Advanced Online Monitoring of In Vitro Human 3D Full-Thickness Skin Equivalents
Abstract
:1. Introduction
2. Materials and Methods
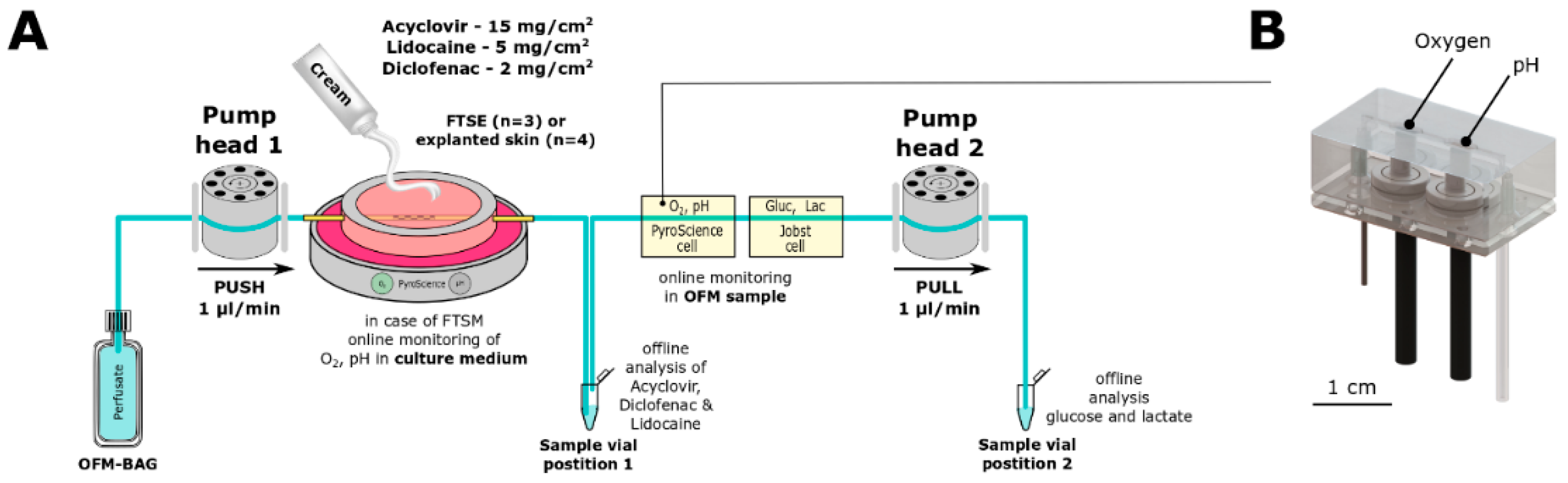
2.1. Ex Vivo Incubator
2.2. OFM Technology
2.3. Online Monitoring
2.4. Full Thickness SKIN Equivalent (FTSE)
2.5. Skin Explants
2.6. Penetration Tests
2.7. Analyses of Active Ingredients by HPLC-MS/MS
2.8. Statistical Analysis
3. Results
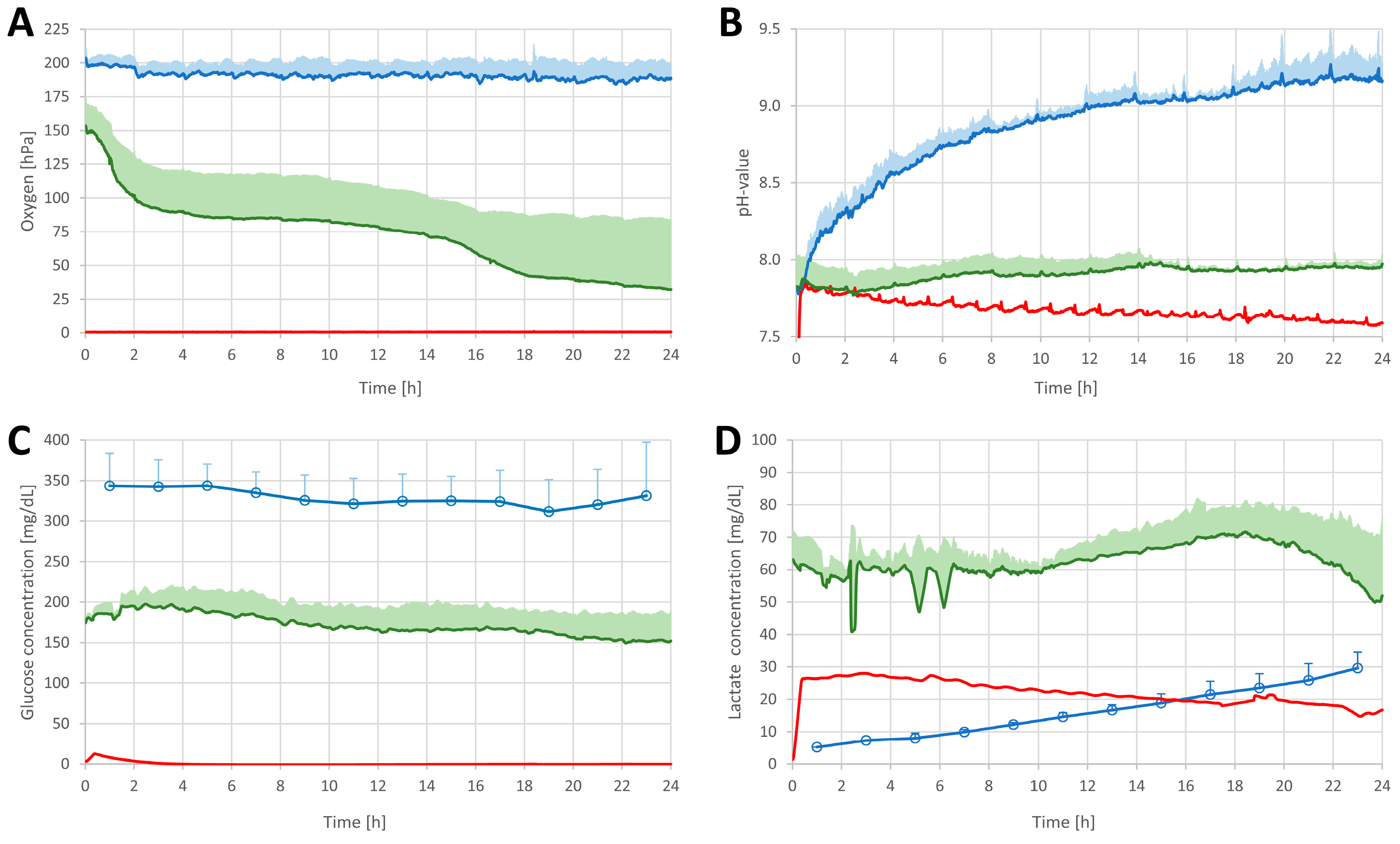
3.1. Online Monitoring of Critical Tissue Parameters
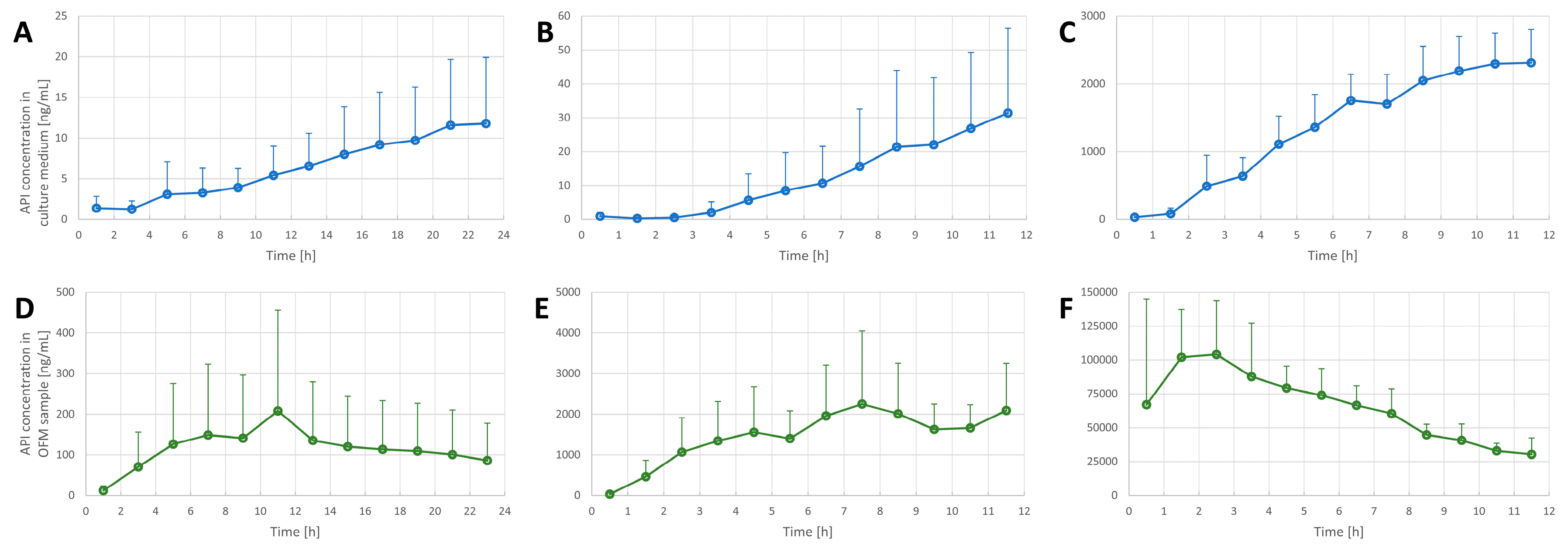
3.2. Penetration Tests in the FTSE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Cell culture based in vitro test systems for anticancer drug screening. Front. Bioeng. Biotechnol. 2020, 8, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Reuter, C.; Walles, H.; Groeber, F. Preparation of a three-dimensional full thickness skin equivalent. In 3D Cell Culture. Methods in Molecular Biology; Koledova, Z., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1612, pp. 191–198. [Google Scholar]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked collagen hydrogel matrix resisting contraction to facilitate full-thickness skin equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [Green Version]
- Schweinlin, M.; Wilhelm, S.; Schwedhelm, I.; Hansmann, J.; Rietscher, R.; Jurowich, C.; Walles, H.; Metzger, M. Development of an advanced primary human in vitro model of the small intestine. Tissue Eng. Part C Methods 2016, 22, 873–883. [Google Scholar] [CrossRef]
- Alzheimer, M.; Svensson, S.L.; König, F.; Schweinlin, M.; Metzger, M.; Walles, H.; Sharma, C.M. A three-dimensional intestinal tissue model reveals factors and small regulatory RNAs important for colonization with Campylobacter jejuni. PLoS Pathog. 2020, 16, e1008304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Lee, S.J.; Cheng, H.-J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Lodes, N.; Seidensticker, K.; Perniss, A.; Nietzer, S.; Oberwinkler, H.; May, T.; Walles, T.; Hebestreit, H.; Hackenberg, S.; Steinke, M. Investigation on ciliary functionality of different airway epithelial cell lines in three-dimensional cell culture. Tissue Eng. Part A 2020, 26, 432–440. [Google Scholar] [CrossRef]
- Weinhart, M.; Hocke, A.; Hippenstiel, S.; Kurreck, J.; Hedtrich, S. 3D organ models—Revolution in pharmacological research? Pharmacol. Res. 2019, 139, 446–451. [Google Scholar] [CrossRef]
- Hwang, D.G.; Choi, Y.-m.; Jang, J. 3D bioprinting-based vascularized tissue models mimicking tissue-specific architecture and pathophysiology for in vitro studies. Front. Bioeng. Biotechnol. 2021, 9, 426. [Google Scholar] [CrossRef]
- Karl, F.; Wußmann, M.; Kreß, L.; Malzacher, T.; Fey, P.; Groeber-Becker, F.; Üçeyler, N. Patient–derived in vitro skin models for investigation of small fiber pathology. Ann. Clin. Transl. Neurol. 2019, 6, 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.F.; Groeber-Becker, F.; Schwab, S.; Thude, S.; Goebeler, M.; Walles, H.; Hansmann, J. A standardized method based on pigmented epidermal models evaluates sensitivity against UV-irradiation. ALTEX-Altern. Anim. Exp. 2018, 35, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.-E.; Chen, C.-W.; Kiesewetter, L.; Györfi, A.-H.; Li, Y.-N.; Trinh-Minh, T.; Xu, X.; Manh, C.T.; van Kuppevelt, T.; Hansmann, J. Vascularised human skin equivalents as a novel in vitro model of skin fibrosis and platform for testing of antifibrotic drugs. Ann. Rheum. Dis. 2019, 78, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Wallstabe, J.; Bussemer, L.; Groeber-Becker, F.; Freund, L.; Alb, M.; Dragan, M.; Waaga-Gasser, A.M.; Jakubietz, R.; Kneitz, H.; Rosenwald, A. Inflammation-Induced Tissue Damage Mimicking GvHD in Human Skin Models as Test Platform for Immunotherapeutics. ALTEX 2020, 37, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.; Kruse, D.; de Mattos, I.B.; Zöphel, S.; Tiltmann, K.-K.; Reigl, A.; Khan, S.; Funk, M.; Bodenschatz, K.; Groeber-Becker, F. A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level. Biomedicines 2021, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Hilzenrat, G.; Gill, E.T.; McArthur, S.L. Imaging approaches for monitoring 3D cell and tissue culture systems. J. Biophotonics 2022, 15, e202100380. [Google Scholar] [CrossRef]
- De León, S.E.; Pupovac, A.; McArthur, S.L. Three-Dimensional (3D) cell culture monitoring: Opportunities and challenges for impedance spectroscopy. Biotechnol. Bioeng. 2020, 117, 1230–1240. [Google Scholar] [CrossRef]
- Ng, S.-F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a static Franz diffusion cell system for in vitro permeation studies. Aaps Pharmscitech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [Green Version]
- Kiesewetter, L.; Littau, L.; Walles, H.; Boccaccini, A.R.; Groeber-Becker, F. Reepithelialization in focus: Non-invasive monitoring of epidermal wound healing in vitro. Biosens. Bioelectron. 2019, 142, 111555. [Google Scholar] [CrossRef] [PubMed]
- Nischwitz, S.; de Mattos, I.B.; Hofmann, E.; Groeber-Becker, F.; Funk, M.; Mohr, G.; Branski, L.; Mautner, S.; Kamolz, L. Continuous pH monitoring in wounds using a composite indicator dressing—A feasibility study. Burns 2019, 45, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.; van den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Kreß, S.; Schaller-Ammann, R.; Feiel, J.; Wegener, J.; Priedl, J.; Dietrich, W.; Kasper, C.; Egger, D. Innovative Platform for the Advanced Online Monitoring of Three-Dimensional Cells and Tissue Cultures. Cells 2022, 11, 412. [Google Scholar] [CrossRef]
- Bodenlenz, M.; Aigner, B.; Dragatin, C.; Liebenberger, L.; Zahiragic, S.; Höfferer, C.; Birngruber, T.; Priedl, J.; Feichtner, F.; Schaupp, L. Clinical applicability of dOFM devices for dermal sampling. Ski. Res. Technol. 2013, 19, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development. Organization for Economic Cooperation and Development. Guidance document for the conduct of skin absorption studies. Env/Jm/Mono 2004, 2, 1–31. [Google Scholar]
- Kemppainen, B.W.; Reifenrath, W.G. Methods for Skin Absorption; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Clowes, H.; Scott, R.; Heylings, J. Skin absorption: Flow-through or static diffusion cells. Toxicol. Vitr. 1994, 8, 827–830. [Google Scholar] [CrossRef]
- Chang, S.; Riviere, J. Percutaneous absorption of parathion in vitro in porcine skin: Effects of dose, temperature, humidity, and perfusate composition on absorptive flux. Toxicol. Sci. 1991, 17, 494–504. [Google Scholar] [CrossRef]
- Schaefer, H.; Redelmeier, T.E. Skin Barrier; Karger Publishers: Basel, Switzerland, 1996. [Google Scholar]
- Fritsch, W.C.; Stoughton, R.B. The effect of temperature and humidity on the penetration of C14 acetylsalicylic acid in excised human skin. J. Investig. Dermatol. 1963, 41, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Altendorfer-Kroath, T.; Schimek, D.; Eberl, A.; Rauter, G.; Ratzer, M.; Raml, R.; Sinner, F.; Birngruber, T. Comparison of cerebral Open Flow Microperfusion and Microdialysis when sampling small lipophilic and small hydrophilic substances. J. Neurosci. Methods 2019, 311, 394–401. [Google Scholar] [CrossRef]
- Schaupp, L.; Ellmerer, M.; Brunner, G.; Wutte, A.; Sendlhofer, G.; Trajanoski, Z.; Skrabal, F.; Pieber, T.; Wach, P. Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, E401–E408. [Google Scholar] [CrossRef] [PubMed]
- Ellmerer, M.; Schaupp, L.; Brunner, G.A.; Sendlhofer, G.; Wutte, A.; Wach, P.; Pieber, T.R. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E352–E356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragatin, C.; Polus, F.; Bodenlenz, M.; Calonder, C.; Aigner, B.; Tiffner, K.I.; Mader, J.K.; Ratzer, M.; Woessner, R.; Pieber, T.R. Secukinumab distributes into dermal interstitial fluid of psoriasis patients as demonstrated by open flow microperfusion. Exp. Dermatol. 2016, 25, 157–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbinger, F.; Loesche, C.; Valentin, M.-A.; Jiang, X.; Cheng, Y.; Jarvis, P.; Peters, T.; Calonder, C.; Bruin, G.; Polus, F. β-Defensin 2 is a responsive biomarker of IL-17A–driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017, 139, 923–932.e928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinert, M.; Kotzbeck, P.; Altendorfer-Kroath, T.; Birngruber, T.; Tschöp, M.H.; Clemmensen, C. Time-resolved hypothalamic open flow micro-perfusion reveals normal leptin transport across the blood–brain barrier in leptin resistant mice. Mol. Metab. 2018, 13, 77–82. [Google Scholar] [CrossRef]
- Schimek, D.; Raml, R.; Francesconi, K.A.; Bodenlenz, M.; Sinner, F. Quantification of acyclovir in dermal interstitial fluid and human serum by ultra-high-performance liquid–high-resolution tandem mass spectrometry for topical bioequivalence evaluation. Biomed. Chromatogr. 2018, 32, e4194. [Google Scholar] [CrossRef]
- Mieremet, A.; Vázquez García, A.; Boiten, W.; van Dijk, R.; Gooris, G.; Bouwstra, J.A.; El Ghalbzouri, A. Human skin equivalents cultured under hypoxia display enhanced epidermal morphogenesis and lipid barrier formation. Sci. Rep. 2019, 9, 7811. [Google Scholar] [CrossRef]
- Min, S.; Ko, I.K.; Yoo, J.J. State-of-the-art strategies for the vascularization of three-dimensional engineered organs. Vasc. Spec. Int. 2019, 35, 77. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Ogiso, T.; Shiraki, T.; Okajima, K.; Tanino, T.; Iwaki, M.; Wada, T. Transfollicular drug delivery: Penetration of drugs through human scalp skin and comparison of penetration between scalp and abdominal skins in vitro. J. Drug Target. 2002, 10, 369–378. [Google Scholar] [CrossRef]
- Frum, Y.; Eccleston, G.M.; Meidan, V.M. Factors influencing hydrocortisone permeation into human hair follicles: Use of the skin sandwich system. Int. J. Pharm. 2008, 358, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Korting, M.; Bock, U.; Diembeck, W.; Düsing, H.-J.; Gamer, A.; Haltner-Ukomadu, E.; Hoffmann, C.; Kaca, M.; Kamp, H.; Kersen, S. The use of reconstructed human epidermis for skin absorption testing: Results of the validation study. Altern. Lab. Anim. 2008, 36, 161–187. [Google Scholar] [CrossRef]
- Weigel, T.; Malkmus, C.; Weigel, V.; Wußmann, M.; Berger, C.; Brennecke, J.; Groeber-Becker, F.; Hansmann, J. Fully Synthetic 3D Fibrous Scaffolds for Stromal Tissues—Replacement of Animal-Derived Scaffold Materials Demonstrated by Multilayered Skin. Adv. Mater. 2022, 34, 2106780. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.F.; Nowakowski, S.; Kluger, P.J. Improvement of a three-layered in vitro skin model for topical application of irritating substances. Front. Bioeng. Biotechnol. 2020, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Bellas, E.; Seiberg, M.; Garlick, J.; Kaplan, D.L. In vitro 3D full-thickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol. Biosci. 2012, 12, 1627–1636. [Google Scholar] [CrossRef] [Green Version]



| Active Ingredients | Registered Trade Name (Manufacturer, City, Country) | Dose | No. Application Sites Explants/FTSE | Penetration Behavior |
|---|---|---|---|---|
| Acyclovir | ACICLOVIR Creme (1A Pharma GmbH, Vienna, Austria) | 15 mg/cm2 | 4/3 | Weak |
| Diclofenac | Voltaren Arthritis Pain Gel 1% (GlaxoSmithKline, Philadelphia, USA) | 2 mg/cm2 | 4/3 | Medium |
| Lidocaine | EMLA Cream 5% (AstraZeneca GmbH, Wedel, Germany) | 5 mg/cm2 | 4/3 | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaller-Ammann, R.; Kreß, S.; Feiel, J.; Schwagerle, G.; Priedl, J.; Birngruber, T.; Kasper, C.; Egger, D. Advanced Online Monitoring of In Vitro Human 3D Full-Thickness Skin Equivalents. Pharmaceutics 2022, 14, 1436. https://doi.org/10.3390/pharmaceutics14071436
Schaller-Ammann R, Kreß S, Feiel J, Schwagerle G, Priedl J, Birngruber T, Kasper C, Egger D. Advanced Online Monitoring of In Vitro Human 3D Full-Thickness Skin Equivalents. Pharmaceutics. 2022; 14(7):1436. https://doi.org/10.3390/pharmaceutics14071436
Chicago/Turabian StyleSchaller-Ammann, Roland, Sebastian Kreß, Jürgen Feiel, Gerd Schwagerle, Joachim Priedl, Thomas Birngruber, Cornelia Kasper, and Dominik Egger. 2022. "Advanced Online Monitoring of In Vitro Human 3D Full-Thickness Skin Equivalents" Pharmaceutics 14, no. 7: 1436. https://doi.org/10.3390/pharmaceutics14071436
APA StyleSchaller-Ammann, R., Kreß, S., Feiel, J., Schwagerle, G., Priedl, J., Birngruber, T., Kasper, C., & Egger, D. (2022). Advanced Online Monitoring of In Vitro Human 3D Full-Thickness Skin Equivalents. Pharmaceutics, 14(7), 1436. https://doi.org/10.3390/pharmaceutics14071436








