Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objectives and Protocol
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection
2.6. Data Synthesis, Risk of Bias Assessment and Critical Appraisal
2.7. Statistical Analysis and Effect Measures
3. Results
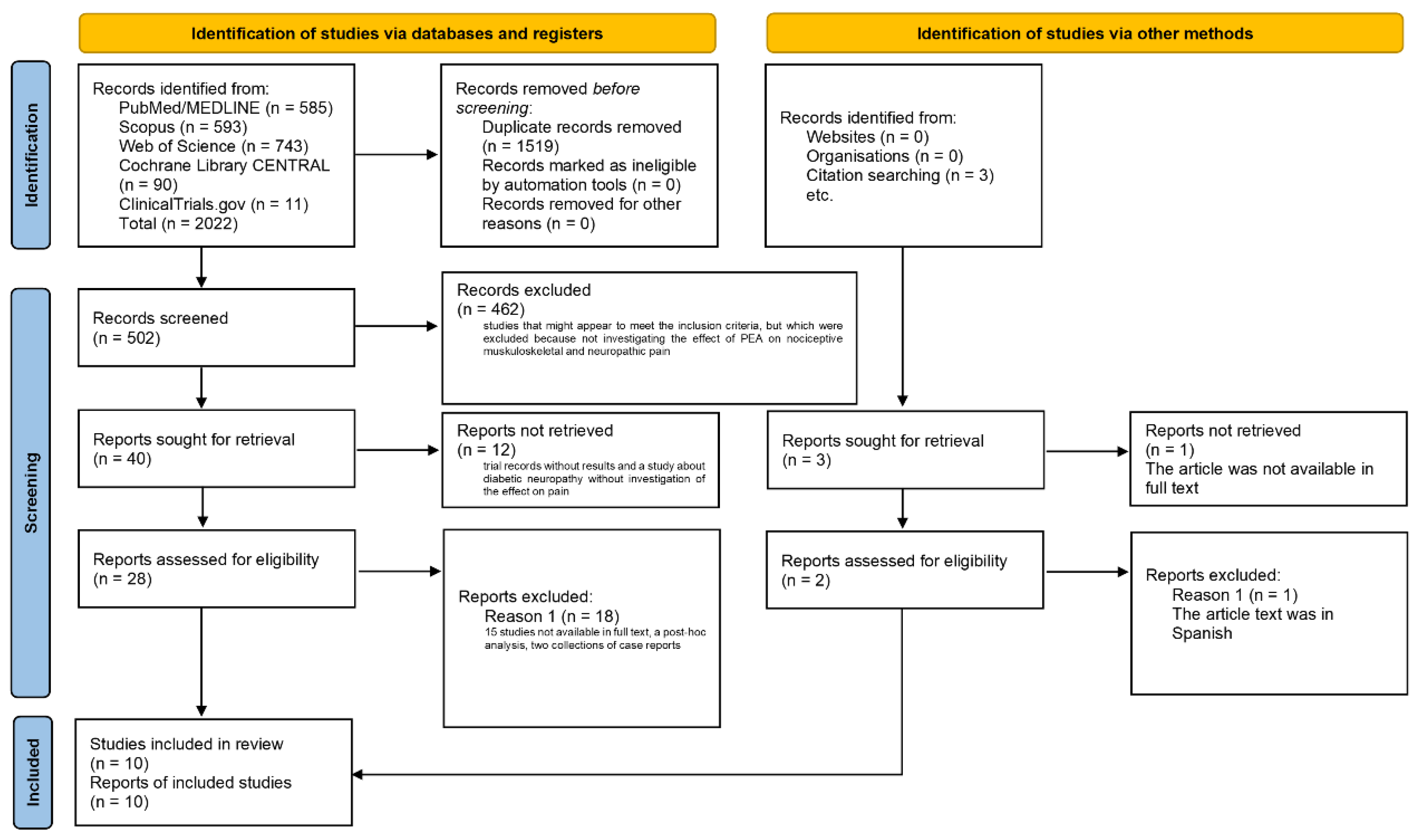
3.1. Extraction of the Studies
3.2. Synthesis of the Studies
3.3. Risk of Bias
3.4. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHFD | Cochin hand functional disability |
| DN4 | Doleur Neuropatique 4 |
| SRs | Evidence-based guideline for Peer Review of Electronic Search Strategies (PRESS) for systematic reviews |
| FAAH | Fatty acid amide hydrolase |
| GOfER | Graphical Overview for Evidence Reviews diagram |
| PROSPERO | International prospective register of systematic reviews |
| LBP-IQ | Low Back Pain Impact Questionnaire |
| MeSH | Medical and subject headings |
| MNSI | Michigan Neuropathy Screening Instrument |
| NIHR | National Institute for Health Research |
| NPQ | Neuropathic Pain Questionnaire |
| NPSI | Neuropathic pain symptom inventory |
| NRSI | Nonrandomized studies of the effects of interventions |
| NF-kB | Nuclear factor kB |
| PPAR-α | Nuclear receptor peroxisome proliferator-activated receptor-α |
| NRS | Numeric rating scale |
| ODQ | Oswestry Disability Questionnaire |
| PEA | Palmitoylethanolamide |
| PICOS | Participants, intervention, control, outcome, study design |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCTs | Randomized clinical trials |
| RoB | Risk of bias |
| ROBINS-I | Risk Of Bias In Non-randomised Studies of Interventions tool |
| TRPV1 | Transient receptor potential channel of the vanilloid type 1 |
| PEA-um | Ultramicronized PEA |
| VAS | Visual analogue scale |
| WOS | Web of Science |
References
- Schappert, S.M.; Burt, C.W. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–2002. Vital Health Stat. Ser. 13 Data Natl. Health Surv. 2006, 159, 1–66. [Google Scholar]
- Deyo, R.A.; Dworkin, S.F.; Amtmann, D.; Andersson, G.; Borenstein, D.; Carragee, E.; Carrino, J.; Chou, R.; Cook, K.; DeLitto, A.; et al. Report of the NIH Task Force on research standards for chronic low back pain. J. Pain 2014, 15, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, S.J.; Haldar, R. Pain management following spinal surgeries: An appraisal of the available options. J. Craniovertebral Junction Spine 2015, 6, 105–110. [Google Scholar] [CrossRef]
- Masri, R.; Keller, A. Chronic pain following spinal cord injury. Adv. Exp. Med. Biol. 2012, 760, 74–88. [Google Scholar] [CrossRef]
- Scuteri, D.; Mantovani, E.; Tamburin, S.; Sandrini, G.; Corasaniti, M.T.; Bagetta, G.; Tonin, P. Opioids in Post-stroke Pain: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 587050. [Google Scholar] [CrossRef]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.; Kulkarni, J.; Boulton, A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef]
- Devor, M. Rethinking the causes of pain in herpes zoster and postherpetic neuralgia: The ectopic pacemaker hypothesis. Pain Rep. 2018, 3, e702. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S. The use of the McGill Pain Questionnaire in assessing arthritis pain. Pain 1984, 19, 305–314. [Google Scholar] [CrossRef]
- Roche, P.A.; Klestov, A.C.; Heim, H.M. Description of stable pain in rheumatoid arthritis: A 6 year study. J. Rheumatol. 2003, 30, 1733–1738. [Google Scholar]
- Koop, S.M.; ten Klooster, P.M.; Vonkeman, H.E.; Steunebrink, L.M.; van de Laar, M.A. Neuropathic-like pain features and cross-sectional associations in rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C. Effect and treatment of chronic pain in inflammatory arthritis. Curr. Rheumatol. Rep. 2013, 15, 300. [Google Scholar] [CrossRef]
- Molton, I.R.; Terrill, A.L. Overview of persistent pain in older adults. Am. Psychol. 2014, 69, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.P.; Martel, M.; Houde, F.; Daguet, I.; Riesco, E.; Léonard, G. Relieving Chronic Musculoskeletal Pain in Older Adults Using Transcranial Direct Current Stimulation: Effects on Pain Intensity, Quality, and Pain-Related Outcomes. Front. Pain Res. 2022, 3, 817984. [Google Scholar] [CrossRef]
- Scuteri, D.; Vulnera, M.; Piro, B.; Bossio, R.B.; Morrone, L.A.; Sandrini, G.; Tamburin, S.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Pattern of treatment of behavioural and psychological symptoms of dementia and pain: Evidence on pharmacoutilization from a large real-world sample and from a centre for cognitive disturbances and dementia. Eur. J. Clin. Pharmacol. 2021, 77, 241–249. [Google Scholar] [CrossRef]
- Scuteri, D.; Garreffa, M.R.; Esposito, S.; Bagetta, G.; Naturale, M.D.; Corasaniti, M.T. Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in Calabria region, Italy. Neural Regen. Res. 2018, 13, 1619–1621. [Google Scholar] [CrossRef]
- Scuteri, D.; Piro, B.; Morrone, L.A.; Corasaniti, M.T.; Vulnera, M.; Bagetta, G. The need for better access to pain treatment: Learning from drug consumption trends in the USA. Funct. Neurol. 2017, 32, 229–230. [Google Scholar] [CrossRef]
- Morrone, L.A.; Scuteri, D.; Rombolà, L.; Mizoguchi, H.; Bagetta, G. Opioids Resistance in Chronic Pain Management. Curr. Neuropharmacol. 2017, 15, 444–456. [Google Scholar] [CrossRef]
- Scuteri, D.; Berliocchi, L.; Rombolà, L.; Morrone, L.A.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Aging on Formalin-Induced Pain Behavior and Analgesic Activity of Gabapentin in C57BL/6 Mice. Front. Pharmacol. 2020, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, A.J.; Hilmer, S.N.; Le Couteur, D.G. Variability in response to medicines in older people: Phenotypic and genotypic factors. Clin. Pharmacol. Ther. 2009, 85, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, A.; Jensen-Dahm, C.; Gasse, C.; Hansen, E.S.; Waldemar, G. Psychotropic Polypharmacy in Patients with Dementia: Prevalence and Predictors. J. Alzheimer’s Dis. 2017, 56, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Tadd, W. Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: Descriptive study. BMJ 2000, 321, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; De Francesco, A.E.; Esposito, S.; Zito, M.; Morrone, L.A.; Bagetta, G.; Tonin, P.; et al. Pattern of triptans use: A retrospective prescription study in Calabria, Italy. Neural Regen. Res. 2020, 15, 1340–1343. [Google Scholar] [CrossRef]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Bagetta, G. Eptinezumab for the treatment of migraine. Drugs Today 2019, 55, 695–703. [Google Scholar] [CrossRef]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Nicotera, P.; Bagetta, G. Role of CGRP pathway polymorphisms in migraine: A systematic review and impact on CGRP mAbs migraine therapy. J. Headache Pain 2021, 22, 87. [Google Scholar] [CrossRef]
- Scuteri, D.; Contrada, M.; Loria, T.; Sturino, D.; Cerasa, A.; Tonin, P.; Sandrini, G.; Tamburin, S.; Bruni, A.C.; Nicotera, P.; et al. Pain and agitation treatment in severe dementia patients: The need for Italian Mobilization-Observation-Behavior-Intensity-Dementia (I-MOBID2) pain scale translation, adaptation and validation with psychometric testing. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 113013. [Google Scholar] [CrossRef]
- Scuteri, D.; Cassano, R.; Trombino, S.; Russo, R.; Mizoguchi, H.; Watanabe, C.; Hamamura, K.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; et al. Development and Translation of NanoBEO, a Nanotechnology-Based Delivery System of Bergamot Essential Oil Deprived of Furocumarins, in the Control of Agitation in Severe Dementia. Pharmaceutics 2021, 13, 379. [Google Scholar] [CrossRef]
- Husebo, B.S.; Vislapuu, M.; Cyndecka, M.A.; Mustafa, M.; Patrascu, M. Understanding Pain and Agitation Through System Analysis Algorithms in People With Dementia. A Novel Explorative Approach by the DIGI.PAIN Study. Front. Pain Res. 2022, 3, 847578. [Google Scholar] [CrossRef]
- Forrester, L.T.; Maayan, N.; Orrell, M.; Spector, A.E.; Buchan, L.D.; Soares-Weiser, K. Aromatherapy for dementia. Cochrane Database Syst. Rev. 2014, CD003150. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Watanabe, C.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Tonin, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Role of 5-HT1A Receptor in the Anxiolytic-Relaxant Effects of Bergamot Essential Oil in Rodent. Int. J. Mol. Sci. 2020, 21, 2597. [Google Scholar] [CrossRef]
- Scuteri, D.; Hamamura, K.; Sakurada, T.; Watanabe, C.; Sakurada, S.; Morrone, L.A.; Rombolà, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Efficacy of Essential Oils in Pain: A Systematic Review and Meta-Analysis of Preclinical Evidence. Front. Pharmacol. 2021, 12, 640128. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Sandrini, G.; Tamburin, S.; Corasaniti, M.T.; Nicotera, P.; Tonin, P.; Bagetta, G. Bergamot rehabilitation AgaINst agitation in dementia (BRAINAID): Study protocol for a randomized, double-blind, placebo-controlled trial to assess the efficacy of furocoumarin-free bergamot loaded in a nanotechnology-based delivery system of the essential oil in the treatment of agitation in elderly affected by severe dementia. Phytother. Res. PTR 2021, 35, 5333–5338. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Rombolà, L.; Crudo, M.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Morrone, L.A.; Tonin, P.; et al. Translational Value of the Transdermal Administration of Bergamot Essential Oil and of Its Fractions. Pharmaceutics 2022, 14, 1006. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Rombolà, L.; Crudo, M.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Tonin, P.; Corasaniti, M.T.; et al. Preclinical Characterization of Antinociceptive Effect of Bergamot Essential Oil and of Its Fractions for Rational Translation in Complementary Therapy. Pharmaceutics 2022, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Bronzuoli, M.R.; Facchinetti, R.; Steardo, L.; Romano, A.; Stecca, C.; Passarella, S.; Steardo, L.; Cassano, T.; Scuderi, C. Palmitoylethanolamide Dampens Reactive Astrogliosis and Improves Neuronal Trophic Support in a Triple Transgenic Model of Alzheimer’s Disease: In vitro and in vivo evidence. Oxidative Med. Cell. Longev. 2018, 2018, 4720532. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Iuvone, T.; Di Marzo, V. N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities. Biochimie 2010, 92, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Calignano, A.; Rana, G.L.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.E.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R.; et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the CB2 receptor. Sci. Rep. 2017, 7, 375. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- LoVerme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The search for the palmitoylethanolamide receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Davis, J.B.; Di Marzo, V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001, 506, 253–256. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D.; et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Iannotta, M.; De Gregorio, D.; Giordano, C.; Belardo, C.; Romano, R.; Palazzo, E.; Scafuro, M.A.; Serra, N.; et al. Palmitoylethanolamide Reduces Neuropsychiatric Behaviors by Restoring Cortical Electrophysiological Activity in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Liao, J.; Macleod, M.; Segelcke, D.; Sena, C.; Thomas, J.; Vollert, J.; et al. Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain 2021, 162, S26–S44. [Google Scholar] [CrossRef] [PubMed]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar] [PubMed]
- Passavanti, M.B.; Alfieri, A.; Pace, M.C.; Pota, V.; Sansone, P.; Piccinno, G.; Barbarisi, M.; Aurilio, C.; Fiore, M. Clinical applications of palmitoylethanolamide in pain management: Protocol for a scoping review. Syst. Rev. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Littlewood, A.; Marshall, C.; Metzendorf, M.-I.; Noel-Storr, A.; Rader, T.; Shokraneh, F.; Thomas, J.; et al. Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 67–107. [Google Scholar]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Hunter, D. Qualitative Research: Consensus methods for medical and health services research. BMJ 1995, 311, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Cochrane Consumers and Communication Review Group. Cochrane Consumers and Communication Review Group: Data Synthesis and Analysis. Available online: http://cccrg.cochrane.org (accessed on 13 March 2019).
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot–Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Lazzari, M.; Gianfelice, V.; Di Paolo, A.; Sabato, E.; Sabato, A.F. Palmitoylethanolamide in the treatment of chronic pain caused by different etiopathogenesis. Pain Med. 2012, 13, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Desio, P. Associazione dell’ossicodone a lenta titolazione con Palmitoiletanolamide per il trattamento del low back pain. Anest. E Med. Crit. 2011, 1, 63–71. [Google Scholar]
- Canteri, L.; Petrosino, S.; Guida, G. Reduction in consumption of anti-inflammatory and analgesic medication in the treatment of chronic neuropathic pain in patients affected by compression lumbocischialgia due to the treatment with Normast 300 mg. DOLOR 2010, 25, 227–234. [Google Scholar]
- De Leo, V.; Cagnacci, A.; Cappelli, V.; Biasioli, A.; Leonardi, D.; Seracchioli, R. Role of a natural integrator based on lipoic acid, palmitoiletanolamide and myrrh in the treatment of chronic pelvic pain and endometriosis. Minerva Ginecol. 2019, 71, 191–195. [Google Scholar] [CrossRef]
- Giugliano, E.; Cagnazzo, E.; Soave, I.; Lo Monte, G.; Wenger, J.M.; Marci, R. The adjuvant use of N-palmitoylethanolamine and transpolydatin in the treatment of endometriotic pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Lo Monte, G.; Soave, I.; Marci, R. Administration of micronized palmitoylethanolamide (PEA)-transpolydatin in the treatment of chronic pelvic pain in women affected by endometriosis: Preliminary results. Minerva Ginecol. 2013, 65, 453–463. [Google Scholar]
- Stochino Loi, E.; Pontis, A.; Cofelice, V.; Pirarba, S.; Fais, M.F.; Daniilidis, A.; Melis, I.; Paoletti, A.M.; Angioni, S. Effect of ultramicronized-palmitoylethanolamide and co-micronized palmitoylethanolamide/polydatin on chronic pelvic pain and quality of life in endometriosis patients: An open-label pilot study. Int. J. Women’s Health 2019, 11, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, E.; Armentano, M.; Giugliano, B.; Sena, T.; Giuliano, P.; Loffredo, C.; Mastrantonio, P. Effectiveness of the Association N-Palmitoylethanolamine and Transpolydatin in the Treatment of Primary Dysmenorrhea. J. Pediatr. Adolesc. Gynecol. 2015, 28, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Iraci Sareri, M.; Casella, E.; Ventura, B.; Fava, V.; Cianci, A. Chronic pelvic pain, quality of life and sexual health of women treated with palmitoylethanolamide and α-lipoic acid. Minerva Ginecol. 2015, 67, 413–419. [Google Scholar] [PubMed]
- Cervigni, M.; Nasta, L.; Schievano, C.; Lampropoulou, N.; Ostardo, E. Micronized Palmitoylethanolamide-Polydatin Reduces the Painful Symptomatology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. BioMed Res. Int. 2019, 2019, 9828397. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.N. Experience with nutraceutical supplements in the treatment of pelvic pain in gynaecology: Case reports. Drugs Context 2022, 11. [Google Scholar] [CrossRef]
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; Trabucco, E.; De Franciscis, P.; Torella, M.; Colacurci, N. Effectiveness of the association micronized N-Palmitoylethanolamine (PEA)-transpolydatin in the treatment of chronic pelvic pain related to endometriosis after laparoscopic assessment: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Pizzigallo, D. Use of micronized palmitoylethanolamide and trans-polydatin in chronic pelvic pain associated with endometriosis. An open-label study. G. Ital. Di Ostet. E Ginecol. 2014, 36, 353–358. [Google Scholar] [CrossRef]
- Giammusso, B.; Di Mauro, R.; Bernardini, R. The efficacy of an association of palmitoylethanolamide and alpha-lipoic acid in patients with chronic prostatitis/chronic pelvic pain syndrome: A randomized clinical trial. Arch. Ital. Di Urol. Androl. Organo Uff. [Di] Soc. Ital. Di Ecogr. Urol. E Nefrol. 2017, 89, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ostardo, E.; Impellizzeri, D.; Cervigni, M.; Porru, D.; Sommariva, M.; Cordaro, M.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; et al. Adelmidrol plus sodium hyaluronate in IC/BPS or conditions associated to chronic urothelial inflammation. A translational study. Pharmacol. Res. 2018, 134, 16–30. [Google Scholar] [CrossRef]
- Bacci, C.; Cassetta, G.; Emanuele, B.; Berengo, M. Randomized split-mouth study on postoperative effects of palmitoylethanolamide for impacted lower third molar surgery. ISRN Surg. 2011, 2011, 917350. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral Investig. 2021, 25, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Rupel, K.; Gobbo, M.; Poropat, A.; Zoi, V.; Faraon, M.; Di Lenarda, R.; Biasotto, M. Efficacy of ultramicronized palmitoylethanolamide in burning mouth syndrome-affected patients: A preliminary randomized double-blind controlled trial. Clin. Oral Investig. 2019, 23, 2743–2750. [Google Scholar] [CrossRef]
- Cremon, C.; Stanghellini, V.; Barbaro, M.R.; Cogliandro, R.F.; Bellacosa, L.; Santos, J.; Vicario, M.; Pigrau, M.; Alonso Cotoner, C.; Lobo, B.; et al. Randomised clinical trial: The analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2017, 45, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.K.; Kopsky, D.J. Treatment of chronic regional pain syndrome type 1 with palmitoylethanolamide and topical ketamine cream: Modulation of nonneuronal cells. J. Pain Res. 2013, 6, 239–245. [Google Scholar] [CrossRef]
- Chirchiglia, D.; Chirchiglia, P.; Signorelli, F. Nonsurgical lumbar radiculopathies treated with ultramicronized palmitoylethanolamide (umPEA): A series of 100 cases. Neurol. I Neurochir. Pol. 2018, 52, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Chirchiglia, D.; Paventi, S.; Seminara, P.; Cione, E.; Gallelli, L. N-Palmitoyl Ethanol Amide Pharmacological Treatment in Patients With Nonsurgical Lumbar Radiculopathy. J. Clin. Pharmacol. 2018, 58, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, R.; Drago, V.; Foster, P.S.; Schievano, C.; Di Marzo, V. Use of palmitoylethanolamide in the entrapment neuropathy of the median in the wrist. Minerva Med. 2011, 102, 141–147. [Google Scholar]
- Degenhardt, B.F.; Darmani, N.A.; Johnson, J.C.; Towns, L.C.; Rhodes, D.C.; Trinh, C.; McClanahan, B.; DiMarzo, V. Role of osteopathic manipulative treatment in altering pain biomarkers: A pilot study. J. Am. Osteopath. Assoc. 2007, 107, 387–400. [Google Scholar] [PubMed]
- Domínguez, C.M.; Martín, A.D.; Ferrer, F.G.; Puertas, M.A.I.; Muro, A.L.; González, J.C.M.; Prieto, J.P.; Taberna, I.R. N-palmitoylethanolamide in the treatment of neuropathic pain associated with lumbosciatica. Pain Manag. 2012, 2, 119–124. [Google Scholar] [CrossRef]
- Evangelista, M.; Cilli, V.; De Vitis, R.; Militerno, A.; Fanfani, F. Ultra-micronized Palmitoylethanolamide Effects on Sleep-wake Rhythm and Neuropathic Pain Phenotypes in Patients with Carpal Tunnel Syndrome: An Open-label, Randomized Controlled Study. CNS Neurol. Disord. Drug Targets 2018, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Germini, F.; Coerezza, A.; Andreinetti, L.; Nobili, A.; Rossi, P.D.; Mari, D.; Guyatt, G.; Marcucci, M. N-of-1 Randomized Trials of Ultra-Micronized Palmitoylethanolamide in Older Patients with Chronic Pain. Drugs Aging 2017, 34, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Morera, C.; Sabates, S.; Jaen, A. Sex differences in N-palmitoylethanolamide effectiveness in neuropathic pain associated with lumbosciatalgia. Pain Manag. 2015, 5, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.Q.; Siepmann, D.; Gralow, I.; Ständer, S. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2010, 8, 88–91. [Google Scholar] [CrossRef]
- Schifilliti, C.; Cucinotta, L.; Fedele, V.; Ingegnosi, C.; Savoca, G.; Leotta, C. Palmitoylethanolamide reduces the symptoms of neuropathic pain in diabetic patients. Shock 2011, 36, 30. [Google Scholar] [CrossRef] [PubMed]
- Passavanti, M.B.; Fiore, M.; Sansone, P.; Aurilio, C.; Pota, V.; Barbarisi, M.; Fierro, D.; Pace, M.C. The beneficial use of ultramicronized palmitoylethanolamide as add-on therapy to Tapentadol in the treatment of low back pain: A pilot study comparing prospective and retrospective observational arms. BMC Anesthesiol. 2017, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Semprini, R.; Martorana, A.; Ragonese, M.; Motta, C. Observational clinical and nerve conduction study on effects of a nutraceutical combination on painful diabetic distal symmetric sensory-motor neuropathy in patients with diabetes type 1 and type 2. Minerva Med. 2018, 109, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Steels, E.; Venkatesh, R.; Steels, E.; Vitetta, G.; Vitetta, L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology 2019, 27, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Biasiotta, A.; Di Stefano, G.; La Cesa, S.; Leone, C.; Cartoni, C.; Federico, V.; Petrucci, M.T.; Cruccu, G. Palmitoylethanolamide restores myelinated-fibre function in patients with chemotherapy-induced painful neuropathy. CNS Neurol. Disord. Drug Targets 2011, 10, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Pieralice, S.; Vari, R.; Minutolo, A.; Maurizi, A.R.; Fioriti, E.; Napoli, N.; Pozzilli, P.; Manfrini, S.; Maddaloni, E. Biomarkers of response to alpha-lipoic acid +/− palmitoiletanolamide treatment in patients with diabetes and symptoms of peripheral neuropathy. Endocrine 2019, 66, 178–184. [Google Scholar] [CrossRef]
- Cruccu, G.; Stefano, G.D.; Marchettini, P.; Truini, A. Micronized Palmitoylethanolamide: A Post Hoc Analysis of a Controlled Study in Patients with Low Back Pain—Sciatica. CNS Neurol. Disord. Drug Targets 2019, 18, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M. Chronic idiopathic axonal neuropathy and pain, treated with the endogenous lipid mediator palmitoylethanolamide: A case collection. Int. Med. Case Rep. J. 2013, 6, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.; Hekker, T.A. Therapeutic utility of palmitoylethanolamide in the treatment of neuropathic pain associated with various pathological conditions: A case series. J. Pain Res. 2012, 5, 437–442. [Google Scholar] [CrossRef]
- Andresen, S.R.; Bing, J.; Hansen, R.M.; Biering-Sorensen, F.; Johannesen, I.L.; Hagen, E.M.; Rice, A.S.C.; Nielsen, J.F.; Bach, F.W.; Finnerup, N.B. Ultramicronized palmitoylethanolamide in spinal cord injury neuropathic pain: A randomized, double-blind, placebo-controlled trial. Pain 2016, 157, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Faig-Marti, J.; Martinez-Catassus, A. Use of palmitoylethanolamide in carpal tunnel syndrome: A prospective randomized study. J. Orthop. Traumatol. 2017, 18, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Lauritano, D.; Ottaviani, G.M.; Fontana, A.; Zambello, A.; Della Gatta, L.; Muto, M.; Carinci, F. Oxygen-Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients. Int. J. Environ. Res. Public Health 2022, 19, 5716. [Google Scholar] [CrossRef]
- Cocito, D.; Peci, E.; Ciaramitaro, P.; Cocito, C.; Merola, A.; Lopiano, L. Short-term efficacy of palmitoylethanolamide in peripheral neuropathic pain. J. Peripher. Nerv. Syst. 2014, 19, S9–S10. [Google Scholar] [CrossRef] [PubMed]
- Paladini, A.; Varrassi, G.; Bentivegna, G.; Carletti, S.; Piroli, A.; Coaccioli, S. Palmitoylethanolamide in the Treatment of Failed Back Surgery Syndrome. Pain Res. Treat. 2017, 2017, 1486010. [Google Scholar] [CrossRef] [PubMed]
- Scaturro, D.; Asaro, C.; Lauricella, L.; Tomasello, S.; Varrassi, G.; Letizia Mauro, G. Combination of Rehabilitative Therapy with Ultramicronized Palmitoylethanolamide for Chronic Low Back Pain: An Observational Study. Pain Ther. 2020, 9, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Schifilliti, C.; Cucinotta, L.; Fedele, V.; Ingegnosi, C.; Luca, S.; Leotta, C. Micronized palmitoylethanolamide reduces the symptoms of neuropathic pain in diabetic patients. Pain Res. Treat. 2014, 2014, 849623. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Ditto, M.C.; Borrelli, R.; Fusaro, E. Efficacy of a fixed combination of palmitoylethanolamide and acetyl-l-carnitine (PEA plus ALC FC) in the treatment of neuropathies secondary to rheumatic diseases. Minerva Med. 2021, 112, 492–499. [Google Scholar] [CrossRef]
- Sievert, K.; Hussain, S.M.; Page, M.J.; Wang, Y.; Hughes, H.J.; Malek, M.; Cicuttini, F.M. Effect of breakfast on weight and energy intake: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 364, l42. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, D. Of apples and oranges, file drawers and garbage: Why validity issues in meta-analysis will not go away. Clin. Psychol. Rev. 1997, 17, 881–901. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolà, L.; Watanabe, C.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Tonin, P.; Russo, R.; Nucci, C.; Morrone, L.A. Impact of nutraceuticals on glaucoma: A systematic review. Prog. Brain Res. 2020, 257, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Esposito, E.; Mazzon, E.; Di Paola, R.; Meli, R.; Bramanti, P.; Piomelli, D.; Calignano, A.; Cuzzocrea, S. Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J. Pharmacol. Exp. Ther. 2008, 326, 12–23. [Google Scholar] [CrossRef]
- Romero, T.R.; Duarte, I.D. N-palmitoyl-ethanolamine (PEA) induces peripheral antinociceptive effect by ATP-sensitive K+-channel activation. J. Pharmacol. Sci. 2012, 118, 156–160. [Google Scholar] [CrossRef]
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996, 300, 227–236. [Google Scholar] [CrossRef]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Mattace Raso, G.; Cuzzocrea, S.; Loverme, J.; Piomelli, D.; et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur. J. Pharmacol. 2009, 613, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; De Martino, M.; De Fabiani, A.; Cantieri, L.; Alexandre, A.; Vassallo, G.; Rogai, M.; Lanaia, F.; Petrosino, S. La palmitoiletanolamida (Normast®) en el dolor neuropático crónico por lumbociatalgia de tipo compresivo: Estudio clínico multicéntrico. Dolor. Investig. Clínica Ter. 2010, 25, 35–42. [Google Scholar]
- Franceschi, M.; Scarcelli, C.; Niro, V.; Seripa, D.; Pazienza, A.M.; Pepe, G.; Colusso, A.M.; Pacilli, L.; Pilotto, A. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: A prospective study of 1756 patients. Drug Saf. 2008, 31, 545–556. [Google Scholar] [CrossRef]
- Riedl, L.; Kiesel, E.; Hartmann, J.; Fischer, J.; Roßmeier, C.; Haller, B.; Kehl, V.; Priller, J.; Trojan, M.; Diehl-Schmid, J. A bitter pill to swallow—Polypharmacy and psychotropic treatment in people with advanced dementia. BMC Geriatr. 2022, 22, 214. [Google Scholar] [CrossRef]
- Landi, F.; Onder, G.; Cesari, M.; Gambassi, G.; Steel, K.; Russo, A.; Lattanzio, F.; Bernabei, R. Pain management in frail, community-living elderly patients. Arch. Intern. Med. 2001, 161, 2721–2724. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients of any age, gender and ethnicity suffering from pain; | Animal studies; |
| Publications reporting on in vitro studies; | |
| Case reports; | |
| Narrative reviews; | |
| Systematic reviews and meta-analysis; | |
| Abstracts and congress communications, proceedings, editorials and book chapters; | |
| Editorials; | |
| No restrictions regarding study duration or follow-up and publication date. | Studies not available in a full-text format or not published in English. |
| Study Report | Study Design | Intervention | Control | Results |
|---|---|---|---|---|
| Andresen et al., 2016 | Randomized, double-blind, placebo-controlled, parallel multicenter trial NCT01851499 | Sublingual ultramicronized PEA (PEA-um) 600 mg (Normast®), twice daily with approximately 12 h between doses for 12 weeks, as add-on therapy. n = 36 | Identical placebo n = 37 | No statistically significant difference in primary outcome (PEA 6.3 ± 1.7 and 0.4 ± 1.4 from baseline, placebo 5.5 ± 1.8 and 0.7 ± 1.4 from baseline); significant reduction in the use of rescue medication; increase in self-reported intensity of spasticity; no statistically significant differences for any of the other secondary outcomes; 5 patients reported serious adverse events; urinary tract infection, paralytic ileus, cholecystolithiasis, erysipelas causing hospitalization, fungus infection, blurred vision |
| Bonetti et al., 2022 | Observational | Combined treatment of oxygen–ozone therapy and oral treatment with alpha-lipoic acid (ALA, 800 mg/day) + palmitoylethanolamide (PEA, 600 mg/day) and myrrh (200 mg). n = 153. Three treatments with oxygen–ozone therapy over 30 days with period of 9 ± 2 days between the first and second therapeutic session and 18 ± 2 between the second and third | Oxygen–ozone treatment alone. n = 165. Three treatments with oxygen–ozone therapy over 30 days with period of 9 ± 2 days between the first and second therapeutic session and 18 ± 2 between the second and third | 116/165 patients in Group A had a complete remission of pain (70.3%), while 21 (12.7%) and 28 (17.0%) had no benefit from the treatment, reporting a partial remission of painful symptoms, while in Group B, 119/153 (77.8%) had a complete remission of pain, 13 (8.5%) considered the outcome of the treatment sufficient and 21 (13.7%) considered it to be insufficient |
| Cocito et al., 2014 | Open-label study | Oral PEA-um treatment was initiated at the doses of 1200 mg/die in sachet formulation for the first 10 days and 1200 mg/die in tablet formulation between the 10th and 40th days. The dosages of all other therapies were maintained during the entire duration of the study. n = 30 | - | Significant decrease in the VAS mean score at the first evaluation (T1; 8.20 ± 1.53 vs. 6.4 ± 1.83, p < 0.002), even more evident at the T2 evaluation (5.80 ± 2.04; p < 0.001). Significant improvement in the NPSI total score, from 5.2 ± 1.5 to the T2 (40 days) values of 3.8 ± 2.1 (p: 0.025), and a similar trend was seen for the EQ-5D mean score, from the T0 value of −0.30 ± 0.65 to the T2 value of 0.50 ± 0.34 (p < 0.001) |
| Faig-Martì and Martinez-Catassus 2017 | Prospective, double-blinded, randomized study | 300 mg of PEA twice a day over 60 days. n = 30 | Placebo with exactly the same appearance twice a day for the same period. n = 31 | No significant differences in any outcomes. VAS 3.76 ± 3.19 (PEA) vs. 3.25 ± 3.18 (Control) |
| Gatti et al., 2012 | Observational study | PEA (600 mg) was administered twice daily for 3 weeks followed by single daily dosing for 4 weeks, in addition to standard analgesic therapies or as single therapy. n = 610 | - | NRS significant decrease from 6.4 ± 1.4 to 2.5 ± 1.3. No treatment-related adverse events or serious adverse events |
| Paladini et al., 2017 | Observational study | Tapentadol and pregabalin at variable doses, for three months in this study. One month after the start of standard treatment, um-PEA (Normast, Epitech Group SpA, Saccolongo, Italia) was added at 1200 mg/day (two 600 mg tablets daily) for one month followed by 600 mg/day for the next month. n = 35 | - | VAS (2–8 months after surgery) 5.7 ± 0.12 vs. VAS 4.3 ± 0.11 after 1 month of treatment (and 2.7 ± 0.09 after two and 1.7 ± 0.11 after 3 months of treatment) (for all measures, 𝑝 < 0.0001) |
| Parisi et al., 2021 | Prospective study | Standard therapy + a fixed combination of PEA (600 mg) + Acetyl-L-Carnitine (500 mg) (Kalanit®) twice a day for 2 weeks and then once a day for 6 months. n = 42 | Standard therapy. n = 40 | Significant improvement in pain VAS: intervention 5.8 ± 1.3 vs. 7.1 ± 1.3 with respect to standard therapy 6.1 ± 0.7 vs. 6.8 ± 0.7. Significant improvement in LBP-IQ and CHFD scores. |
| Passavanti et al., 2017 | Pilot, observational study | Prospective arm: PEA-um as add-on therapy to tapentadol for 6 months. Paracetamol (1000 mg) was habitually used as rescue drug in the case of exacerbation of pain. n = 30 | Retrospective arm: tapentadol for 6 months. Paracetamol (1000 mg) was habitually used as rescue drug in case of exacerbation of pain. n = 25 | VAS significant reduction from 7.4 ± 0.08 to 4.5 ± 0.09 in the prospective group vs. from 7.7 ± 0.10 to 5.9 ± 0.09 in the retrospective group. DN4 mean score reduction from 6.1 ± 0.14 to 3.2 ± 0.13 with PEA vs. from 6.1 ± 0.09 to 5.0 ± 0.04 in the retrospective group. Prospective group presented ODQ reduction from 56.9 ± 1.55 to 37.7 ± 2.38 vs. retrospective group going from 54.6 ± 2.20 to 44.6 ± 3.02. PEA significantly reduced the dosage of tapentadol and the use of paracetamol. No serious side effects |
| Scaturro et al., 2020 | Observational Study | PEA-um 600 mg twice a day in combination with a daily functional rehabilitation session + a decontracting massage for 20 consecutive days, followed by 600 mg of umPEA once a day for 40 days in addition to standard therapy. n = 120 | - | NRS decreased significantly from 6.3 ± 0.1 at baseline to 3.7 ± 0.09 and 2 ± 0.09 at 30 and 60 days, respectively. Significant improvement in quality of life and mental component |
| Schifilliti et al., 2014 | Open-label study | Micronized palmitoylethanolamide (300 mg twice daily) for 60 days. n = 30 | - | Significant reduction in the pain symptoms characteristic of diabetic neuropathy after only 30 days (MNSI, TSS, NPSI). No serious adverse events |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuteri, D.; Guida, F.; Boccella, S.; Palazzo, E.; Maione, S.; Rodríguez-Landa, J.F.; Martínez-Mota, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence. Pharmaceutics 2022, 14, 1672. https://doi.org/10.3390/pharmaceutics14081672
Scuteri D, Guida F, Boccella S, Palazzo E, Maione S, Rodríguez-Landa JF, Martínez-Mota L, Tonin P, Bagetta G, Corasaniti MT. Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence. Pharmaceutics. 2022; 14(8):1672. https://doi.org/10.3390/pharmaceutics14081672
Chicago/Turabian StyleScuteri, Damiana, Francesca Guida, Serena Boccella, Enza Palazzo, Sabatino Maione, Juan Francisco Rodríguez-Landa, Lucia Martínez-Mota, Paolo Tonin, Giacinto Bagetta, and Maria Tiziana Corasaniti. 2022. "Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence" Pharmaceutics 14, no. 8: 1672. https://doi.org/10.3390/pharmaceutics14081672
APA StyleScuteri, D., Guida, F., Boccella, S., Palazzo, E., Maione, S., Rodríguez-Landa, J. F., Martínez-Mota, L., Tonin, P., Bagetta, G., & Corasaniti, M. T. (2022). Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence. Pharmaceutics, 14(8), 1672. https://doi.org/10.3390/pharmaceutics14081672









