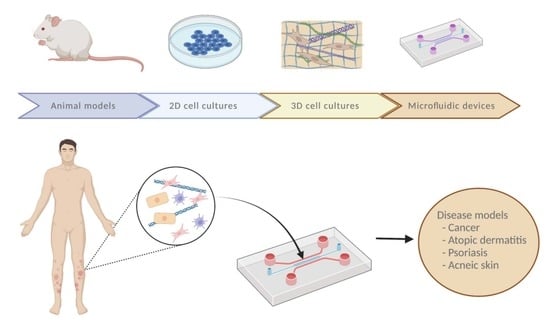
Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies
Abstract
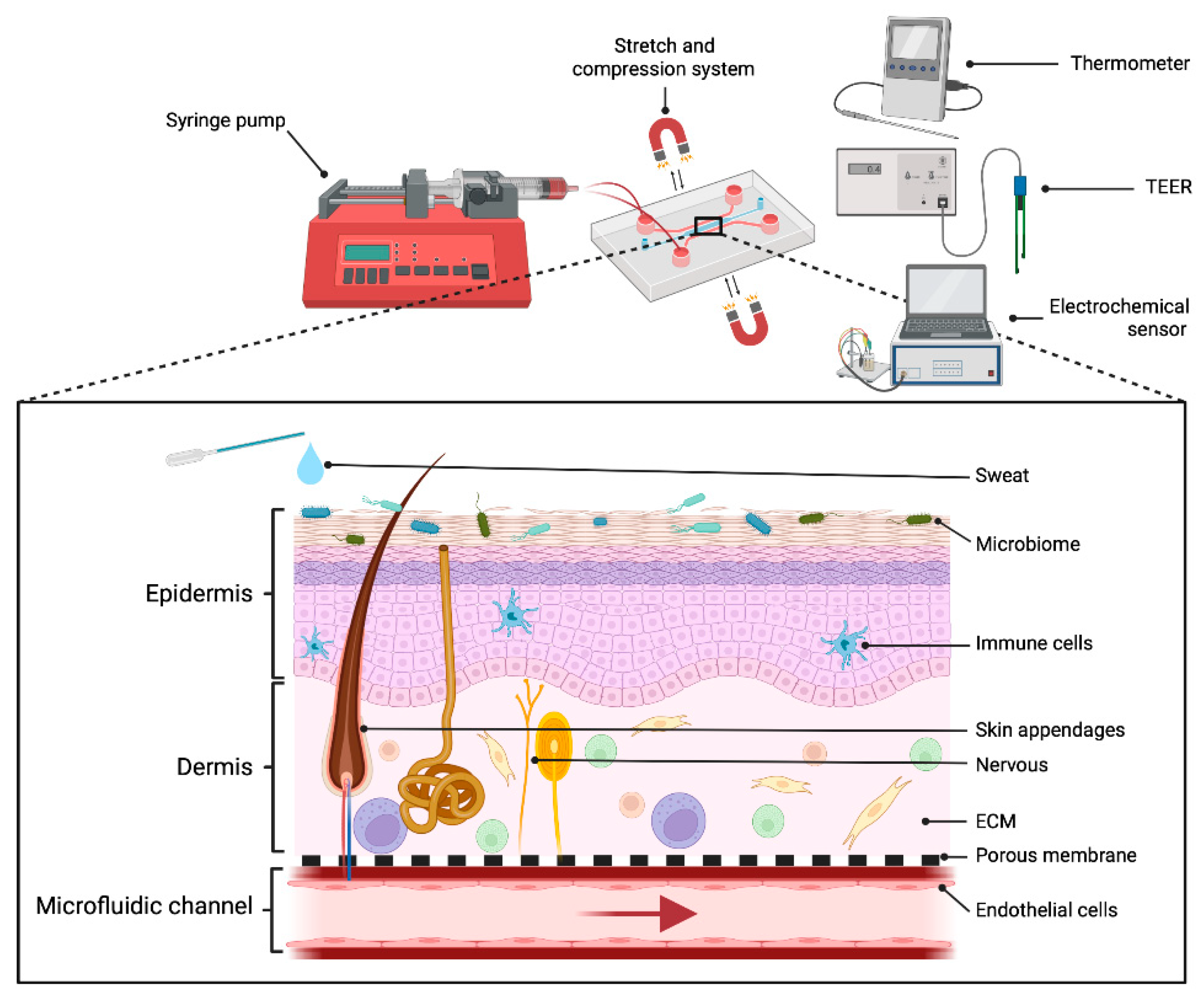
:1. Introduction
2. Conventional Skin Models Do Not Completely Reproduce Natural Skin
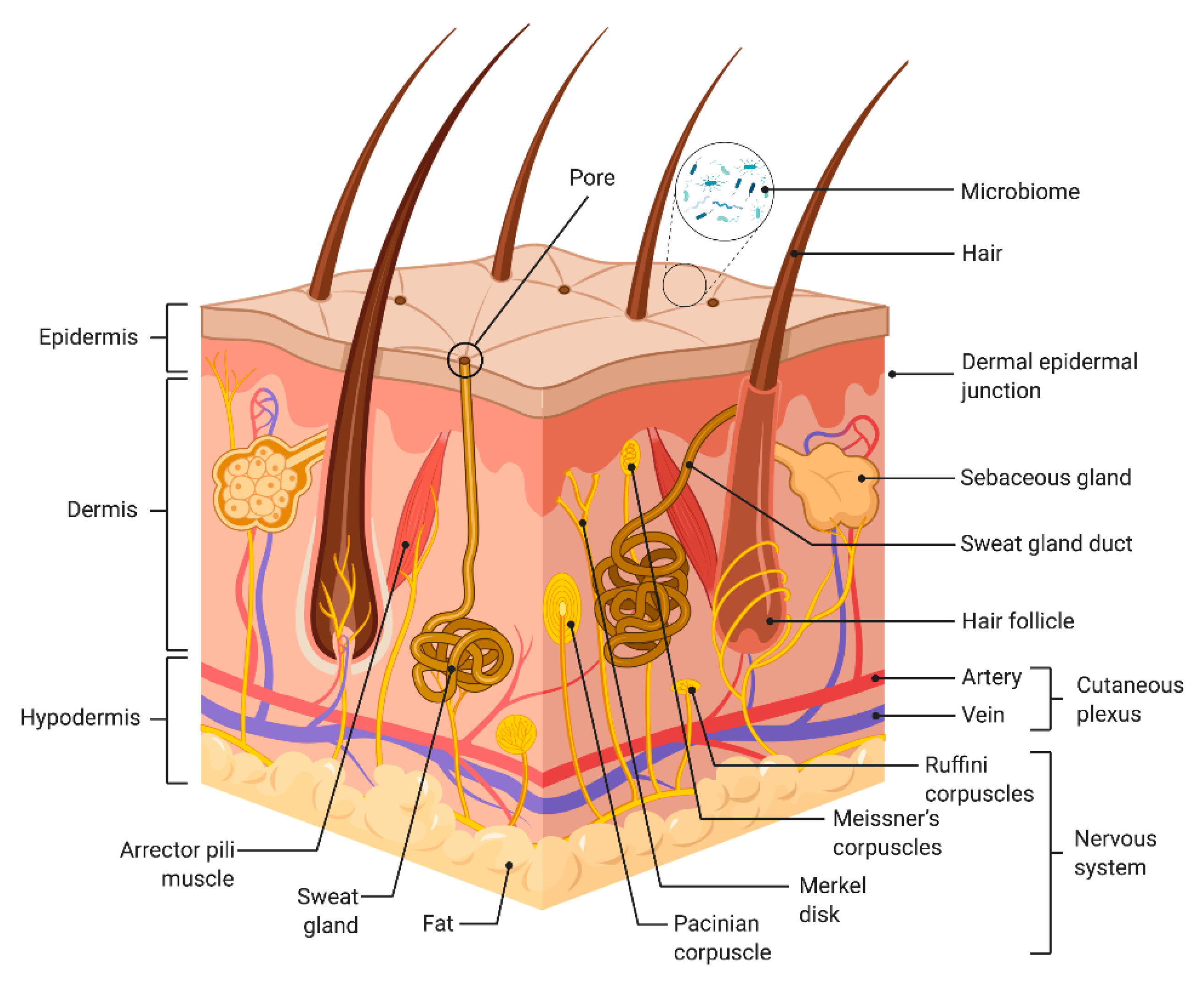
3. Biological Requirements for Skin-on-Chip (SoC) Devices
3.1. The Epidermis
3.2. The Dermal–Epidermal Junction
3.3. The Dermis
3.4. The Skin Vasculature
3.5. Immune System
3.6. Microbiome
3.7. Nerves
| Biological Requirement | Device Material | Cell Types | Main Characteristics | Refs. |
|---|---|---|---|---|
| Epidermis | 4PMMA layers | NHK | 1 µm pore size PET membrane (density of 2 × 106 pores/cm2). Differentiation of different layers of the epidermis. Correctly distributed epidermal proteins. Efficient debubble chip. Integration of perfusion and TEER. | [38] |
| Epidermis and dermis | 2 PDMS layers | HK HDF | Transwell®-cut membrane. Differentiation of different layers of the epidermis. Gravity flow helps to reduce shrinkage of the hydrogel. | [46,47] |
| Epidermis and dermis | PDMS | Human primary keratinocytes Human primary fibroblasts | Devices fabricated by soft lithography. Useful for analyzing the effects of drugs or cosmetic products. | [48] |
| Epidermis and dermis | 8 vinyl layers, PDMS layer 2PMMA layers | HaCaT HDF | PC membrane (5 µm pore size). Epidermis and dermis layer. Fibrin hydrogels with a thickness like that of human dermis, fairly homogeneous. Automatization and standardization of the hydrogel loading process. Presence of shear stress. | [49] |
| Epidermis and dermis | 2 PDMS layers | HEK HDF | Permanent magnet inserted into a cavity. Presence of mechanical forces. | [50] |
| Epidermis, dermis, and vascular layer | 2 PDMS layers separated by membrane | HaCaT HDF HUVEC HL-60 | Presence of immune system. Perfusion (10 μL/min) or gravity-driven flow. | [53] |
| Epidermis, dermis, and vascular layer | PDMS channels | HaCaT HS27 HUVEC | Fabricated using soft lithography. PET membranes (obtained from Transwell®). Model for the study of inflammation and edema. | [54] |
| Dermis, vascular layer, and immune system | PDMS | HDF HUVECs M1 and M2 macrophages | Devices fabricated by soft lithography. Three main channels: 2 laterals for 2D monolayer and inner channel for 3D coculture. Simple model to simulate early inflammation phase. Useful for analyzing the effects of drugs | [56] |
| Epidermis and dermis | PDMS | Human volunteer’s abdominoplasty | Devices fabricated by soft lithography. Human full thickness skin sample. Blood loading channel 1.5 mm (1 mm inlet/outlet channels). Crossing the endothelial membrane simulated by the filter system. Useful device for migration studies. | [57] |
| Epidermis and dermis | 2 PDMS layers | NHEK HDF | 0.4 μm porous membrane. Prevention of shrinkage of the dermal scaffold by functionalization of the surface. | [58] |
| Epidermis, nerves, and liver | PDMS | HEK hNSC hiPSC-HEP | Devices fabricated by soft lithography. Four sections for each cell type. Representation of the effect of substances at different levels of the organism. Reproducibility. | [67] |
3.8. Skin Appendages
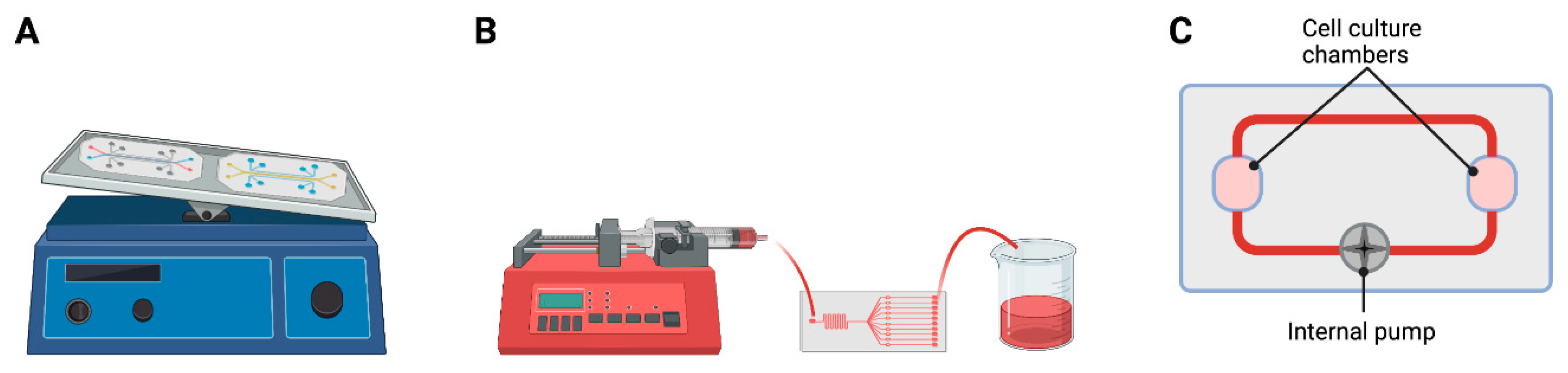
4. Mechanical Components
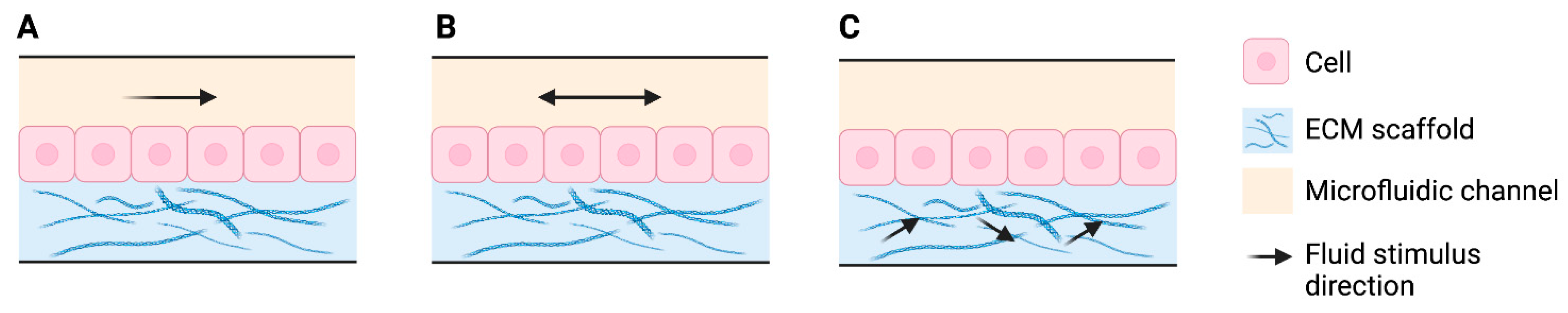
4.1. Flow and Shear Stress
4.2. Compression and Stretch
4.3. Integrated Sensors
| Mechanical Requirement | Device Material | Cell Types | Main Characteristics | Ref |
|---|---|---|---|---|
| Perfusion TEER | PMMA 1 µm pore size PET membrane | NHK | Differentiation of different layers of the epidermis. Correctly distributed epidermal proteins. Efficient debubble chip. | [38] |
| Pumpless, gravity driven | PDMS Transwell®-cut membrane | Primary human keratinocyes HDF embedded in rat tail collagen hydrogel | Differentiation of different epidermal layers. Epidermal and dermal layers. Gravity flow helps to reduce shrinkage of the hydrogel. Cell–cell and cell–matrix interactions. | [46,47] |
| Perfusion | PDMS | Human keratinocyes Human fibroblasts | Presence of mechanical forces and their effect on cell behavior. Epidermal and dermal layers. | [50] |
| Parallel flow controlled with syringe pump | PDMS PMMA Vinyl layer, PC membrane | HaCaT HDF embedded in fibrin hydrogel | Epidermal and dermal layers. Hydrogels with a thickness like that of human dermis. Automatization and standardization of the hydrogel loading process. Complementation between mathematical model and experimental model. | [49] |
| Gravity driven | PDMS PET membranes | HaCaT HS27 fibroblasts HUVECs | Epidermal, dermal, and vascular layers. Study of inflammation and edema. | [54] |
| Perfusion gravity driven | PDMS | HaCaT or primary keratinocytes HDF HUVECs HL-60 | Epidermal, dermal, and vascular layer.Immune system presence. | [53] |
| Gravity driven | PDMS porous membrane | NHEK HDF | Epidermal and dermal layers. Prevention of shrinkage of dermal scaffold by functionalization of the surface. | [58] |
| Pulsatile flow, micropump | PDMS | EpiDerm®, human skin explant, and hair follicle explant | Epidermis, dermis, and skin appendage (hair follicle). Two simultaneous microfluidic circuits. | [68] |
| Perfusion TEER | PMMA Porous membrane | N/TERT-1 HDF | Epidermal and dermal layers. Real-time monitoring. | [42] |
| Syringe pump | PDMS | NHEK | Epidermal layer. | [80] |
| Syringe pump | PDMS | HaCaT | Epidermal layer. Study of mechanotransduction. | [79] |
| Double-side perfused | PDMS, polystyrene, and membrane or scaffold | HDF HEKn | Epidermal and dermal layers. Uniform current density. Real-time assessment. Low cost. | [91] |
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- van den Broek, L.J.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.-W. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-W.; LeGrand, C.F.; Kinnear, B.F.; Sobota, R.M.; Ramalingam, R.; Dye, D.E.; Raghunath, M.; Lane, E.B.; Coombe, D.R. In Vitro Expansion of Keratinocytes on Human Dermal Fibroblast-Derived Matrix Retains Their Stem-Like Characteristics. Sci. Rep. 2019, 9, 18561. [Google Scholar] [CrossRef] [Green Version]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Aleemardani, M.; Trikić, M.Z.; Green, N.H.; Claeyssens, F. The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review. Bioengineering 2021, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Schimek, K.; Hsu, H.-H.; Boehme, M.; Kornet, J.J.; Marx, U.; Lauster, R.; Pörtner, R.; Lindner, G. Bioengineering of a Full-Thickness Skin Equivalent in a 96-Well Insert Format for Substance Permeation Studies and Organ-on-a-Chip Applications. Bioengineering 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, K.; Borgia, S.L.; Korting, H.; Mewes, K.; Schäfer-Korting, M. The Phenion® Full-Thickness Skin Model for Percutaneous Absorption Testing. Ski. Pharmacol. Physiol. 2010, 23, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asbill, C.; Kim, N.; El-Kattan, A.; Creek, K.; Wertz, P.; Michniak, B. Evaluation of a human bio-engineered skin equivalent for drug permeation studies. Pharm. Res. 2000, 17, 1092–1097. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, A. The Flaws and Human Harms of Animal Experimentation. Camb. Q. Healthc. Ethics. 2015, 24, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.R.; Sung, J.H. Effect of culture condition on cell viability and gel contraction in a skin chip. J. Ind. Eng. Chem. 2020, 87, 60–67. [Google Scholar] [CrossRef]
- Höfer, T.; Gerner, I.; Gundert-Remy, U.; Liebsch, M.; Schulte, A.; Spielmann, H.; Vogel, R.; Wettig, K. Animal testing and alternative approaches for the human health risk assessment under the proposed new European chemicals regulation. Arch. Toxicol. 2004, 78, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, B.; Bunge, A. Animal Models: A Comparison of Permeability Coefficients for Excised Skin from Humans and Animals. Dermal Absorpt. Models Toxicol. Pharmacol. 2005, 24, 305–367. [Google Scholar]
- Gattu, S.; Maibach, H. Enhanced Absorption through Damaged Skin: An Overview of the in vitro Human Model. Ski. Pharmacol. Physiol. 2010, 23, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Beißner, N.; Albero, A.B.; Füller, J.; Kellner, T.; Lauterboeck, L.; Liang, J.; Böl, M.; Glasmacher, B.; Müller-Goymann, C.C.; Reichl, S. Improved in vitro models for preclinical drug and formulation screening focusing on 2D and 3D skin and cornea constructs. Eur. J. Pharm. Biopharm. 2018, 126, 57–66. [Google Scholar] [CrossRef]
- Lee, S.; Jin, S.-P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 2017, 19, 22–36. [Google Scholar] [CrossRef]
- Ryan, C.A.; Kimber, I.; Basketter, D.A.; Pallardy, M.; Gildea, L.A.; Gerberick, G.F. Dendritic cells and skin sensitization: Biological roles and uses in hazard identification. Toxicol. Appl. Pharmacol. 2007, 221, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Van Gele, M.; Geusens, B.; Brochez, L.; Speeckaert, R.; Lambert, J. Three-dimensional skin models as tools for transdermal drug delivery: Challenges and limitations. Expert Opin. Drug Deliv. 2011, 8, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.K. Cells, tissues, and organs on chips: Challenges and opportunities for the cancer tumor microenvironment. Integr. Biol. 2013, 5, 1096–1109. [Google Scholar] [CrossRef]
- Pissarenko, A.; Meyers, M.A. The materials science of skin: Analysis, characterization, and modeling. Prog. Mater. Sci. 2020, 110, 100634. [Google Scholar] [CrossRef]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Bergfelt, D. Anatomy and Physiology of the Mare. In Equine Breeding Management and Artificial Insemination; WB Saunders: Philadelphia, PA, USA, 2009; Chapter 11; pp. 113–131. [Google Scholar]
- Chu, D.H. Fitzpatrick’s Dermatology in General Medicine; Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffell, D.J., Wolff, K., Eds.; McGraw Hill: New York, NY, USA, 2012; 8e. [Google Scholar]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Adv. Dermatol. Allergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Rheinwatd, J.G.; Green, H. Seria cultivation of strains of human epidemal keratinocytes: The formation keratinizin colonies from single cell is. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Poumay, Y.; Coquette, A. Modelling the human epidermis in vitro: Tools for basic and applied research. Arch. Dermatol. Res. 2006, 298, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Boyce, S.T.; Ham, R.G. Calcium-Regulated Differentiation of Normal Human Epidermal Keratinocytes in Chemically Defined Clonal Culture and Serum-Free Serial Culture. J. Investig. Dermatol. 1983, 81 (Suppl. S1), S33–S40. [Google Scholar] [CrossRef] [Green Version]
- Minner, F.; Herphelin, F.; Poumay, Y. Study of Epidermal Differentiation in Human Keratinocytes Cultured in Autocrine Conditions. Methods Mol. Biol. 2010, 585, 71–82. [Google Scholar]
- Sugihara, H.; Toda, S.; Miyabara, S.; Kusaba, Y.; Minami, Y. Reconstruction of the skin in three-dimensional collagen gel matrix culture. Vitr. Cell. Dev. Biol. 1991, 27, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Gazel, A.; Ramphal, P.; Rosdy, M.; De Wever, B.; Tornier, C.; Hosein, N.; Lee, B.; Tomic-Canic, M.; Blumenberg, M. Transcriptional Profiling of Epidermal Keratinocytes: Comparison of Genes Expressed in Skin, Cultured Keratinocytes, and Reconstituted Epidermis, Using Large DNA Microarrays. J. Investig. Dermatol. 2003, 121, 1459–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, F.-X.; Pedretti, N.; Rosdy, M.; Deguercy, A. Comparison of gene expression profiles in human keratinocyte mono-layer cultures, reconstituted epidermis and normal human skin; transcriptional effects of retinoid treatments in reconstituted human epidermis. Exp. Dermatol. 2002, 11, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Pellevoisin, C.; Videau, C.; Briotet, D.; Grégoire, C.; Tornier, C.; Alonso, A.; Rigaudeau, A.S.; Bouez, C.; Seyler, N. SkinEthicTM RHE for in vitro evaluation of skin irritation of medical device extracts. Toxicol. In Vitro 2018, 50, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.-Z.; Zhang, Y.-Y.; Wang, X.-C.; Ouyang, J.; Zhu, J.-F.; Yan, Y.-C.; Sun, X.-W.; Wang, F.; Li, X.-R.; et al. Construction of a high fidelity epidermis-on-a-chip for scalable In Vitro irritation evaluation. Lab Chip 2021, 21, 3804–3818. [Google Scholar] [CrossRef]
- Nguyen, A.v.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Helling, A.L.; Viswanathan, P.; Cheliotis, K.S.; Mobasseri, S.A.; Yang, Y.; El Haj, A.J.; Watt, F.M. Dynamic Culture Substrates That Mimic the Topography of the Epidermal–Dermal Junction. Tissue Eng. Part A 2019, 25, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Goletz, S.; Zillikens, D.; Schmidt, E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp. Dermatol. 2017, 26, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2017, 21, 326–340. [Google Scholar] [CrossRef]
- Malara, M.M. Engineering the Dermal-Epidermal Junction; The Ohio State University: Columbus, OH, USA, 2020. [Google Scholar]
- Zeng, Q.; Macri, L.K.; Prasad, A.; Clark, R.A.F.; Zeugolis, D.I.; Hanley, C.; Garcia, Y.; Pandit, A. 5.534-Skin Tissue Engineering. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 467–499. [Google Scholar]
- Schmidt, F.F.; Nowakowski, S.; Kluger, P.J. Improvement of a Three-Layered in vitro Skin Model for Topical Application of Irritating Substances. Front. Bioeng. Biotechnol. 2020, 8, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Sung, J.H.; Sung, G.Y. Fabrication of a pumpless, microfluidic skin chip from different collagen sources. J. Ind. Eng. Chem. 2017, 56, 375–381. [Google Scholar] [CrossRef]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Lee, T.-Y.; Sung, J.H.; Sung, G.Y. Development of 3D skin-equivalent in a pump-less microfluidic chip. J. Ind. Eng. Chem. 2018, 60, 355–359. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2020, 21, 3898. [Google Scholar] [CrossRef] [PubMed]
- Valencia, L.; Canalejas-Tejero, V.; Clemente, M.; Fernaud, I.; Holgado, M.; Jorcano, J.L.; Velasco, D. A new microfluidic method enabling the generation of multi-layered tissues-on-chips using skin cells as a proof of concept. Sci. Rep. 2021, 11, 13160. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Kim, J.; Song, H.J.; Kim, K.; Choi, K.C.; Park, S.; Sung, G.Y. Development of wrinkled skin-on-a-chip (WSOC) by cyclic uniaxial stretching. J. Ind. Eng. Chem. 2018, 68, 238–245. [Google Scholar] [CrossRef]
- Sanchez, B.; Li, L.; Dulong, J.; Aimond, G.; Lamartine, J.; Liu, G.; Sigaudo-Roussel, D. Impact of Human Dermal Microvascular Endothelial Cells on Primary Dermal Fibroblasts in Response to Inflammatory Stress. Front. Cell Dev. Biol. 2019, 7, 44–54. [Google Scholar] [CrossRef]
- Rimal, R.; Marquardt, Y.; Nevolianis, T.; Djeljadini, S.; Marquez, A.B.; Huth, S.; Chigrin, D.N.; Wessling, M.; Baron, J.M.; Möller, M.; et al. Dynamic flow enables long-term maintenance of 3-D vascularized human skin models. Appl. Mater. Today 2021, 25, 101213. [Google Scholar] [CrossRef]
- Kwak, B.S.; Jin, S.; Kim, S.J.; Kim, E.J.; Chung, J.H.; Sung, J.H. Microfluidic skin chip with vasculature for recapitulating the immune response of the skin tissue. Biotechnol. Bioeng. 2020, 117, 1853–1863. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.H.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S.H. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471–37483. [Google Scholar] [CrossRef] [Green Version]
- Akagi, T.; Nagura, M.; Hiura, A.; Kojima, H.; Akashi, M. Construction of Three-Dimensional Dermo–Epidermal Skin Equivalents Using Cell Coating Technology and Their Utilization as Alternative Skin for Permeation Studies and Skin Irritation Tests. Tissue Eng. Part A 2017, 23, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Biglari, S.; Le, T.Y.L.; Tan, R.P.; Wise, S.G.; Zambon, A.; Codolo, G.; Bernard, M.D.; Warkiani, M.; Schindeler, A.; Naficy, S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Healthc. Mater. 2019, 8, e1801307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Ellett, F.; Thomas, C.N.; Jalali, F.; Anderson, R.R.; Irimia, D.; Raff, A.B. A microscale, full-thickness, human skin on a chip assay simulating neutrophil responses to skin infection and antibiotic treatments. Lab Chip 2019, 19, 3094–3103. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-on-a-Chip. Int. J. Mol. Sci. 2022, 23, 2116. [Google Scholar] [CrossRef] [PubMed]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R.; et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 147, 955–966.e16. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, H.K. The role of skin and orogenital microbiota in protective immunity and chronic immune-mediated inflammatory disease. Front. Immunol. 2018, 8, 1955–1968. [Google Scholar] [CrossRef] [Green Version]
- Holland, D.B.; Bojar, R.A.; Jeremy, A.H.T.; Ingham, E.; Holland, K.T. Microbial colonization of an in vitro model of a tissue engineered human skin equivalent—A novel approach. FEMS Microbiol. Lett. 2008, 279, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Laclaverie, M.; Rouaud-Tinguely, P.; Grimaldi, C.; Jugé, R.; Marchand, L.; Aymard, E.; Closs, B. Development and characterization of a 3D in vitro model mimicking acneic skin. Exp. Dermatol. 2020, 30, 347–357. [Google Scholar] [CrossRef]
- Rouaud-Tinguely, P.; Boudier, D.; Marchand, L.; Barruche, V.; Bordes, S.; Coppin, H.; Roth, M.P.; Closs, B. From the morphological to the transcriptomic characterization of a compromised three-dimensional in vitro model mimicking atopic dermatitis. Br. J. Dermatol. 2015, 173, 1006–1014. [Google Scholar] [CrossRef]
- Lebonvallet, N.; Boulais, N.; le Gall, C.; Pereira, U.; Gauché, D.; Gobin, E.; Pers, J.-O.; Jeanmaire, C.; Danoux, L.; Pauly, G.; et al. Effects of the re-innervation of organotypic skin explants on the epidermis. Exp. Dermatol. 2012, 21, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Martorina, F.; Casale, C.; Urciuolo, F.; Netti, P.A.; Imparato, G. In Vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 2016, 113, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, J.; Cui, B.; Kim, S.K.; Cho, S.-A.; An, S.; Cho, S.-W. Hybrid skin chips for toxicological evaluation of chemical drugs and cosmetic compounds. Lab Chip 2021, 22, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Ataç, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A.G.; Azar, R.P.; Lindner, G. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013, 13, 3555–3561. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.; Iván, K.; Erdő, F. Development of Skin-on-a-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kwon, S. Mechanical load increase–induced changes in cytoskeletal structure and cellular barrier function in human cerebral endothelial cells. Biotechnol. Bioeng. 2018, 115, 2624–2631. [Google Scholar] [CrossRef]
- Hudon, V.; Berthod, F.; Black, A.F.; Damour, O.; Germain, L.; Auger, F.A. A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br. J. Dermatol. 2003, 148, 1094–1104. [Google Scholar] [CrossRef]
- Hendrickx, B.; Vranckx, J.J.; Luttun, A. Cell-Based Vascularization Strategies for Skin Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, P.L.; Berthod, F.; Germain, L.; Auger, F.A. In Vitro Evaluation of the Angiostatic Potential of Drugs Using an Endothelialized Tissue-Engineered Connective Tissue. J. Pharmacol. Exp. Ther. 2005, 315, 510–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samatov, T.R.; Shkurnikov, M.U.; Tonevitskaya, S.A.; Tonevitsky, A.G. Modelling the metastatic cascade by in vitro microfluidic platforms. Prog. Histochem. Cytochem. 2015, 49, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kaarj, K.; Yoon, J.-Y. Methods of Delivering Mechanical Stimuli to Organ-on-a-Chip. Micromachines 2019, 10, 700. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, T.; Narayana, G.H.; Banerjee, I. Keratinocytes are mechanoresponsive to the microflow-induced shear stress. Cytoskeleton 2019, 76, 209–218. [Google Scholar] [CrossRef]
- O’Neill, A.T.; Monteiro-Riviere, N.A.; Walker, G.M. Characterization of microfluidic human epidermal keratinocyte culture. Cytotechnology 2008, 56, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Polacheck, W.J.; Li, R.; Uzel, S.G.M.; Kamm, R.D. Microfluidic platforms for mechanobiology. Lab Chip 2013, 13, 2252–2267. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Patel, L.; Mitra, K.; Bit, A. Fibroblast Derived Skin Wound Healing Modeling on Chip under the Influence of Micro-Capillary Shear Stress. Micromachines 2022, 13, 305. [Google Scholar] [CrossRef]
- Chavoshnejad, P.; More, S.; Razavi, M.J. From surface microrelief to big wrinkles in skin: A mechanical in-silico model. Extreme Mech. Lett. 2020, 36, 100647. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Perfusable and stretchable 3D culture system for skin-equivalent. Biofabrication 2018, 11, 011001. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Gao, Y.; Shan, S.; Li, Q. EZH2 Regulates the Correlation between Skin Regeneration and the Duration of Mechanical Stretch. J. Investig. Dermatol. 2021, 141, 894–902.e9. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Takada, H.; Furuya, K.; Sokabe, M.; Ogawa, R. Role of Skin Stretch on Local Vascular Permeability in Murine and Cell Culture Models. Plast. Reconstr. Surg.-Glob. Open 2022, 10, e4084–e4094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, L.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, T.; Navaee, F.; Stradolini, F.; Renaud, P.; Carrara, S. Organs-on-chip monitoring: Sensors and other strategies. Microphysiological Syst. 2018, 2, 4689. [Google Scholar] [CrossRef]
- Alexander, F.A.; Eggert, S.; Wiest, J. Skin-on-a-chip: Transepithelial electrical resistance and extracellular acidification measurements through an automated air-liquid interface. Genes 2018, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Yeste, J.; Illa, X.; Gutiérrez, C.; Solé, M.; Guimerà, A.; Villa, R. Geometric correction factor for transepithelial electrical resistance measurements in transwell and microfluidic cell cultures. J. Phys. D Appl. Phys. 2016, 49, 375401. [Google Scholar] [CrossRef] [Green Version]
- Zoio, P.; Lopes-Ventura, S.; Oliva, A. Barrier-on-a-chip with a modular architecture and integrated sensors for real-time measurement of biological barrier function. Micromachines 2021, 12, 816. [Google Scholar] [CrossRef]
- Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-chip device for ex vivo monitoring of transdermal delivery of drugs—design, fabrication, and testing. Pharmaceutics 2019, 11, 445. [Google Scholar] [CrossRef] [Green Version]
- Dornhof, J.; Kieninger, J.; Muralidharan, H.; Maurer, J.; Urban, G.A.; Weltin, A. Oxygen and lactate monitoring in 3d breast cancer organoid culture with sensor-integrated microfluidic platform. In Proceedings of the 21st International Conference on Solid-State Sensors, Actuators and Microsystems, Transducers, Orlando, FL, USA, 20–24 June 2021; pp. 703–706. [Google Scholar]


| Represented Layers | Commercial Model | Application |
|---|---|---|
| Epidermis | SkinEthicTM (EpiSkin, L’Oréal Lyon France) | Skin irritation Skin corrosion Medical devices UV exposure DNA damage Bacterial adhesion Omics Permeability |
| Open Source Reconstructed Epidermis (OS-Rep) (Henkel, Düsseldorf, Germany) | Skin irritation Skin corrosion | |
| StratiCELL (StratiCELL, Les Isnes, Belgium) | Skin aging Barrier function Damage related to light Acute inflammation Pigmentation Pollution | |
| StrataTestV® (Stratatech, Madison, WI, USA) | Skin irritation Skin corrosion Toxicological assessments | |
| LabCyte Epi-model (LabCyte, Gamagori, Japan) | Skin irritation Skin corrosion | |
| Epidermis and dermis | Vitrolife-SkinTM (Kyoto, Japan) | Skin irritation Skin corrosion |
| Phenion® (Henkel, Düsseldorf, Germany) | Skin physiology Skin biochemistry Clinical dermatology Transdermal drug delivery studies Wound healing Toxicological assessment of chemicals Analysis of environmental effects on skin physiology | |
| EpiDerm-FTTM (Mattek, Ashland, OR, USA) | Anti-aging Wound healing Skin hydration UV protection | |
| CELLnTEC (CELLnTEC, Berne, Switzerland) | Skin irritation Skin corrosion Toxicological assessments Omics | |
| Biomimiq (Biomimiq, Leiden, the Netherlands) | Toxicological assessments Drug development Omics |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Carro, E.; Angenent, M.; Gracia-Cazaña, T.; Gilaberte, Y.; Alcaine, C.; Ciriza, J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022, 14, 1417. https://doi.org/10.3390/pharmaceutics14071417
Fernandez-Carro E, Angenent M, Gracia-Cazaña T, Gilaberte Y, Alcaine C, Ciriza J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics. 2022; 14(7):1417. https://doi.org/10.3390/pharmaceutics14071417
Chicago/Turabian StyleFernandez-Carro, Estibaliz, Maricke Angenent, Tamara Gracia-Cazaña, Yolanda Gilaberte, Clara Alcaine, and Jesús Ciriza. 2022. "Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies" Pharmaceutics 14, no. 7: 1417. https://doi.org/10.3390/pharmaceutics14071417
APA StyleFernandez-Carro, E., Angenent, M., Gracia-Cazaña, T., Gilaberte, Y., Alcaine, C., & Ciriza, J. (2022). Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics, 14(7), 1417. https://doi.org/10.3390/pharmaceutics14071417