Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable
Abstract
:1. Introduction
2. Biological Challenges of Glioblastoma Therapy: More Than Meets the Eye
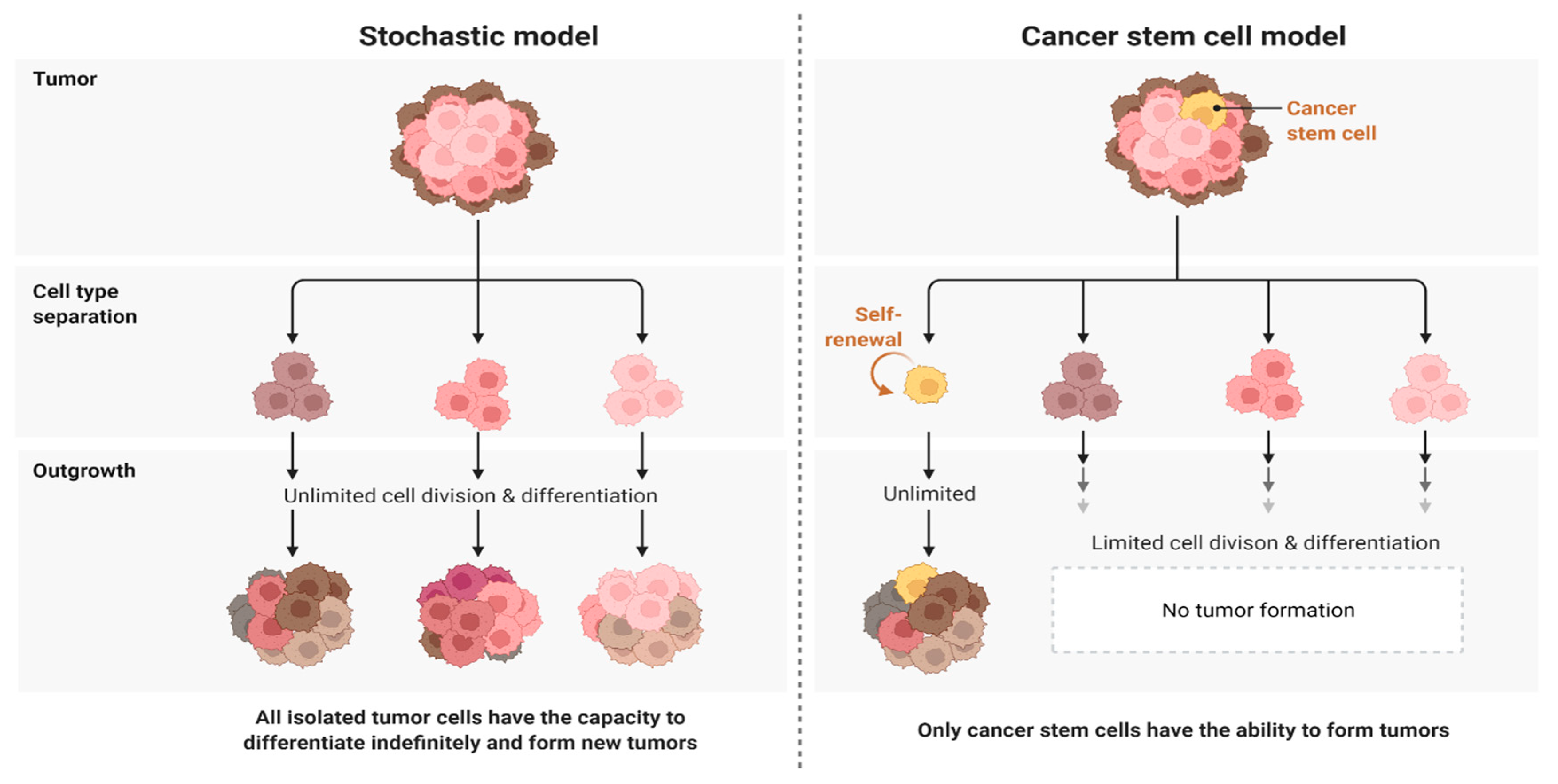
2.1. Challenge 1. Heterogeneity as a Big Challenge Ahead in Targeting Glioblastoma
2.2. Challenge 2. Tumor Microenvironment
2.3. Challenge 3. Blood–Brain Barrier (BBB) vs. Blood–Tumor Barrier (BTB)
3. Multimodality Therapeutic Approaches in Glioblastoma
3.1. Current Treatment
3.2. Nanotechnology as the Potential Therapeutic Strategy for Drug Delivery to Glioblastoma
4. Combination Therapy for Glioblastoma
4.1. Nano-Chemotherapies
4.2. Nano-Chemotherapy–Radiotherapy
4.3. Nano-Chemotherapy–Immunotherapy
5. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.T.; Nguyen, T.T.D.; Vo, T.K.; Nguyen, M.K.; Van Vo, T.; Van Vo, G. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Jin, X.; Kim, L.J.Y.; Wu, Q.; Wallace, L.C.; Prager, B.C.; Sanvoranart, T.; Gimple, R.C.; Wang, X.; Mack, S.C.; Miller, T.E.; et al. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat. Med. 2017, 23, 1352–1361. [Google Scholar] [CrossRef]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef]
- Parrish, K.E.; Cen, L.; Murray, J.; Calligaris, D.; Kizilbash, S.; Mittapalli, R.K.; Carlson, B.L.; Schroeder, M.A.; Sludden, J.; Boddy, A.V.; et al. Efficacy of PARP inhibitor rucaparib in orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol. Cancer Ther. 2015, 14, 2735–2743. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The blood clearance kinetics and pathway of polymeric micelles in cancer drug delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Kingwell, K. New targets for drug delivery across the BBB. Nat. Rev. Drug Discov. 2016, 15, 84–85. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar] [CrossRef]
- Zuchero, Y.J.Y.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of novel blood-brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57. [Google Scholar] [CrossRef]
- Jensen, S.A.; Day, E.S.; Ko, C.H.; Hurley, L.A.; Luciano, J.P.; Kouri, F.M.; Stegh, A.H. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013, 5, 209ra152. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C.; Fitch, S.; Yang, F. Targeting tumor hypoxia using nanoparticle-engineered CXCR4-overexpressing adipose-derived stem cells. Theranostics 2018, 8, 1350–1360. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Barani, I.J.; Larson, D.A. Radiation therapy of glioblastoma. In Current Understanding and Treatment of Gliomas; Springer: Berlin/Heidelberg, Germany, 2015; pp. 49–73. [Google Scholar]
- Clarke, J.; Butowski, N.; Chang, S. Recent advances in therapy for glioblastoma. Arch. Neurol. 2010, 67, 279–283. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Gilbert, M.R.; Chakravarti, A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J. Clin. Oncol. 2007, 25, 4127–4136. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Vijayaraghavan, P.; Chiang, W.-H.; Chen, H.-H.; Liu, T.-I.; Shen, M.-Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.-C. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef]
- Hutóczki, G.; Virga, J.; Birkó, Z.; Klekner, A. Novel concepts of glioblastoma therapy concerning its heterogeneity. Int. J. Mol. Sci. 2021, 22, 10005. [Google Scholar] [CrossRef]
- Habib, A.; Pease, M.; Kodavali, C.V.; Amankulor, N.; Zinn, P.O. A contemporary update on glioblastoma: Molecular biology, current management, and a vision towards bio-adaptable personalized care. J. Neuro-Oncol. 2021, 151, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Nørøxe, D.S.; Poulsen, H.S.; Lassen, U. Hallmarks of glioblastoma: A systematic review. ESMO Open 2016, 1, e000144. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Hara, A.; Kunisada, T.; Yoshimura, S.I.; Iwama, T.; Park, D.M. The evidence of glioblastoma heterogeneity. Sci. Rep. 2015, 5, 7979. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I. Genetic secrets of long-term glioblastoma survivors. Bosn. J. Basic Med. Sci. 2019, 19, 116–124. [Google Scholar] [CrossRef]
- Abels, E.R.; Maas, S.L.; Tai, E.; Ting, D.T.; Broekman, M.L.; Breakefield, X.O.; El Khoury, J. GlioM&M: Web-based tool for studying circulating and infiltrating monocytes and macrophages in glioma. Sci. Rep. 2020, 10, 9898. [Google Scholar]
- Roesch, S.; Rapp, C.; Dettling, S.; Herold-Mende, C. When immune cells turn bad—Tumor-associated microglia/macrophages in glioma. Int. J. Mol. Sci. 2018, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef]
- Locarno, C.V.; Simonelli, M.; Carenza, C.; Capucetti, A.; Stanzani, E.; Lorenzi, E.; Persico, P.; Della Bella, S.; Passoni, L.; Mavilio, D.; et al. Role of myeloid cells in the immunosuppressive microenvironment in gliomas. Immunobiology 2020, 225, 151853. [Google Scholar] [CrossRef]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the immune system in glioblastoma. Br. J. Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Fanelli, G.; Grassini, D.; Ortenzi, V.; Pasqualetti, F.; Montemurro, N.; Perrini, P.; Naccarato, A.; Scatena, C. Decipher the glioblastoma microenvironment: The first milestone for new groundbreaking therapeutic strategies. Genes 2021, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.; Synowitz, M. CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol. 2014, 128, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-brain barrier, blood-brain tumor barrier, and fluorescence-guided neurosurgical oncology: Delivering optical labels to brain tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Wu, S.-K.; Tsai, C.-L.; Huang, Y.; Hynynen, K. Focused ultrasound and microbubbles-mediated drug delivery to brain tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Luo, H.; Shusta, E.V. Blood–brain barrier modulation to improve glioma drug delivery. Pharmaceutics 2020, 12, 1085. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB heterogeneity: A new paradigm for drug delivery to brain tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–brain barrier in brain tumors: Biology and clinical relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; de Sampaio, E.; Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2015, 8, 418. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: An update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Todorova, P.K.; Fletcher-Sananikone, E.; Mukherjee, B.; Kollipara, R.; Vemireddy, V.; Xie, X.-J.; Guida, P.M.; Story, M.D.; Hatanpaa, K.; Habib, A.A.; et al. Radiation-induced DNA damage cooperates with heterozygosity of TP53 and PTEN to generate high-grade gliomas. Cancer Res. 2019, 79, 3749–3761. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg. Rev. 2018, 41, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Yeini, E.; Ofek, P.; Albeck, N.; Ajamil, D.R.; Neufeld, L.; Eldar-Boock, A.; Kleiner, R.; Vaskovich, D.; Koshrovski-Michael, S.; Dangoor, S.I.; et al. Targeting glioblastoma: Advances in drug delivery and novel therapeutic approaches. Adv. Ther. 2021, 4, 2000124. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: Singapore, 2020; pp. 61–91. [Google Scholar]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Merkel, O.M.; Urbanics, R.; Bedőcs, P.; Rozsnyay, Z.; Rosivall, L.; Toth, M.; Kissel, T.; Szebeni, J. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 2011, 32, 4936–4942. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Andersen, A.J.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.L.; Hunter, A.C. Material properties in complement activation. Adv. Drug Deliv. Rev. 2011, 63, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Raucher, D.; Dragojevic, S.; Ryu, J. Macromolecular drug carriers for targeted glioblastoma therapy: Preclinical studies, challenges, and future perspectives. Front. Oncol. 2018, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, A.B.; Talaie, Z.; Jusheghani, F.; Łos, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, A.; Mardani, R.; Roudi, B.; Taghizadeh, M.; Banfshe, H.R.; Ghaderi, A.; Davoodvandi, A.; Shamollaghamsari, S.; Hamblin, M.R.; Mirzaei, H. Combination therapy with nanomicellar-curcumin and temozolomide for in vitro therapy of glioblastoma multi-forme via Wnt signaling pathways. J. Mol. Neurosci. 2020, 70, 1471–1483. [Google Scholar] [CrossRef]
- Behrooz, A.B.; Vazifehmand, R.; Tajudin, A.A.; Masarudin, M.J.; Sekawi, Z.; Masomian, M.; Syahir, A. Tailoring drug co-delivery nanosystem for mitigating U-87 stem cells drug resistance. Drug Deliv. Transl. Res. 2022, 12, 1253–1269. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kimura, Y.; Enatsu, R.; Mikami, T.; Wanibuchi, M.; Mikuni, N. Advantages and disadvantages of combined chemotherapy with carmustine wafer and bevacizumab in patients with newly diagnosed glioblastoma: A single-institutional experience. World Neurosurg. 2018, 113, e508–e514. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Mortezazadeh, T.; Aryafar, T.; Gharepapagh, E.; Majdaeen, M.; Farhood, B. Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: A mechanistic review. Cancer Cell Int. 2021, 21, 391. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Junior, E.D.O.; Granja, S.; Boni, F.I.; Ferreira, L.M.; Cury, B.S.; Santos, L.C.; Reis, R.M.; Lima, E.M.; Baltazar, F.; et al. Nose-to-brain co-delivery of drugs for glioblastoma treatment using nanostructured system. Int. J. Pharm. 2021, 603, 120714. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor re-gression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/methotrexate co-loaded PLGA nanoparticles in glioblastoma treatment: Formulation development and in vitro antitumor activity evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Michael, J.S.; Lee, B.-S.; Zhang, M.; Yu, J.S. Nanotechnology for treatment of glioblastoma multiforme. J. Transl. Intern. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Bi, H.; Yang, D.; Zhang, C. Glioblastoma precision therapy: From the bench to the clinic. Cancer Lett. 2020, 475, 79–91. [Google Scholar] [CrossRef]
- Stafford, J.H.; Hirai, T.; Deng, L.; Chernikova, S.B.; Urata, K.; West, B.L.; Brown, J.M. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-Oncology 2016, 18, 797–806. [Google Scholar] [CrossRef]
- Tamborini, M.; Locatelli, E.; Rasile, M.; Monaco, I.; Rodighiero, S.; Corradini, I.; Franchini, M.C.; Passoni, L.; Matteoli, M. A combined approach employing chlorotoxin-nanovectors and low dose radiation to reach infiltrating tumor niches in glioblastoma. ACS Nano 2016, 10, 2509–2520. [Google Scholar] [CrossRef]
- Liang, P.; Ballou, B.; Lv, X.; Si, W.; Bruchez, M.P.; Huang, W.; Dong, X. Monotherapy and combination therapy using anti-angiogenic nanoagents to fight cancer. Adv. Mater. 2021, 33, 2005155. [Google Scholar] [CrossRef]
- Lan, J.; Wan, X.-L.; Deng, L.; Xue, J.-X.; Wang, L.-S.; Meng, M.-B.; Ling, H.; Zhang, X.; Mo, X.-M.; Lu, Y. Ablative hypofractionated radiotherapy normalizes tumor vasculature in Lewis lung carcinoma mice model. Radiat. Res. 2013, 179, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Iovenitti, G.; Mancini, A.; Gravina, G.L.; Chebbi, M.; Caruana, M.; Vignaroli, G.; Orofino, F.; Rango, E.; Angelucci, A.; et al. AuNP pyrazolo[3,4-d]pyrimidine nanosystem in combination with radiotherapy against glioblastoma. ACS Med. Chem. Lett. 2020, 11, 664–670. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Z.; Ding, Y.; Xue, K.; Wang, X.; Yang, R.; An, Y.; Liu, D.; Hu, C.; Tang, Q. LRP1-mediated pH-sensitive polymersomes facilitate combination therapy of glioblastoma in vitro and in vivo. J. Nanobiotechnol. 2021, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.M.; Je, J.; Cha, S.H.; Oh, Y.; Cho, W.H. Synergistic combination of chemo-phototherapy based on temozolomide/ICG-loaded iron oxide nanoparticles for brain cancer treatment. Oncol. Rep. 2019, 42, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Chibh, S.; Katoch, V.; Singh, M.; Prakash, B.; Panda, J.J. Miniatured fluidics-mediated modular self-assembly of anticancer drug–amino acid composite microbowls for combined chemo-photodynamic therapy in glioma. ACS Biomater. Sci. Eng. 2021, 7, 5654–5665. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Dash, B.S.; Lu, Y.-J.; Chen, H.-A.; Chuang, C.-C.; Chen, J.-P. Magnetic and GRPR-targeted reduced graphene oxide/doxorubicin nanocomposite for dual-targeted chemo-photothermal cancer therapy. Mater. Sci. Eng. C 2021, 128, 112311. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Sambri, L.; Tortorella, S.; Šturm, S.; Kostevšek, N.; Franchini, M.C. Synthesis of ultrasmall single-crystal gold–silver alloy nanotriangles and their application in photothermal therapy. Nanomaterials 2021, 11, 912. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, A.; Wang, S.; Sun, Y.; Chu, L.; Zhou, L.; Yang, X.; Liu, X.; Sha, C.; Sun, K.; et al. Efficacy of temozolomide-conjugated gold nanoparticle photothermal therapy of drug-resistant glioblastoma and its mechanism study. Mol. Pharm. 2022, 19, 1219–1229. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Zhang, W.; Lee, C.-S.; Wang, P. Hypocrellin-based multifunctional phototheranostic agent for NIR-triggered targeted chemo/photodynamic/photothermal synergistic therapy against glioblastoma. ACS Appl. Bio Mater. 2020, 3, 3817–3826. [Google Scholar] [CrossRef]
- Simón, M.; Jørgensen, J.T.; Juhl, K.; Kjaer, A. The use of a uPAR-targeted probe for photothermal cancer therapy prolongs survival in a xenograft mouse model of glioblastoma. Oncotarget 2021, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S.; et al. Brain-targeted aggregation-induced-emission nanoparticles with near-infrared imaging at 1550 nm boosts ortho-topic glioblastoma theranostics. Adv. Mater. 2022, 34, 2106082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Mohammadniaei, M.; Zheng, T.; Zhang, Q.; Ashley, J.; Liu, S.; Sun, Y.; Tang, B.Z. Upregulating aggregation-induced-emission nanoparticles with blood–tumor-barrier permeability for precise photothermal eradication of brain tumors and induction of local immune responses. Adv. Mater. 2021, 33, 2008802. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.-C.; Cheng, P.-H.; Cheng, X.-J.; Chuang, C.-C.; Huang, Y.-T.; Anilkumar, T.S.; Liu, C.-H.; Lu, Y.-J.; Wu, K.C.-W. MCP-1-functionalized, core–shell gold nanorod@ iron-based metal–organic framework (MCP-1/GNR@ MIL-100 (Fe)) for photothermal therapy. ACS Appl. Mater. Interfaces 2021, 13, 52092–52105. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Da Silva, A.; Estève, M.-A. Photothermal therapy for the treatment of glioblastoma: Potential and preclinical challenges. Front. Oncol. 2021, 10, 610356. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Youn, Y.S. Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors. Adv. Mater. 2017, 29, 1605563. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, X.; Yan, X.; Wang, Y.; Guo, J.; Jacobson, O.; Liu, D.; Szajek, L.P.; Zhu, W.; Niu, G.; et al. Chelator-free 64Cu-integrated gold nanomaterials for positron emission tomography imaging guided photothermal cancer therapy. ACS Nano 2014, 8, 8438–8446. [Google Scholar] [CrossRef]
- Day, E.S.; Thompson, P.A.; Zhang, L.; Lewinski, N.A.; Ahmed, N.; Drezek, R.A.; Blaney, S.M.; West, J.L. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J. Neuro-Oncol. 2011, 104, 55–63. [Google Scholar] [CrossRef]
- Nie, L.; Wang, S.; Wang, X.; Rong, P.; Ma, Y.; Liu, G.; Huang, P.; Lu, G.; Chen, X. In vivo volumetric photoacoustic molecular angiography and therapeutic monitoring with targeted plasmonic nanostars. Small 2014, 10, 1585–1593. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Dai, Y.; Chen, J.; Wang, F.; Lin, L.; Liu, Y.; Zhang, F.; Yu, G.; Zhou, Z.; et al. Self-assembly of semiconducting-plasmonic gold nanoparticles with enhanced optical property for photoacoustic imaging and photothermal therapy. Theranostics 2017, 7, 2177–2185. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Graphene nanomesh promises extremely efficient in vivo photothermal therapy. Small 2013, 9, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, J.; Vargas, E.; Wei, J.; Martinez-Zaguilan, R.; Sennoune, S.R.; Pantoya, M.L.; Wang, S.; Chaudhuri, J.; Qiu, J. Porphyrin immobilized nanographene oxide for enhanced and targeted photothermal therapy of brain cancer. ACS Biomater. Sci. Eng. 2016, 2, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Lu, Y.-J.; Lin, K.-J.; Hsu, S.-C.; Huang, C.-Y.; She, S.-H.; Liu, H.-L.; Lin, C.-W.; Xiao, M.-C.; Wey, S.-P.; et al. EGRF conjugated PEGylated nanographene oxide for targeted chemotherapy and photothermal therapy. Biomaterials 2013, 34, 7204–7214. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, Y.; Zhao, N.; Liu, F.; Xu, F.-J. Multifunctional pDNA-conjugated polycationic Au nanorod-coated Fe3O4 hierarchical nanocomposites for trimodal imaging and combined photothermal/gene therapy. Small 2016, 12, 2459–2468. [Google Scholar] [CrossRef]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4@Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Zhou, Z.; Zhou, P.; Yan, Y.; Wang, M.-W.; Yang, H.; Zhang, Y.; Yang, S. MR/SPECT imaging guided photothermal therapy of tumor-targeting Fe@Fe3O4 nanoparticles in vivo with low mononuclear phagocyte uptake. ACS Appl. Mater. Interfaces 2016, 8, 19872–19882. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, B.; Zheng, C.; Ji, R.; Ren, X.; Guo, F.; Sun, S.; Shi, J.; Zhang, H.; Zhang, Z.; et al. The tumor-targeting core–shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J. Control. Release 2015, 220, 545–555. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Guo, Q.; Wang, L.; Liu, D.; Wei, Z.; Zhou, J. Novel Cs-based upconversion nanoparticles as dual-modal CT and UCL imaging agents for chemo-photothermal synergistic therapy. Theranostics 2016, 6, 1491. [Google Scholar] [CrossRef]
- Duan, S.; Yang, Y.; Zhang, C.; Zhao, N.; Xu, F.-J. NIR-responsive polycationic gatekeeper-cloaked hetero-nanoparticles for multimodal imaging-guided triple-combination therapy of cancer. Small 2017, 13, 1603133. [Google Scholar] [CrossRef]
- Duan, S.; Li, J.; Zhao, N.; Xu, F.-J. Multifunctional hybrids with versatile types of nanoparticles via self-assembly for complementary tumor therapy. Nanoscale 2018, 10, 7649–7657. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Sun, X.; Liu, N.; Zhou, Z.; Yu, F.; Zhang, X.; Chen, X. Radiolabeled angiogenesis-targeting croconaine nanoparticles for trimodality imaging guided photothermal therapy of glioma. ACS Appl. Nano Mater. 2018, 1, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, G.; Hu, J.; Ren, Q.; Yang, K.; Wan, C.; Huang, A.; Li, P.; Feng, J.-P.; Chen, J.; et al. Melittin-containing hybrid peptide hydrogels for enhanced photothermal therapy of glioblastoma. ACS Appl. Mater. Interfaces 2017, 9, 25755–25766. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, P.; Jacobson, O.; Wang, Z.; Liu, Y.; Lin, L.; Lin, J.; Lu, N.; Zhang, H.; Tian, R.; et al. Biomineralization-Inspired Synthesis of Copper Sulfide-Ferritin Nanocages as Cancer Theranostics. ACS Nano 2016, 10, 3453–3460. [Google Scholar] [CrossRef]
- Zha, Z.; Wang, J.; Qu, E.; Zhang, S.; Jin, Y.; Wang, S.; Dai, Z. Polypyrrole hollow microspheres as echogenic photothermal agent for ultrasound imaging guided tumor ablation. Sci. Rep. 2013, 3, srep02360. [Google Scholar] [CrossRef]
- Zhu, M.; Sheng, Z.; Jia, Y.; Hu, D.; Liu, X.; Xiaobing, W.; Liu, C.; Wang, P.; Wang, X.; Zheng, H. Indocyanine green-holo-transferrin nanoassemblies for tumor-targeted dual-modal imaging and photothermal therapy of glioma. ACS Appl. Mater. Interfaces 2017, 9, 39249–39258. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Li, A.; Xu, S.; Manghnani, P.; Liu, C.; Guo, L.; Zheng, H.; Liu, B. Molecular engineering of conjugated polymers for biocompatible organic nanoparticles with highly efficient photoacoustic and photothermal performance in cancer theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar] [CrossRef]
- Madsen, S.J.; Christie, C.; Hong, S.J.; Trinidad, A.; Peng, Q.; Uzal, F.A.; Hirschberg, H. Nanoparticle-loaded macrophage-mediated photothermal therapy: Potential for glioma treatment. Lasers Med. Sci. 2015, 30, 1357–1365. [Google Scholar] [CrossRef]
- Lu, W.; Melancon, M.P.; Xiong, C.; Huang, Q.; Elliott, A.; Song, S.; Zhang, R.; Flores, L.G.; Gelovani, J.G.; Wang, L.V.; et al. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 2011, 71, 6116–6121. [Google Scholar] [CrossRef]
- Day, E.S.; Zhang, L.; Thompson, P.A.; Zawaski, J.A.; Kaffes, C.C.; Gaber, M.W.; Blaney, S.M.; West, J.L. Vascular-targeted photothermal therapy of an orthotopic murine glioma model. Nanomedicine 2012, 7, 1133–1148. [Google Scholar] [CrossRef]
- Dong, H.; Jin, M.; Liu, Z.; Xiong, H.; Qiu, X.; Zhang, W.; Guo, Z. In vitro and in vivo brain-targeting chemo-photothermal therapy using graphene oxide conjugated with transferrin for Gliomas. Lasers Med. Sci. 2016, 31, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Du, Y.; Wang, S.; Li, C.; Jiang, H.; Shi, W.; Chen, J.; Wang, Y.; Wagner, E.; Huang, R. Highly crystalline multicolor carbon nanodots for dual-modal imaging-guided photothermal therapy of glioma. ACS Appl. Mater. Interfaces 2018, 10, 4031–4040. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Qian, M.; Wang, Y.; Huang, J.; Chen, J.; Ni, L.; Huang, Q.; Liu, Q.; Gong, P.; Hou, S.; et al. Combination glioma therapy mediated by a dual-targeted delivery system constructed using OMCN–PEG–Pep22/DOX. Small 2018, 14, 1801905. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv. Mater. 2018, 30, e1802591. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 2018, 13, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Cabada, T.F.; de Pablo, C.S.L.; Serrano, A.M.; del Pozo Guerrero, F.; Olmedo, J.J.S.; Gomez, M.R. Induction of cell death in a glioblastoma line by hyperthermic therapy based on gold nanorods. Int. J. Nanomed. 2012, 7, 1511. [Google Scholar]
- Gonçalves, D.P.; Rodriguez, R.D.; Kurth, T.; Bray, L.J.; Binner, M.; Jungnickel, C.; Gür, F.N.; Poser, S.W.; Schmidt, T.L.; Zahn, D.R.; et al. Enhanced targeting of invasive glioblastoma cells by peptide-functionalized gold nanorods in hydrogel-based 3D cultures. Acta Biomater. 2017, 58, 12–25. [Google Scholar] [CrossRef]
- Bernardi, R.J.; Lowery, A.R.; Thompson, P.A.; Blaney, S.M.; West, J.L. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J. Neuro-Oncol. 2008, 86, 165–172. [Google Scholar] [CrossRef]
- Baek, S.-K.; Makkouk, A.R.; Krasieva, T.; Sun, C.-H.; Madsen, S.J.; Hirschberg, H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J. Neuro-Oncol. 2011, 104, 439–448. [Google Scholar] [CrossRef]
- Christie, C.; Madsen, S.J.; Peng, Q.; Hirschberg, H. Photothermal therapy employing gold nanoparticle-loaded macrophages as delivery vehicles: Comparing the efficiency of nanoshells versus nanorods. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 229–235. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Zhang, F.; Xu, H.; Chen, W.; Jiang, T. A novel nanocomposite based on fluorescent turn-on gold nanostars for near-infrared photothermal therapy and self-theranostic caspase-3 imaging of glioblastoma tumor cell. Colloids Surf. B Biointerfaces 2018, 170, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sheng, Z.; Li, P.; Wu, M.; Zhang, N.; Yu, X.-F.; Wang, Y.; Hu, D.; Zheng, H.; Wang, G.P. Indocyanine green-loaded gold nanostars for sensitive SERS imaging and subcellular monitoring of photothermal therapy. Nanoscale 2017, 9, 11888–11901. [Google Scholar] [CrossRef] [PubMed]
- Botella, P.; Ortega, Í.; Quesada, M.; Madrigal, R.F.; Muniesa, C.; Fimia, A.; Fernández, E.; Corma, A. Multifunctional hybrid materials for combined photo and chemotherapy of cancer. Dalton Trans. 2012, 41, 9286–9296. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; Sancey, L.; Palermo, G.; Termine, R.; De Luca, A.; Szerb, E.I.; Aiello, I.; Ghedini, M.; Strangi, G.; La Deda, M. Plasmon-mediated cancer phototherapy: The combined effect of thermal and photodynamic processes. Nanoscale 2017, 9, 19279–19289. [Google Scholar] [CrossRef]
- Popov, A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser-synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Li, Z.-J.; Li, C.; Zheng, M.-G.; Pan, J.-D.; Zhang, L.-M.; Deng, Y.-F. Functionalized nano-graphene oxide particles for targeted fluorescence imaging and photothermy of glioma U251 cells. Int. J. Clin. Exp. Med. 2015, 8, 1844–1852. [Google Scholar]
- Markovic, Z.M.; Harhaji-Trajkovic, L.; Marković, B.T.; Kepić, D.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramicanin, M.; Trajkovic, V. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef]
- Kargar, S.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R. Evaluation of the combined effect of NIR laser and ionizing radiation on cellular damages induced by IUdR-loaded PLGA-coated Nano-graphene oxide. Photodiagn. Photodyn. Ther. 2018, 21, 91–97. [Google Scholar] [CrossRef]
- Eldridge, B.N.; Bernish, B.W.; Fahrenholtz, C.D.; Singh, R. Photothermal therapy of glioblastoma multiforme using multiwalled carbon nanotubes optimized for diffusion in extracellular space. ACS Biomater. Sci. Eng. 2016, 2, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Maziukiewicz, D.; Grześkowiak, B.F.; Coy, E.; Jurga, S.; Mrówczyński, R. NDs@PDA@ICG conjugates for photothermal therapy of glioblastoma multiforme. Biomimetics 2019, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xing, D.; Zhou, F.; Wu, B.; Chen, W.R. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol. Pharm. 2011, 8, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Rad, J.K.; Mahdavian, A.R.; Khoei, S.; Shirvalilou, S. Enhanced photogeneration of reactive oxygen species and targeted photothermal therapy of C6 glioma brain cancer cells by folate-conjugated gold–photoactive polymer nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19483–19493. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Tanaka, S.; Louis, D.N.; Curry, W.T.; Batchelor, T.T.; Dietrich, J. Diagnostic and therapeutic avenues for glioblastoma: No longer a dead end? Nat. Rev. Clin. Oncol. 2013, 10, 14–26. [Google Scholar] [CrossRef]
- Elsamadicy, A.A.; Chongsathidkiet, P.; Desai, R.; Woroniecka, K.; Farber, S.H.; Fecci, P.; Sampson, J.H. Prospect of rindopepimut in the treatment of glioblastoma. Expert Opin. Biol. Ther. 2017, 17, 507–513. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Kang, X.; Wu, A.; Yin, W.; Tang, Y.; Wang, J.; Zhang, M.; Duan, Y.; Huang, Y. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem. Sci. 2018, 9, 2674–2689. [Google Scholar] [CrossRef]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.-P.; Tao, J.; Liu, C.-J.; He, X.-H.; Zhang, X.-Z. iRGD modified chemo-immunotherapeutic nanoparticles for enhanced immunotherapy against glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Kadiyala, P.; Li, D.; Nuñez, F.M.; Altshuler, D.; Doherty, R.; Kuai, R.; Yu, M.; Kamran, N.; Edwards, M.; Moon, J.J.; et al. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano 2019, 13, 1365–1384. [Google Scholar] [CrossRef]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J.; et al. Traceable nanoparticles with dual targeting and ROS response for RNAi-based immunochemotherapy of intracranial glioblastoma treatment. Adv. Mater. 2018, 30, e1705054. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.L.; Braubach, O.; et al. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019, 10, 3850. [Google Scholar] [CrossRef] [PubMed]
- Phuengkham, H.; Song, C.; Lim, Y.T. A designer scaffold with immune nanoconverters for reverting immunosuppression and enhancing immune checkpoint blockade therapy. Adv. Mater. 2019, 31, e1903242. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, Z. The application of nanotechnology in immune checkpoint blockade for cancer treatment. J. Control. Release 2018, 290, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhao, X.; Xiao, S. Application prospect of peptide-modified nano targeting drug delivery system combined with PD-1/PD-L1 based immune checkpoint blockade in glioblastoma. Int. J. Pharm. 2020, 589, 119865. [Google Scholar] [CrossRef]
- Chen, M.H.; Liu, T.Y.; Chen, Y.C.; Chen, M.H. Combining augmented radiotherapy and immunotherapy through a nano-gold and bacterial outer-membrane vesicle complex for the treatment of glioblastoma. Nanomaterials 2021, 11, 1661. [Google Scholar] [CrossRef]
- Li, T.-F.; Li, K.; Wang, C.; Liu, X.; Wen, Y.; Xu, Y.-H.; Zhang, Q.; Zhao, Q.-Y.; Shao, M.; Li, Y.-Z.; et al. Harnessing the cross-talk between tumor cells and tumor-associated macrophages with a nano-drug for modulation of glioblastoma immune microenvironment. J. Control. Release 2017, 268, 128–146. [Google Scholar] [CrossRef]
- Li, T.F.; Li, K.; Zhang, Q.; Wang, C.; Yue, Y.; Chen, Z.; Yuan, S.-J.; Liu, X.; Wen, Y.; Han, M.; et al. Dendritic cell-mediated delivery of doxorubicin-polyglycerol-nanodiamond composites elicits enhanced anti-cancer immune response in glioblastoma. Biomaterials 2018, 181, 35–52. [Google Scholar] [CrossRef]
- Li, T.-F.; Xu, Y.-H.; Li, K.; Wang, C.; Liu, X.; Yue, Y.; Chen, Z.; Yuan, S.-J.; Wen, Y.; Zhang, Q.; et al. Doxorubicin-polyglycerol-nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomater. 2019, 86, 381–394. [Google Scholar] [CrossRef]
- Zhang, P.; Miska, J.; Lee-Chang, C.; Rashidi, A.; Panek, W.K.; An, S.; Zannikou, M.; Lopez-Rosas, A.; Han, Y.; Xiao, T.; et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 23714–23723. [Google Scholar] [CrossRef]
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R.; et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv. Drug Deliv. Rev. 2021, 171, 108–138. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Nandi, S.; Bhattacharjee, S. Combination therapy to checkmate glioblastoma: Clinical challenges and advances. Clin. Transl. Med. 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Mathios, D.; Kim, J.E.; Mangraviti, A.; Phallen, J.; Park, C.-K.; Jackson, C.M.; Garzon-Muvdi, T.; Kim, E.; Theodros, D.; Polanczyk, M.; et al. Anti–PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci. Transl. Med. 2016, 8, 370ra180. [Google Scholar] [CrossRef] [PubMed]




| Molecules | Suggested Mechanism of Action | Result |
|---|---|---|
| TMZ + RT | - | Longer survival |
| Bevacizumab + TMZ + RT | Suppresses the proliferation and angiogenesis of vascular endothelial cells | Therapeutic effectiveness |
| IR + PLX3397 | - | Longer lifespan |
| Ag-PNP-CTX | Reduce the extracellular activity of MMP-2 | - |
| CTX nanovector + RT | Increases the accumulation of nanovector therapeutic cargo in GBM cells | Synergistic effect suppresses by about 50% |
| DC101 + RT | Lowering hypoxia | Tumor growth suppression |
| AuNPs-SI306 + RT | - | Inhibition of tumor cell growth |
| Au-DOX@PO-ANG NPs + RT | Increase BBB-crossing capacity | Reduction in tumor volume |
| Photoabsorbing Agent | PTT Laser and Treatment Conditions | Preclinical Model | Model | Reference | ||
|---|---|---|---|---|---|---|
| Power (W/cm2) | Exposure Time (min) | Administration Regimen/Route | ||||
| RVG29-SiO2-PEG-AuNR | 1.5 | 5 | iv | N2a neuroblastoma | [88] | |
| 4Cu-RGD-Au NR | 1 | 10 | iv | U87 MG | [89] | |
| AuNS | 4 | 3 | iv | U373 GBM | [90] | |
| RGD-AuNSt | 1 | 10 | iv, multiple | U87 MG | [91] | |
| PPDI-PEG-Au NP | 0.3 | 5 | iv | U87 MG | [92] | |
| rGONM-PEG-Cy7-RGD | 0.1 | 7 | iv | U87 MG | [93] | |
| PNG-RGD | 2.5 | 5 | it | U87 MG | [94] | |
| C225-EPI-PEG-NGO | 2 | 2 | iv | U87 MG | [95] | |
| rGO-AuNRVe-DOX | 0.25 | 5 | iv | U87 MG | [96] | |
| pDNA-loaded AuNR-Fe3O4NS | 2 | 5 | it, multiple | U87 MG | [97] | |
| C225-Au-MNP | 0.3 | 30 | pt, multiple | C6 | [98] | |
| I-RGD-PEG-MNP | 0.5 | 5 | iv, multiple | U251 | [99] | |
| ANG-Au-PLGA-DTX NPs | 1.5 | 1.5 | iv, multiple | U87 MG | [100] | |
| UCNP-PEG-ICG-TOS-RGD | 0.5 | 5 | iv, multiple | U87 MG | [101] | |
| ASQ-DOX-PGEA2/p53 nanohybrids | 2 | 5 | it, multiple | C6 | [102,103] | |
| I RGD-CR780-PEG NPs | 0.5 | 10 | iv | U87 MG | [104] | |
| melittin/ICG peptide nanofiber hydrogel | 2 | 8 | it | C6 | [105] | |
| CuS–Fn NCs | 0.8 | 5 | iv | U87 MG | [106] | |
| PPyHMs | 0.64 | 10 | it | U87 MG | [107] | |
| holo-Tf-ICG | 0.8 | 5 | iv | U87 MG | [108] | |
| CPNP | 0.8 | 5 | iv | U87 MG | [109] | |
| Ma-AuNS | N/A | 10 | it | C6 | [110] | |
| cRGD-PEG-HAuNS | 16 | 3 | iv | U87 MG-Luc | [111] | |
| VEGF-AuNS | 3 | 6 | iv | U373 GBM | [112] | |
| Tf-TPGD | 2.5 | 5 | iv, multiple | C6 | [113] | |
| HCCD | 1 | 5 | iv, multiple | U87 MG | [114] | |
| OMCN–PEG–Pep22/doxycycline | N/A | 5 | iv, multiple | C6 | [115] | |
| ANG-IMNPs | 0.21 | 3 | iv | ALTS1C1 astrocytoma | [20] | |
| cRGD-CPNP | 0.8 | 5 | iv | U87 MG-Luc | [116] | |
| BLIPO-ICG | 1 | 5 | iv | C6-Luc | [117] | |
| AuNR | 1.2 W * | 1321N1 human astrocytoma | 2D | [118] | ||
| Nes-AuNR | 0.5 | X01 GBM, X01 GBM-BMP | 2D, 3D | [119] | ||
| AuNS | 80 | U373, U87 MG | 2D | [120] | ||
| Ma-AuNS | 2, 7, 14, or 28 | ACBT human glioma | 2D, 3D | [121,122] | ||
| AuNSt@probe | 2 | U87 MG | 2D | [123] | ||
| AuNSt-ICG-BSA | 1 | U87 MG | 2D | [124] | ||
| CPT-GNC | 76 ** | 42 MG-BA | 2D | [125] | ||
| r1-AuSiO2 NP | 4 | U87 MG | 2D | [126] | ||
| TiN NP | 4.4 | U87 MG | 2D, 3D | [127] | ||
| nano-rGO-RGD | 15.3 | U87 MG | 2D | [128] | ||
| nanoGO-Tf-FITC | 7.5 | U251 glioma | 2D | [129] | ||
| PVP-G | 2 | U251 glioma | 2D | [130] | ||
| DOX-GMS-PI | 6 | U251 glioma | 2D | [131] | ||
| IUdR-PLGA-NGO | 2 | U87 MG | 2D | [132] | ||
| MWCNTS | 3 | U87 MG, U373, D54 | 2D, 3D | [133] | ||
| PDA-ICG-NDs | 2 W * | U-118 MG | 2D | [134] | ||
| ICG-PL-PEG | 0.75 to 3.25 | U87 MG | 2D | [135] | ||
| FA-Au-NP | 8.5 | C6 glioma | 2D | [136] | ||
| Molecules | Suggested Mechanism of Action | Results |
|---|---|---|
| Fe3O4-TMZ-ICG MNPs | Effects on Bcl-2-associated X protein, Bcl-2, cytochrome c, caspase-3, Fas-associated via the death domain, and caspase-8 genes | Increased anticancer effects |
| Doxorubicin–curcumin–amino acid (CMBs) | Drug carrier for cancer treatment | Treatment using CMBs on two- and three-dimensional (2D) spheroids of C6 glioma cells |
| mrGOG-DOX | DOX coupled to mrGO (mrGOG) through the binding of π-π stacking | Tumor reduction, long-term survival |
| Gold-silver nanotri-angles (AuAgNTrs) | Becomes nontoxic to cells | Cell viability decreased by >80% |
| anti-EphA3-TMZ@GNPs | Boosts TMZ’s cytotoxicity and apoptosis | Increase in the production of antiapoptotic signaling molecules and cell-cycle inhibitors |
| DCHB-TMZ-C18 | Cross the BBB and target tumors directly | Targeted chemo/photodynamic/photothermal synergistic treatment with little harm |
| ICG-Glu-Glu-AE105 | Targeting plasminogen activator receptor (uPAR) | Tumor death and prolonged survival |
| ApoE-Ph NPs | Increases PTT efficiency | Increases the survival of mice with orthotopic GBM |
| MCP-1/GNR@MIL-100 (Fe) | Boost cellular absorption and biocompatibility | Antitumor effectiveness |
| BK@AIE NPs-NIR | Removal and release of tissue necrosis factor and tumor-associated antigens by NIR irradiation | Improving GBM clearance and activating local brain immune privilege |
| Molecules | Suggested Mechanism of Action | Results |
|---|---|---|
| Rindopepimut (CDX-110) | EGFRvIII | Multi-immunotherapy/enhances chemotherapy effectiveness |
| Doxorubicin + (1-methyltryptophan, 1MT) | Immune checkpoint inhibitor | Drug accumulation in orthotopic brain tumors |
| DTX-sHDL-CpG nanodisc + IR | Antitumor CD8+ T-cell responses in the brain tumor microenvironment (TME) | Tumor reduction, long-term survival |
| Angiopep LipoPCB (TMZ + BAP/siTGF-β), ALBTA | Chemotherapy + RNAi-based immunomodulation | Boosts TMZ’s cytotoxicity/improves gene silencing efficacy of siTGF-β ALBTA |
| ALBTA’s zwitterionic lipid (distearoyl phos phoethanol-amine-polycarboxybetaine lipid + TMZ) | Boosts TMZ’s cytotoxicity and improves gene silencing efficacy of siTGF-β by promoting endosomal/lysosomal escape | Increases the susceptibility of GBM cells to chemotherapeutic agents/regulated the tumor microenvironment |
| Immunoconjugates (NICs) + a-CTLA-4 or a-PD-1 | Checkpoint blockade drug delivered across BBB to the tumor location | Induction of a systemic and local immune response in glioblastoma therapy |
| Resiquimod + doxorubicin | Activation of neoantigen-specific T cells | Polarization of immunosuppressive tumor-associated macrophages (TAMs) |
| Chemotherapy + anti-PD-1 | Improves antitumor immune responses | Prolong overall survival in glioblastoma treatment |
| AuNPs + OMVs-(Au–OMV) | Induces radiosensitizing and immunomodulatory effects | Reduced tumor development |
| Chemotaxis + TNF-α | ||
| Immunosuppressive microenvironment + doxorubicin (Nano-DOX) | Increasing the immunogenicity of GBM cells (GC) | Initiation of anti-GBM immune responses |
| Nano-DOX + dendritic cells (DC) | Increases GC immunogenicity via activation of autophagy | Alteration of the GBM immune microenvironment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzegar Behrooz, A.; Talaie, Z.; Syahir, A. Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable. Pharmaceutics 2022, 14, 1697. https://doi.org/10.3390/pharmaceutics14081697
Barzegar Behrooz A, Talaie Z, Syahir A. Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable. Pharmaceutics. 2022; 14(8):1697. https://doi.org/10.3390/pharmaceutics14081697
Chicago/Turabian StyleBarzegar Behrooz, Amir, Zahra Talaie, and Amir Syahir. 2022. "Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable" Pharmaceutics 14, no. 8: 1697. https://doi.org/10.3390/pharmaceutics14081697
APA StyleBarzegar Behrooz, A., Talaie, Z., & Syahir, A. (2022). Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable. Pharmaceutics, 14(8), 1697. https://doi.org/10.3390/pharmaceutics14081697






