Nanotopographical 3D-Printed Poly(ε-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of TPS
2.2. Characterization of Substrate Surface of TPS and BPS
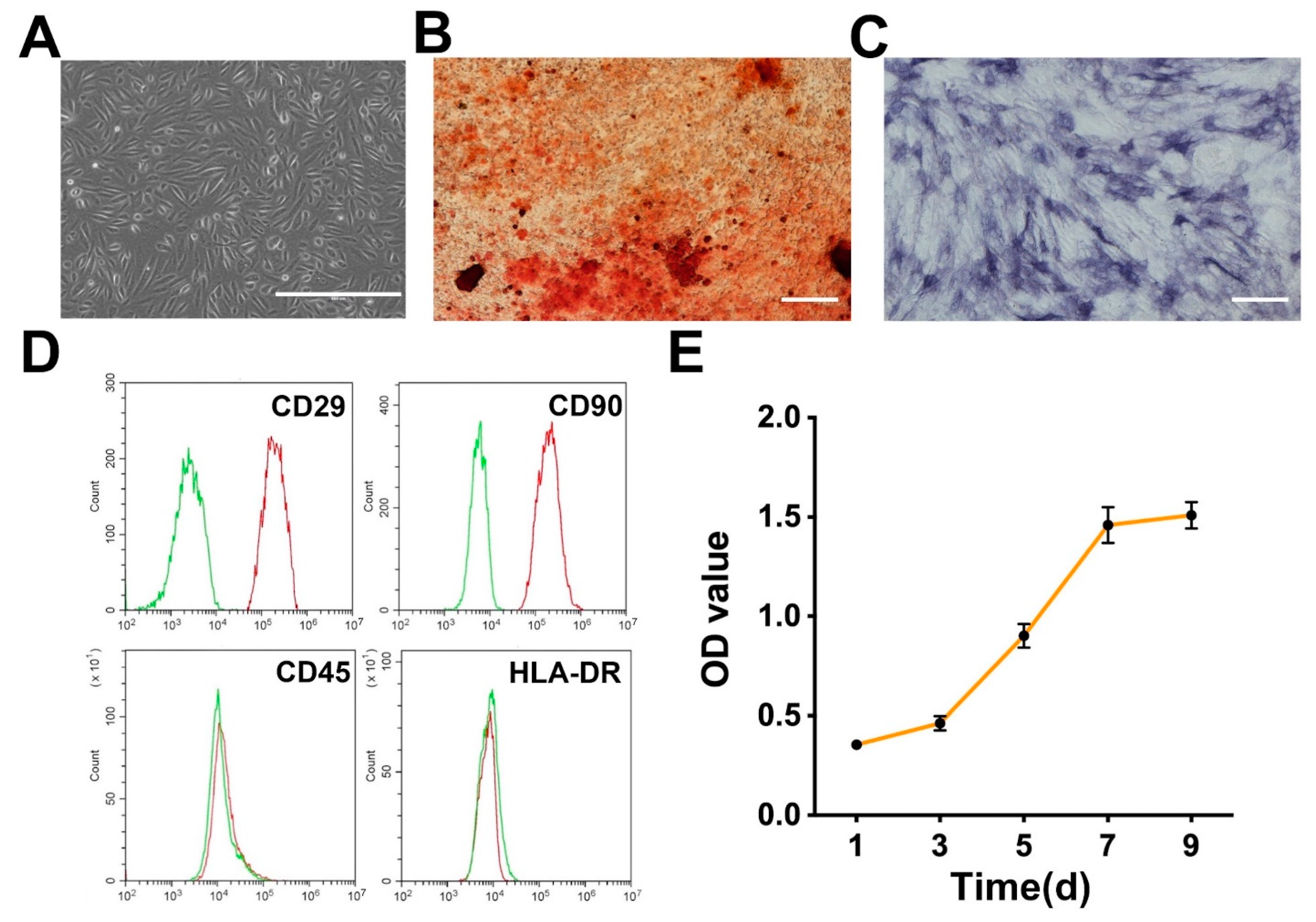
2.3. Isolation and Identification of USCs
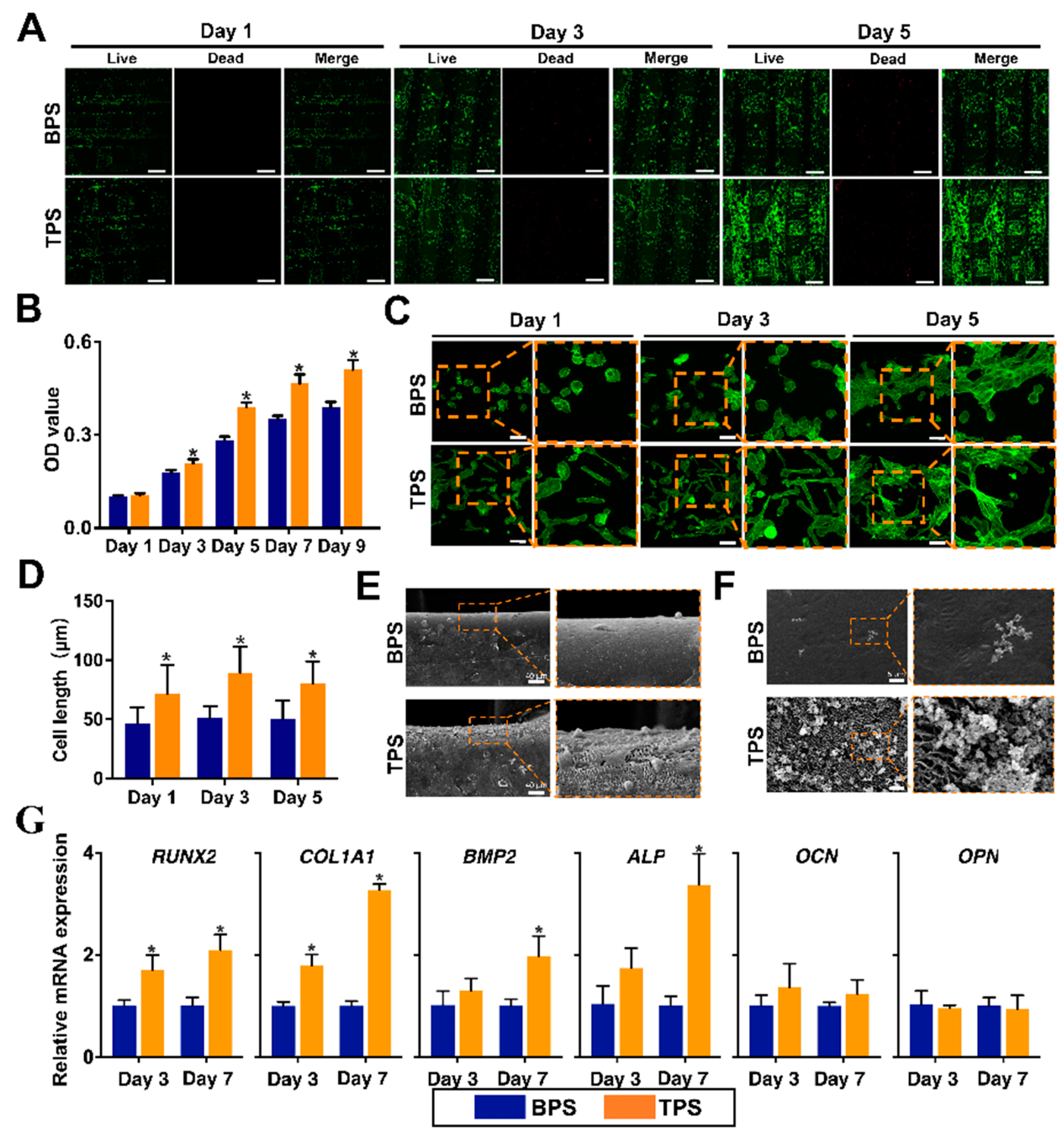
2.4. Biological Behaviors of USCs on the Surface of TPS and BPS
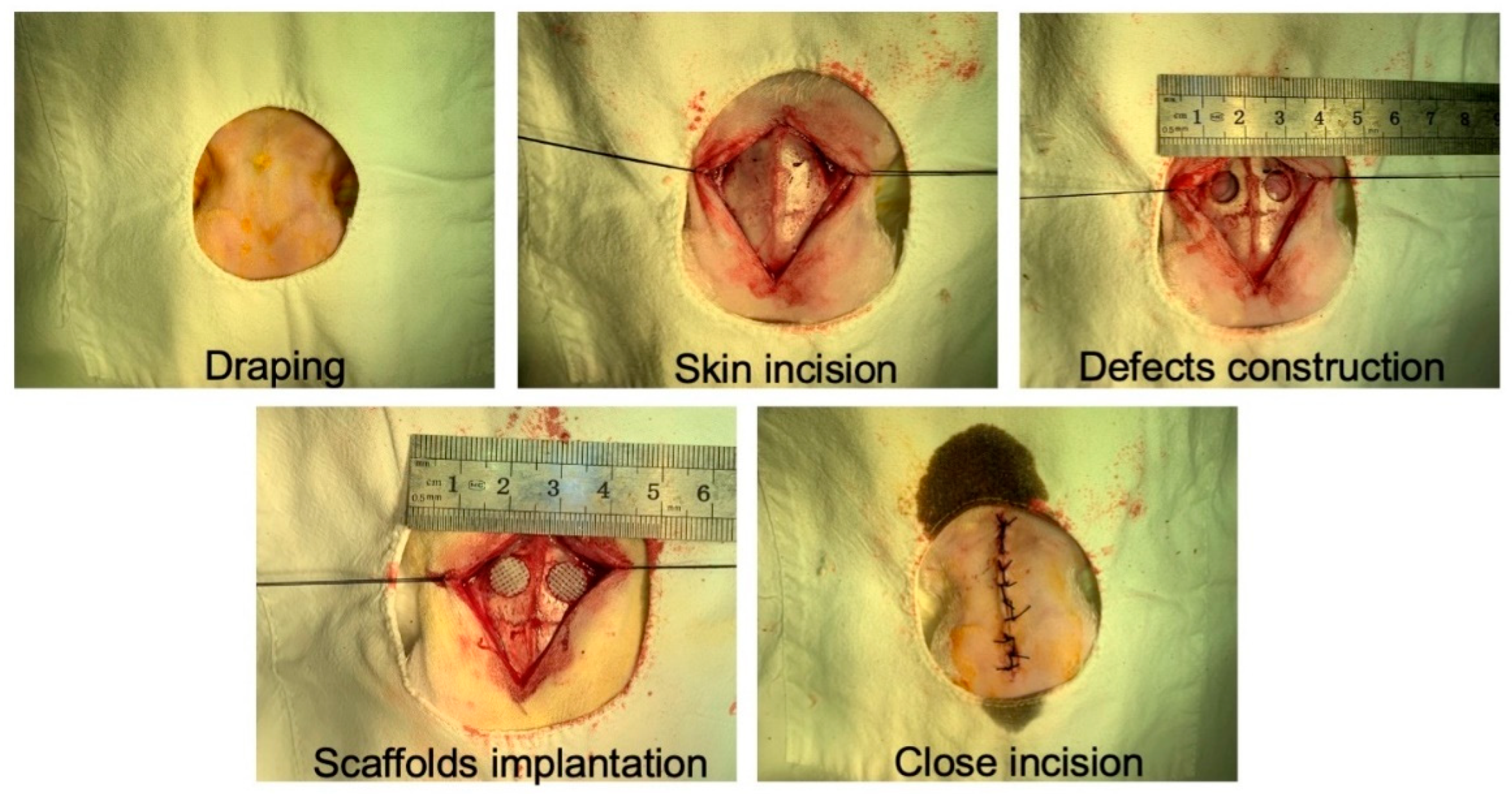
2.5. In Vivo Bone Regeneration Evaluation of TPS Loaded with USCs
2.6. Statistical Analysis
3. Results
3.1. Fabrication and Characterization of TPS
3.2. Isolation and Identification of USCs
3.3. Biological Behaviors of USCs on the Surface of TPS and BPS
3.4. Radiologic Evaluation of Scaffolds after Transplantation In Vivo
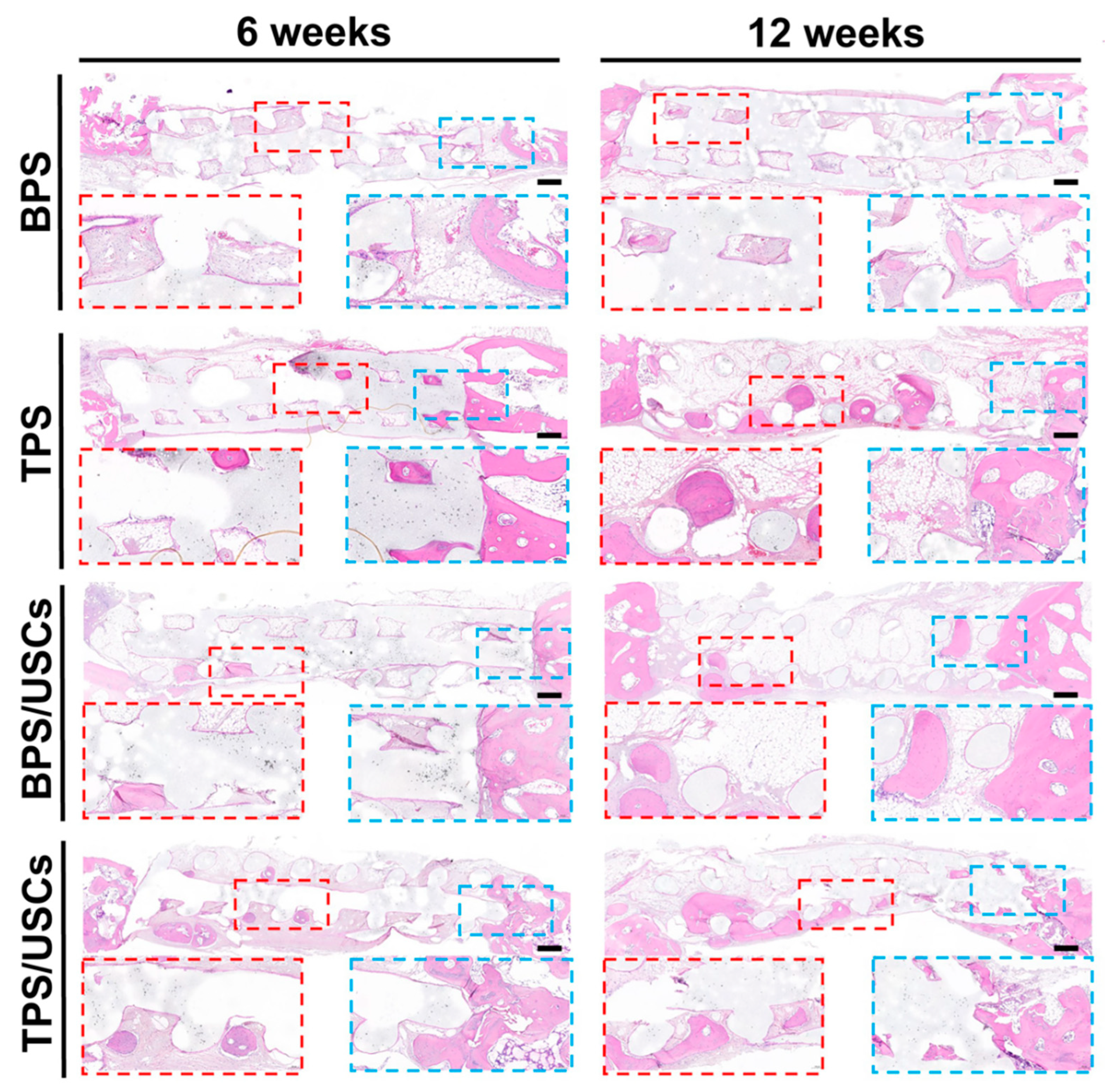
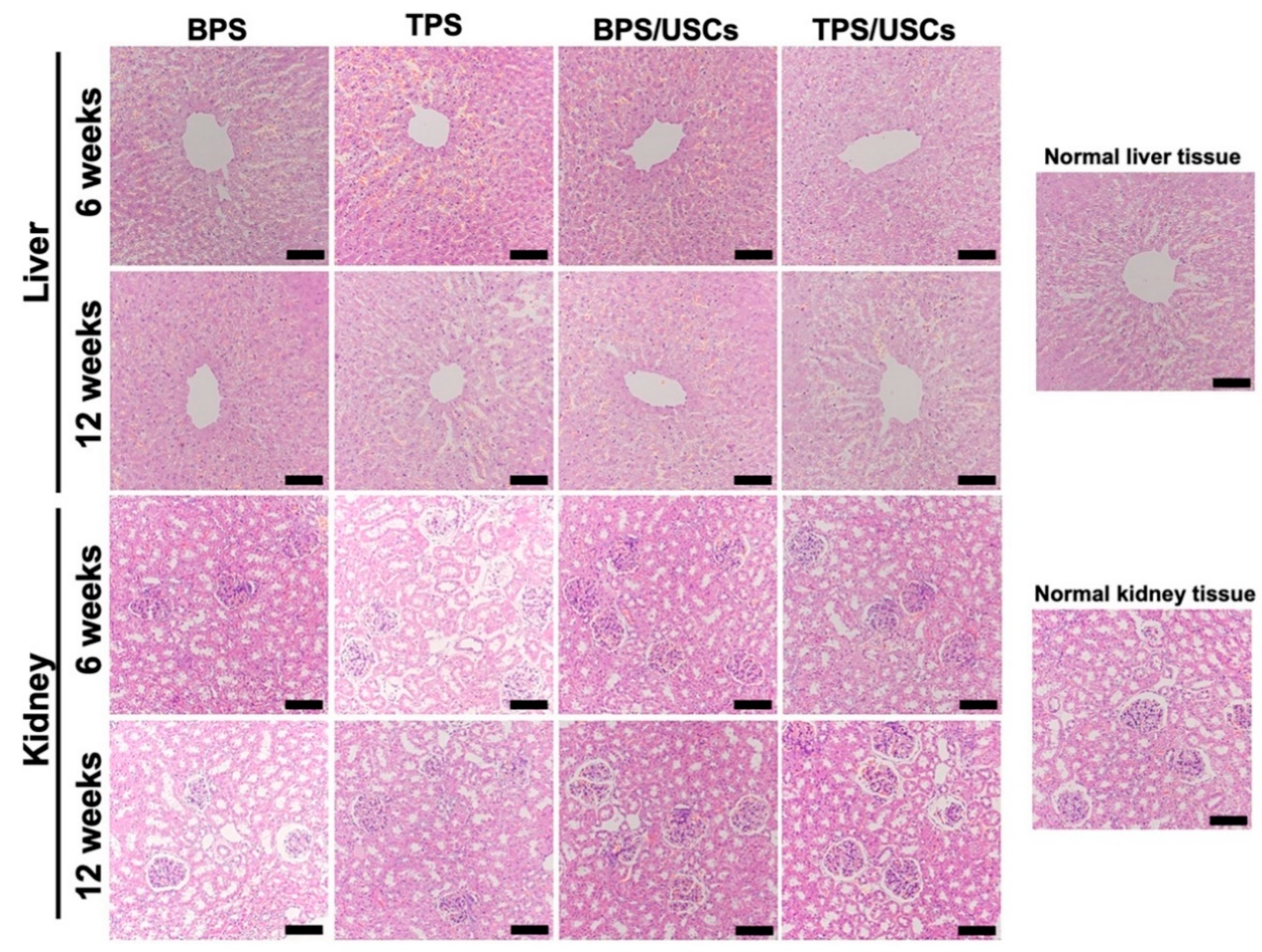
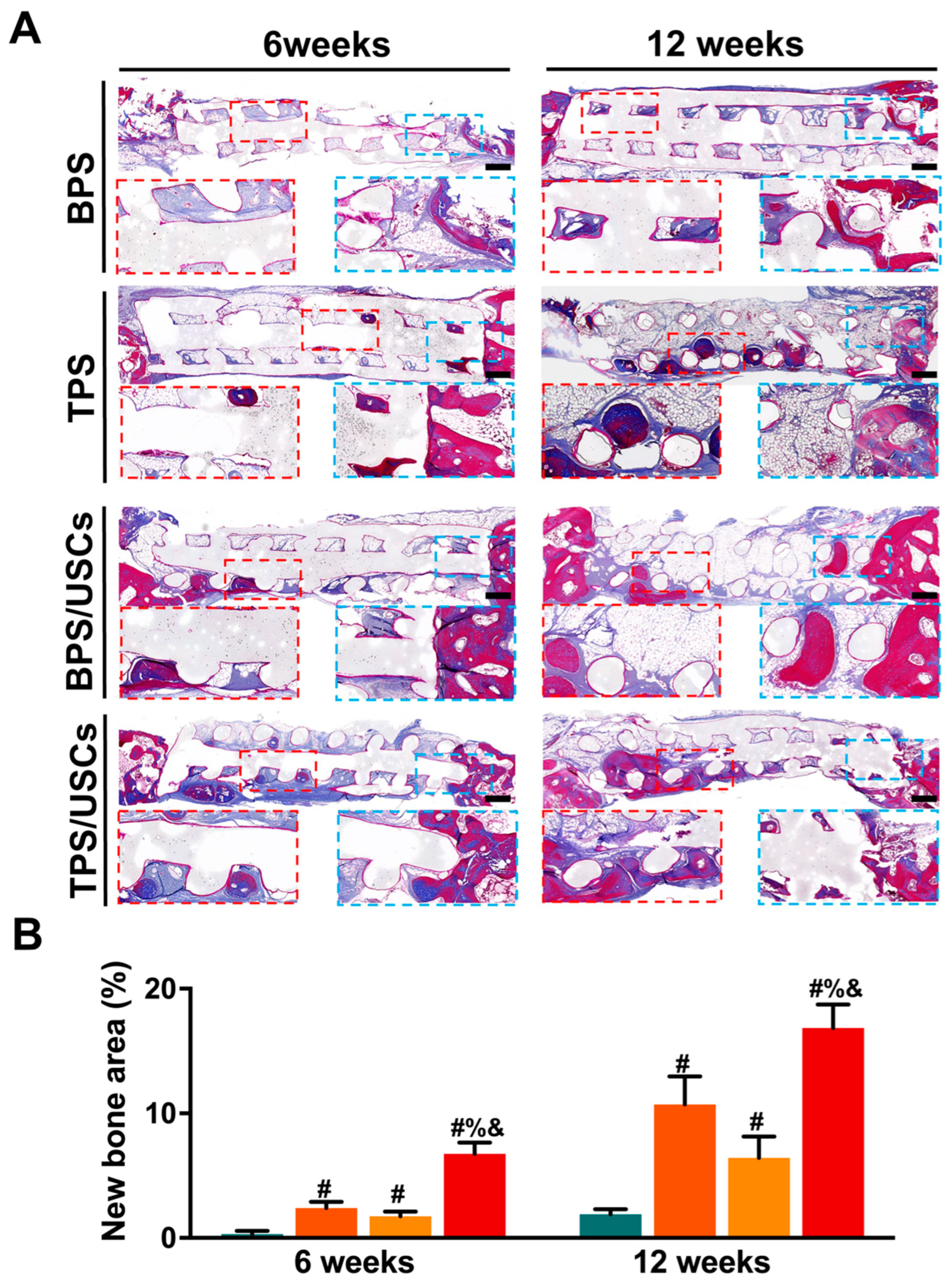
3.5. Histological Evaluation of Scaffolds after Transplantation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbeck, M.; Kühnel, L.; Witte, F.; Pissarek, J.; Precht, C.; Xiong, X.; Krastev, R.; Wegner, N.; Walther, F.; Jung, O. Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane. Int. J. Mol. Sci. 2020, 21, 3098. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. J. Bone Jt. Surg. Am. Vol. 2011, 93, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Grassi, F.R.; Grassi, R.; Vivarelli, L.; Dallari, D.; Govoni, M.; Nardi, G.M.; Kalemaj, Z.; Ballini, A. Design Techniques to Optimize the Scaffold Performance: Freeze-dried Bone Custom-made Allografts for Maxillary Alveolar Horizontal Ridge Augmentation. Materials 2020, 13, 1393. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; David, H.; Denvid, L. Nanoengineering in biomedicine: Current development and future perspectives. Nanotechnol. Rev. 2020, 9, 700–715. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N. Natural Medicinal Compounds in Bone Tissue Engineering. Trends Biotechnol. 2020, 38, 404–417. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856. [Google Scholar] [CrossRef]
- Sun, B.; Luo, X.; Yang, C.; Liu, P.; Yang, Y.; Dong, X.; Yang, Z.; Xu, J.; Zhang, Y.; Li, L. Therapeutic Effects of Human Urine-Derived Stem Cells in a Rat Model of Cisplatin-Induced Acute Kidney Injury In Vivo and In Vitro. Stem Cells Int. 2019, 2019, 8035076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liao, J.; Su, X.; Li, W.; Bi, Z.; Wang, J.; Su, Q.; Huang, H.; Wei, Y.; Gao, Y.; et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics 2020, 10, 9561–9578. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Li, L.; Sun, J.; Liu, G.; Duan, X.; Chen, J.; Liu, M.; Long, Y.; Xiang, Z. Surface mineralized biphasic calcium phosphate ceramics loaded with urine-derived stem cells are effective in bone regeneration. J. Orthop. Surg. Res. 2019, 14, 419. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Xing, F.; Yang, Y.; Gong, M.; Liu, G.; Wu, S.; Luo, R.; Duan, X.; Liu, M.; et al. Graphene oxide-modified silk fibroin/nanohydroxyapatite scaffold loaded with urine-derived stem cells for immunomodulation and bone regeneration. Stem Cell Res. Ther. 2021, 12, 591. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Gong, M.; Xing, F.; Wu, S.; Xiang, Z. Urine-derived stem cells loaded onto a chitosan-optimized biphasic calcium-phosphate scaffold for repairing large segmental bone defects in rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2014–2029. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, L.; Xing, F.; Peng, J.; Peng, K.; Wang, Y.; Xiang, Z. Human Urine-Derived Stem Cells: Potential for Cell-Based Therapy of Cartilage Defects. Stem Cells Int. 2018, 2018, 4686259. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Chen, Z.; Yu, X.; Duan, X.; Chen, J.; Liu, G.; Gong, M.; Xing, F.; Sun, J.; Huang, S.; et al. A sustained release of BMP2 in urine-derived stem cells enhances the osteogenic differentiation and the potential of bone regeneration. Regen. Biomater. 2022, 9, rbac015. [Google Scholar] [CrossRef]
- Sun, J.; Xing, F.; Zou, M.; Gong, M.; Li, L.; Xiang, Z. Comparison of chondrogenesis-related biological behaviors between human urine-derived stem cells and human bone marrow mesenchymal stem cells from the same individual. Stem Cell Res. Ther. 2021, 12, 366. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- He, L.; Si, G.; Huang, J.; Samuel, A.D.T.; Perrimon, N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 2018, 555, 103–106. [Google Scholar] [CrossRef]
- Kang, H.; Yang, B.; Zhang, K.; Pan, Q.; Yuan, W.; Li, G.; Bian, L. Immunoregulation of macrophages by dynamic ligand presentation via ligand-cation coordination. Nat. Commun. 2019, 10, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Zhang, K.; Jung, H.J.; Yang, B.; Chen, X.; Pan, Q.; Li, R.; Xu, X.; Li, G.; Dravid, V.P.; et al. An In Situ Reversible Heterodimeric Nanoswitch Controlled by Metal-Ion-Ligand Coordination Regulates the Mechanosensing and Differentiation of Stem Cells. Adv. Mater. 2018, 30, e1803591. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.; Frischauf, I.; Olbrich, M.; Peterbauer, T.; Hering, S.; Preiner, J.; Hinterdorfer, P.; Romanin, C.; Heitz, J. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials 2008, 29, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [Green Version]
- Shcherbakov, M.R.; Liu, S.; Zubyuk, V.V.; Vaskin, A.; Vabishchevich, P.P.; Keeler, G.; Pertsch, T.; Dolgova, T.V.; Staude, I.; Brener, I.; et al. Ultrafast all-optical tuning of direct-gap semiconductor metasurfaces. Nat. Commun. 2017, 8, 17. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, B.; Yang, Y.; Liu, H.; Huang, G.; Han, L.; Li, F.; Xu, F. Microchannel Stiffness and Confinement Jointly Induce the Mesenchymal-Amoeboid Transition of Cancer Cell Migration. Nano Lett. 2019, 19, 5949–5958. [Google Scholar] [CrossRef]
- Bucaro, M.A.; Vasquez, Y.; Hatton, B.D.; Aizenberg, J. Fine-Tuning the Degree of Stem Cell Polarization and Alignment on Ordered Arrays of High-Aspect-Ratio Nanopillars. ACS Nano 2012, 6, 6222–6230. [Google Scholar] [CrossRef]
- Anish, C.; Dinesh, K.M.; Sanjay, S.; Gaurav, K.S.; Umesh, G.; Gaurav, G. 3D Printing Technology: A New Milestone in the Development of Pharmaceuticals. Curr. Pharm. Des. 2019, 25, 937–945. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Lovecchio, J.; Cortesi, M.; Zani, M.; Govoni, M.; Dallari, D.; Giordano, E. Fiber Thickness and Porosity Control in a Biopolymer Scaffold 3D Printed through a Converted Commercial FDM Device. Materials 2022, 15, 2394. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-Printed PCL/rGO Conductive Scaffolds for Peripheral Nerve Injury Repair. Artif. Organs 2019, 43, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar] [CrossRef]
- Melzer, C.; Jacobs, R.; Dittmar, T.; Pich, A.; von der Ohe, J.; Yang, Y.; Hass, R. Reversible Growth-Arrest of a Spontaneously-Derived Human MSC-Like Cell Line. Int. J. Mol. Sci. 2020, 21, 4752. [Google Scholar] [CrossRef]
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef]
- Matsuguma, C.; Wakiguchi, H.; Suzuki, Y.; Okada, S.; Furuta, T.; Ohnishi, Y.; Azuma, Y.; Ohga, S.; Hasegawa, S. Dynamics of immunocyte activation during intravenous immunoglobulin treatment in Kawasaki disease. Scand. J. Rheumatol. 2019, 48, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Yin, H.-M.; Liu, W.; Huang, Y.-F.; Ren, Y.; Xu, L.; Xu, J.-Z.; Zhao, B.; Li, Z.-M. Surface Epitaxial Crystallization-Directed Nanotopography for Accelerating Preosteoblast Proliferation and Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2019, 11, 42956–42963. [Google Scholar] [CrossRef]
- Mesdaghinia, A.; Pourpak, Z.; Naddafi, K.; Nodehi, R.N.; Alizadeh, Z.; Rezaei, S.; Mohammadi, A.; Faraji, M. An in vitro method to evaluate hemolysis of human red blood cells (RBCs) treated by airborne particulate matter (PM10). MethodsX 2019, 6, 156–161. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, K.; Wang, Q.; Huang, Y.; Ji, J.; Peng, Y.; Zhang, X.; Zheng, T.; Zhang, Z.; Chong, D.; et al. The transcription factor RUNX2 fuels YAP1 signaling and gastric cancer tumorigenesis. Cancer Sci. 2021, 112, 3533–3544. [Google Scholar] [CrossRef]
- Mu, S.; Guo, S.; Wang, X.; Zhan, Y.; Li, Y.; Jiang, Y.; Zhang, R.; Zhang, B. Effects of deferoxamine on the osteogenic differentiation of human periodontal ligament cells. Mol. Med. Rep. 2017, 16, 9579–9586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.H.C. BMP-2 mediates PGE2-induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J. Orthop. Res. 2012, 30, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, W.; Dai, J.; Wang, X.; Shen, S.G. Overexpression of Dlx2 enhances osteogenic differentiation of BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and Alp. Int. J. Oral Sci. 2019, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimando, M.G.; Wu, H.-H.; Liu, Y.-A.; Lee, C.-W.; Kuo, S.-W.; Lo, Y.-P.; Tseng, K.-F.; Liu, Y.-S.; Lee, O.K.-S. Glucocorticoid receptor and Histone deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of mesenchymal stromal cells (MSCs). Sci. Rep. 2016, 6, 37371. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Zhu, L.; Ding, L.; Yuan, J.; Du, L.; Pan, M.; Xue, F.; Xiao, H. MiR-135-5p promotes osteoblast differentiation by targeting HIF1AN in MC3T3-E1 cells. Cell. Mol. Biol. Lett. 2019, 24, 51. [Google Scholar] [CrossRef] [Green Version]
- Ralis, Z.A.; Watkins, G. Modified Tetrachrome Method for Osteoid and Defectively Mineralized Bone in Paraffin Sections. Biotech. Histochem. 1992, 67, 339–345. [Google Scholar] [CrossRef]
- Saleh, R.; Toor, S.M.; Taha, R.Z.; Al-Ali, D.; Sasidharan Nair, V.; Elkord, E. DNA methylation in the promoters of PD-L1, MMP9, ARG1, galectin-9, TIM-3, VISTA and TGF-β genes in HLA-DR– myeloid cells, compared with HLA-DR+ antigen-presenting cells. Epigenetics 2020, 15, 1275–1288. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Rao, S.-S.; Tan, Y.-J.; Luo, M.-J.; Hu, X.-K.; Yin, H.; Huang, J.; Hu, Y.; Luo, Z.-W.; Liu, Z.-Z.; et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, K.-G.; Hwang, J.-H.; Cho, Y.S.; Lee, K.-S.; Jeong, H.-J.; Park, S.-H.; Park, Y.; Cho, Y.-S.; Lee, B.-K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C 2019, 98, 949–959. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Thissen, H.; Kingshott, P. Modulation of human multipotent and pluripotent stem cells using surface nanotopographies and surface-immobilised bioactive signals: A review. Acta Biomater. 2016, 45, 31–59. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical Aspects of Cell Culture Substrates: Topography, Roughness, and Elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Kingshott, P.; Thissen, H.; Pera, M.; Wang, P.-Y. Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: A review. Biotechnol. Bioeng. 2017, 114, 260–280. [Google Scholar] [CrossRef]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.C.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 Nanotube Surfaces: 15 nm—An Optimal Length Scale of Surface Topography for Cell Adhesion and Differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Liu, W.; Yin, H.-M.; Shi, A.; Sun, W.-J.; Wu, D.-W.; Huang, S.; Zhao, B.; Xu, J.-Z.; Li, Z.-M. Surface-Directed Self-Epitaxial Crystallization of Poly(ε-caprolactone) from Isotropic to Highly Orientated Lamellae. Macromolecules 2020, 53, 1736–1744. [Google Scholar] [CrossRef]
- Mei, S.; Staub, M.; Li, C.Y. Directed Nanoparticle Assembly through Polymer Crystallization. Chem.—Eur. J. 2020, 26, 349–361. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface Roughness and Substrate Stiffness Synergize To Drive Cellular Mechanoresponse. Nano Lett. 2020, 20, 748–757. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Gui, N.; Xu, W.; Myers, D.E.; Shukla, R.; Tang, H.P.; Qian, M. The effect of ordered and partially ordered surface topography on bone cell responses: A review. Biomater. Sci. 2018, 6, 250–264. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Q.; Wang, S.; Huo, B. Spreading Shape and Area Regulate the Osteogenesis of Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2019, 16, 573–583. [Google Scholar] [CrossRef]
- Speight, P.; Kofler, M.; Szászi, K.; Kapus, A. Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFβ-regulated Smad3. Nat. Commun. 2016, 7, 11642. [Google Scholar] [CrossRef] [PubMed]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Xing, F.; Xiang, Z.; Rommens, P.M.; Ritz, U. 3D Bioprinting for Vascularized Tissue-Engineered Bone Fabrication. Materials 2020, 13, 2278. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. Part B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [Green Version]


| Target Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) | Length of Amplicon |
|---|---|---|---|
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC | 91 |
| BMP2 | ACCCGCTGTCTTCTAGCGT | TTTCAGGCCGAACATGCTGAG | 180 |
| RUNX2 | CCAACCCACGAATGCACTATC | TAGTGAGTGGTGGCGGACATAC | 91 |
| ALP | ACCACCACGAGAGTGAACCA | CGTTGTCTGAGTACCAGTCCC | 79 |
| OCN | CCCCCTCTAGCCTAGGACC | ACCAGGTAATGCCAGTTTGC | 169 |
| COL1A1 | GCCCAGAAGAACTGGTACATCAG | CGCCATACTCGAACTGGAATC | 97 |
| OPN | GCCGAGGTGATAGTGTGGTT | TGAGGTGATGTCCTCGTCTG | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, F.; Yin, H.-M.; Zhe, M.; Xie, J.-C.; Duan, X.; Xu, J.-Z.; Xiang, Z.; Li, Z.-M. Nanotopographical 3D-Printed Poly(ε-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration. Pharmaceutics 2022, 14, 1437. https://doi.org/10.3390/pharmaceutics14071437
Xing F, Yin H-M, Zhe M, Xie J-C, Duan X, Xu J-Z, Xiang Z, Li Z-M. Nanotopographical 3D-Printed Poly(ε-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration. Pharmaceutics. 2022; 14(7):1437. https://doi.org/10.3390/pharmaceutics14071437
Chicago/Turabian StyleXing, Fei, Hua-Mo Yin, Man Zhe, Ji-Chang Xie, Xin Duan, Jia-Zhuang Xu, Zhou Xiang, and Zhong-Ming Li. 2022. "Nanotopographical 3D-Printed Poly(ε-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration" Pharmaceutics 14, no. 7: 1437. https://doi.org/10.3390/pharmaceutics14071437
APA StyleXing, F., Yin, H.-M., Zhe, M., Xie, J.-C., Duan, X., Xu, J.-Z., Xiang, Z., & Li, Z.-M. (2022). Nanotopographical 3D-Printed Poly(ε-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration. Pharmaceutics, 14(7), 1437. https://doi.org/10.3390/pharmaceutics14071437







