Hanes, J.J. Large porous particle impingement on lung epithelial cell monolayers—Toward improved particle characterization in the lung. Pharm. Res. 2003, 20, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Krouse, M.; Moon, S.; Wine, J.J.J. Most basal I (SC) in Calu-3 human airway cells is bicarbonate-dependent Cl-secretion. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1997, 272, L690–L698. [Google Scholar] [CrossRef] [PubMed]
- Inglis, S.K.; Corboz, M.R.; Ballard, S.T.J. Effect of anion secretion inhibitors on mucin content of airway submucosal gland ducts. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1998, 274, L762–L766. [Google Scholar] [CrossRef]
- Hussain, A.; Arnold, J.J.; Khan, M.A.; Ahsan, F.J. Absorption enhancers in pulmonary protein delivery. J. Control. Release 2004, 94, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal penetration enhancers—How do they really work? J. Control. Release 2005, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Uehara, Y.; Iwanaga, K.; Kakemi, M.; Ohashi, Y.; Tanaka, A.; Nakai, Y.J. Influence of absorption enhancers (bile salts) and the preservative (benzalkonium chloride) on mucociliary function and permeation barrier function in rabbit tracheas. Eur. J. Pharm. Sci. 1998, 6, 225–230. [Google Scholar] [CrossRef]









| Formulation | L-Leucine | Tiotropium Bromide | Sodium Glycocholate | Content Uniformity |
|---|---|---|---|---|
| (mg) | (mg) | (mg) | (%) | |
| SDL 1 | 1100 | 400 | - | 93.7 ± 0.3 |
| SDL 2 | 1000 | 400 | 100 | 92.1 ± 4.3 |
| SDL 3 | 900 | 400 | 200 | 94.5 ± 4.4 |
| SDL 4 | 700 | 400 | 400 | 95.8 ± 1.5 |
| PM | 700 | 400 | 400 | - |
| SDL 1 | SDL 2 | SDL 3 | SDL 4 | |
|---|---|---|---|---|
| Dv (10, µm) | 2.13 | 1.04 | 0.92 | 0.91 |
| Dv (50, µm) | 5.36 | 2.68 | 2.8 | 2.55 |
| Dv (90, µm) | 10.5 | 4.39 | 4.4 | 4.4 |
| Span | 1.57 | 1.25 | 1.24 | 1.37 |
| Formulation | ED% | FPF% < 4.4 µm | MMAD | GSD |
|---|---|---|---|---|
| SDL 1 | 98.62 ± 0.68 | 59.75 ± 2.54 | 2.27 ± 0.31 | 1.41 ± 0.11 |
| SDL 2 | 96.86 ± 0.89 | 68.00 ± 6.51 | 1.90 ± 0.18 | 2.15 ± 0.60 |
| SDL 3 | 98.04 ± 0.75 | 69.12 ± 6.82 | 1.93 ± 0.05 | 3.32 ± 1.02 |
| SDL 4 | 94.81 ± 1.25 | 62.99 ± 6.87 | 1.88 ± 0.18 | 2.01 ± 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-B.; Kang, J.-H.; Kim, Y.-J.; Kim, D.-W.; Lee, S.-H.; Park, C.-W. Preparation and Evaluation of Mucus-Penetrating Inhalable Microparticles of Tiotropium Bromide Containing Sodium Glycocholate. Pharmaceutics 2022, 14, 1409. https://doi.org/10.3390/pharmaceutics14071409
Kwon Y-B, Kang J-H, Kim Y-J, Kim D-W, Lee S-H, Park C-W. Preparation and Evaluation of Mucus-Penetrating Inhalable Microparticles of Tiotropium Bromide Containing Sodium Glycocholate. Pharmaceutics. 2022; 14(7):1409. https://doi.org/10.3390/pharmaceutics14071409
Chicago/Turabian StyleKwon, Yong-Bin, Ji-Hyun Kang, Young-Jin Kim, Dong-Wook Kim, Sung-Hoon Lee, and Chun-Woong Park. 2022. "Preparation and Evaluation of Mucus-Penetrating Inhalable Microparticles of Tiotropium Bromide Containing Sodium Glycocholate" Pharmaceutics 14, no. 7: 1409. https://doi.org/10.3390/pharmaceutics14071409
APA StyleKwon, Y.-B., Kang, J.-H., Kim, Y.-J., Kim, D.-W., Lee, S.-H., & Park, C.-W. (2022). Preparation and Evaluation of Mucus-Penetrating Inhalable Microparticles of Tiotropium Bromide Containing Sodium Glycocholate. Pharmaceutics, 14(7), 1409. https://doi.org/10.3390/pharmaceutics14071409






