Dual Antimelanogenic Effect of Nicotinamide-Stabilized Phloretin Nanocrystals in Larval Zebrafish
Abstract
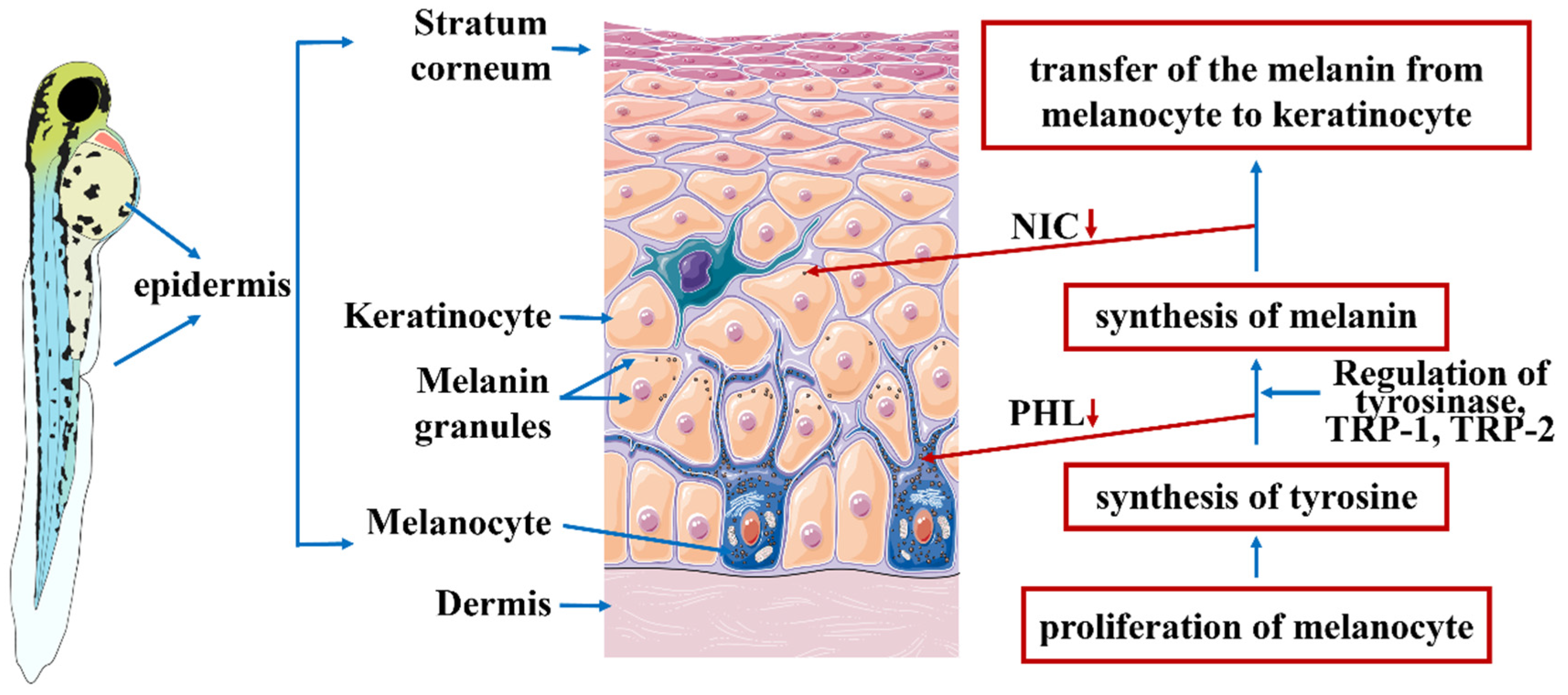
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication PHL-NIC-NCs
2.3. Investigation on the Stability of NCs
2.4. Characterization of NCs
2.5. In Vitro Drug Release
2.6. Efficacy Validation of Zebrafish
2.6.1. Screening of Drug Concentration
2.6.2. Transport of NCs in Embryonic Zebrafish In Vivo
2.6.3. Zebrafish Embryo Exposure Experiment
2.6.4. Determination of Tyrosinase Activity in Zebrafish Embryos
2.6.5. Determination of Melanin Content in Zebrafish Embryos
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
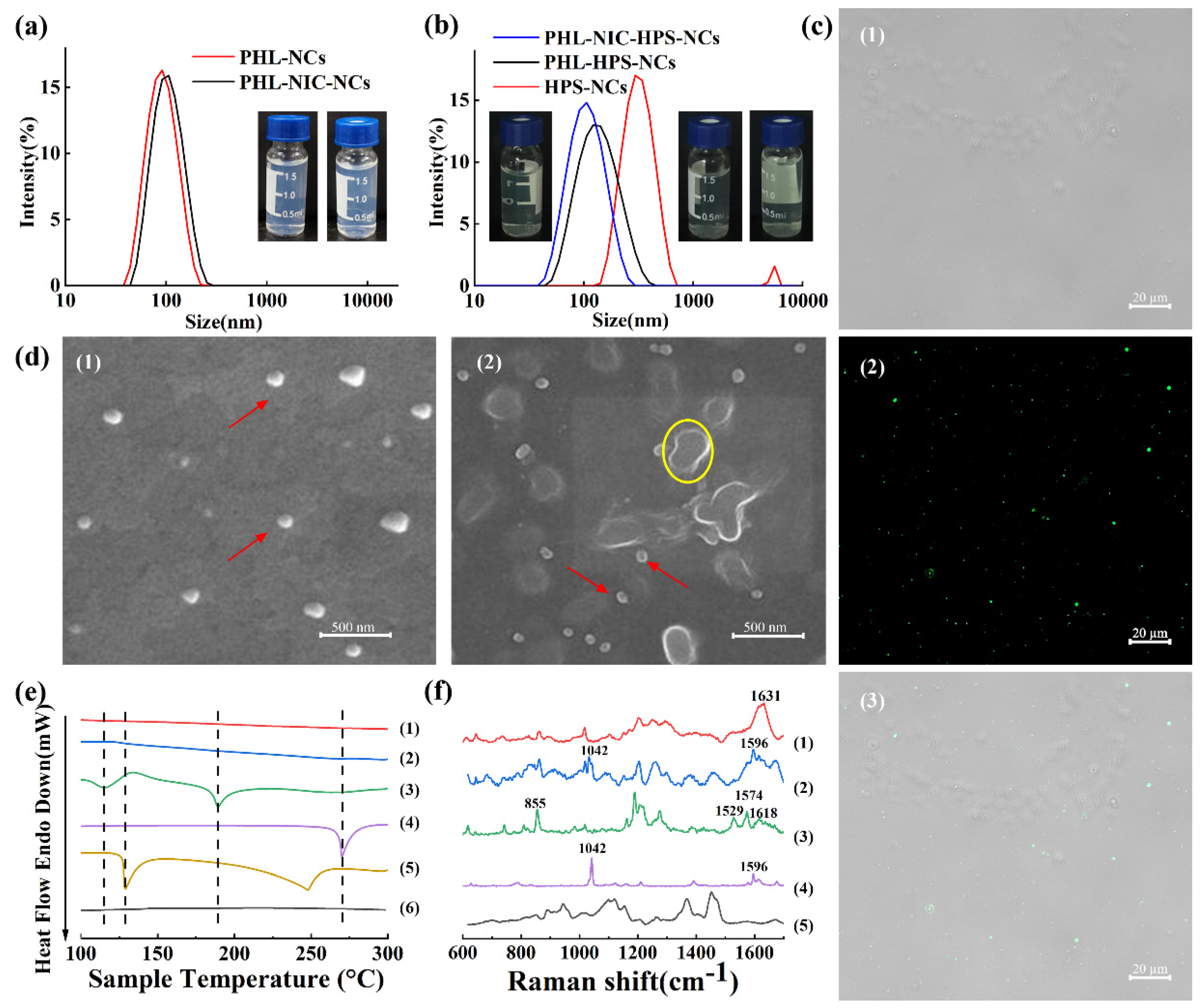
3.1. Characterization of NCs
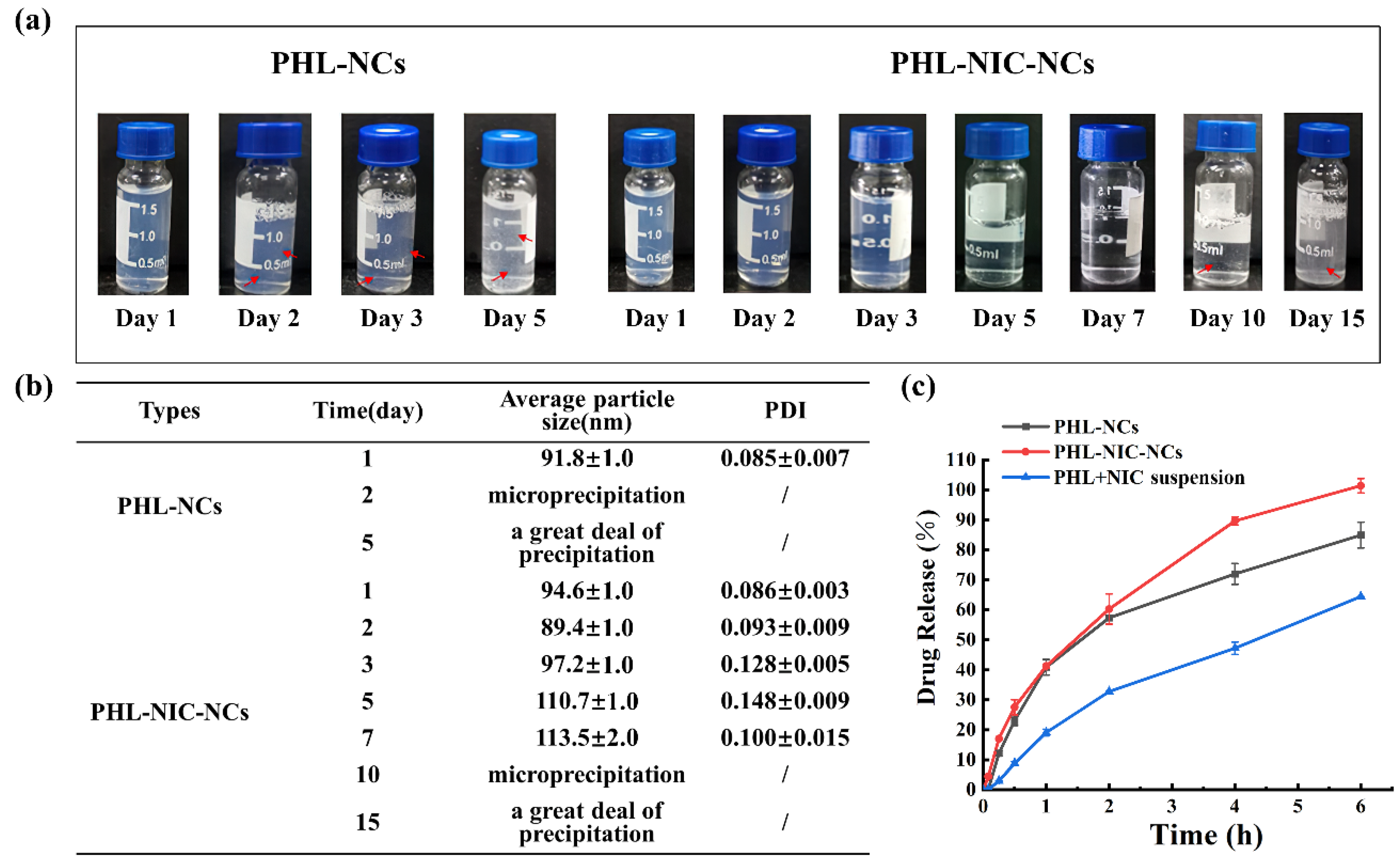
3.2. The Stability of NCs
3.3. In Vitro Drug Release Study
3.4. Effect of NCs on Melanin Synthesis
3.4.1. Screening of Drug Concentrations
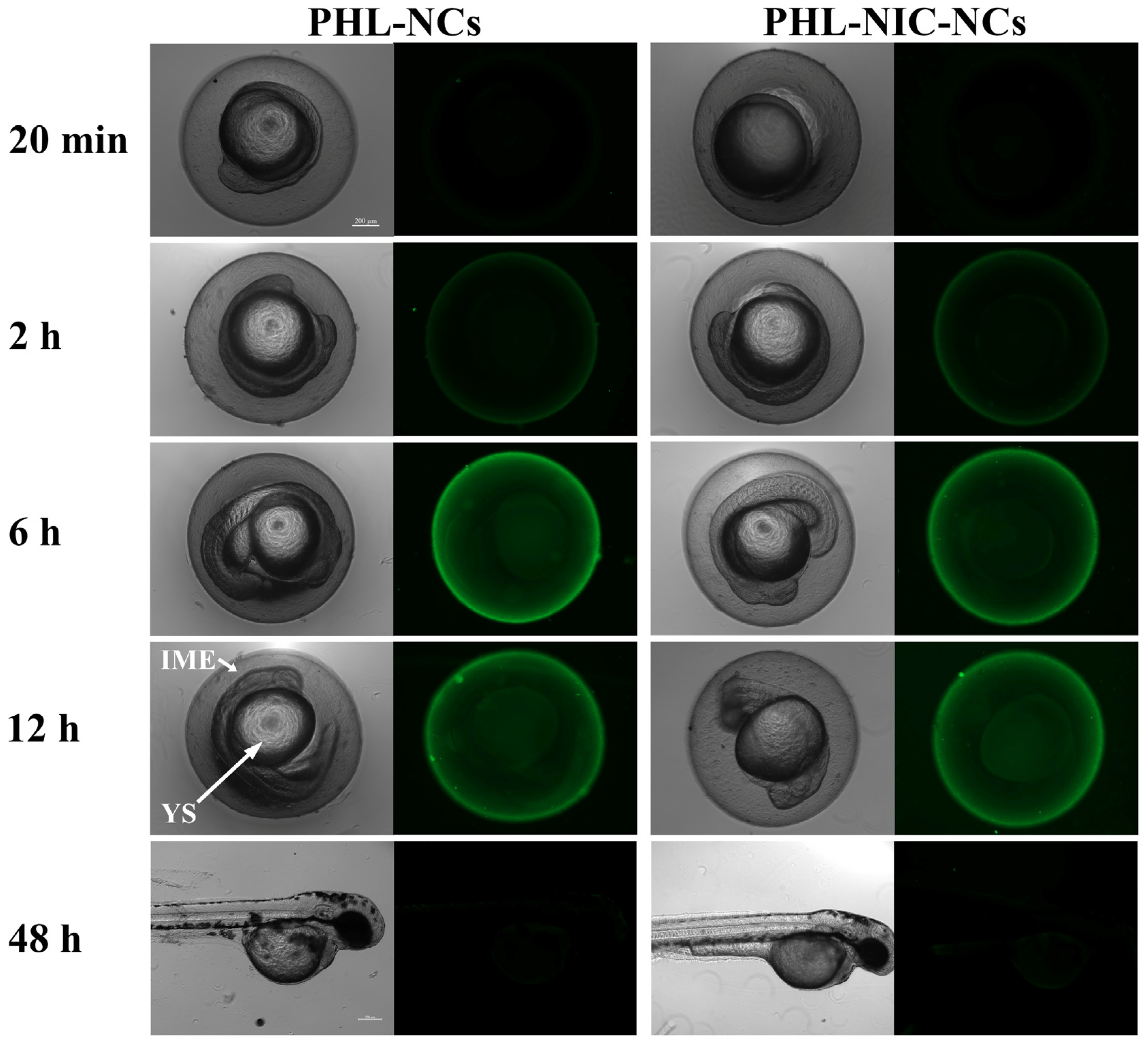
3.4.2. Transport of NCs in Embryonic Zebrafish In Vivo
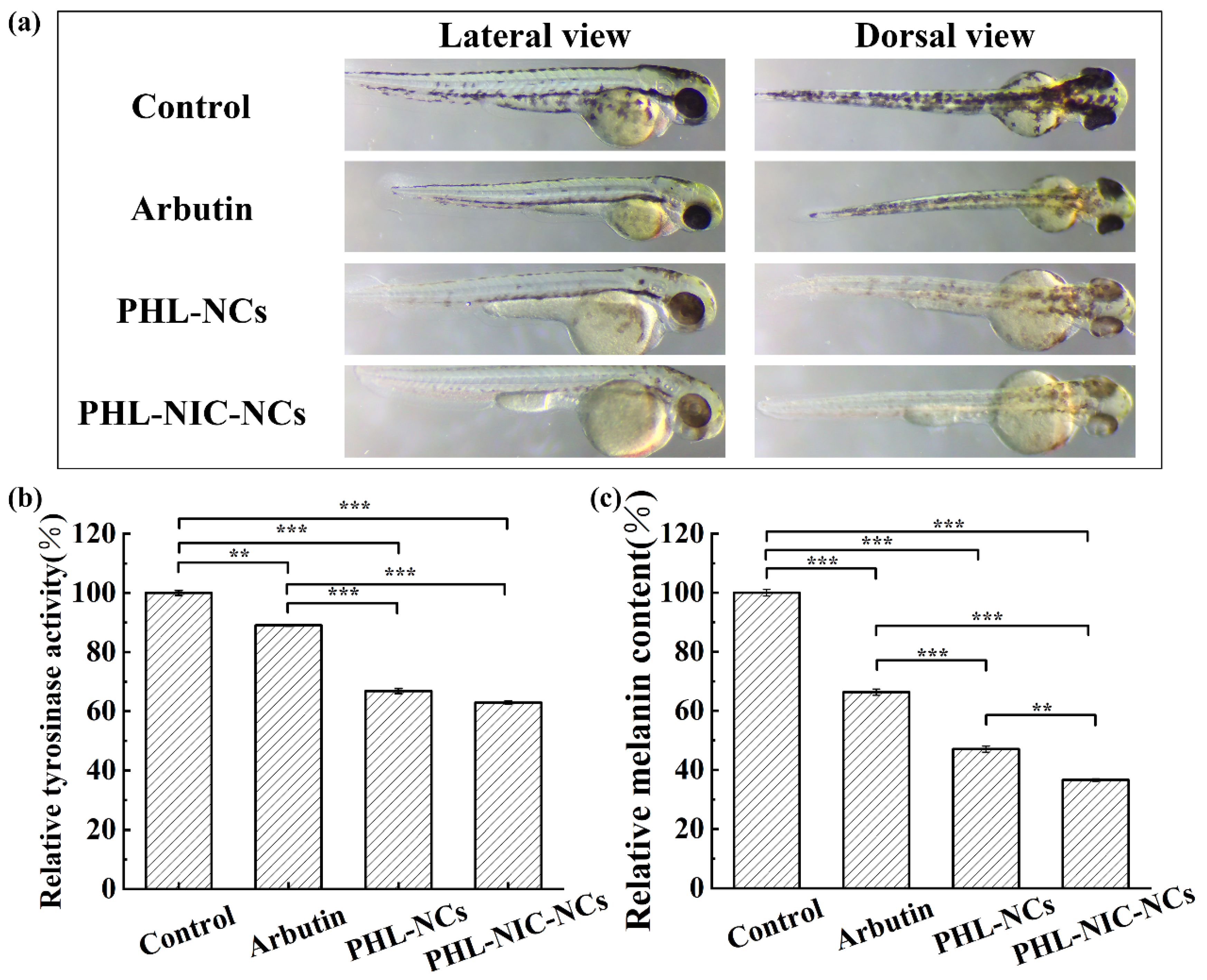
3.4.3. Influence of NCs on Tyrosinase Activity and Relative Melanin Content in Zebrafish Embryos
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPMC | Hydroxypropyl methylcellulose |
| NCs | Nanocrystals |
| PHL | Phloretin |
| NIC | Nicotinamide |
| HPS | 1,1,2,3,4,5-Hexaphenylsilole |
| PHL-NCs | Phloretin-nanocrystals |
| PHL-NIC-NCs | Phloretin-nicotinamide-nanocrystals |
| PHL-HPS-NCs | Phloretin-1,1,2,3,4,5-hexaphenylsilole-nanocrystals |
| PHL-NIC-HPS-NCs | Phloretin-nicotinamide-1,1,2,3,4,5-hexaphenylsilole- nanocrystals |
| PDI | Polydispersity index |
| SC | Stratum corneum |
| NPs | Nanoparticles |
| DDSs | Drug delivery systems |
| dpf | Days post-fertilization |
| hpf | Hours post-fertilization |
| AIE | Aggregation-induced emission |
| HPLC | High-performance liquid chromatography |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| PHL-NCs-L | Phloretin-nanocrystals-low |
| PHL-NCs-M | Phloretin-nanocrystals-medium |
| PHL-NCs-H | Phloretin-nanocrystals-high |
| IME | Inner mass of embryos |
| YS | Yolk sac |
| RPE | Retinal pigment epithelium |
References
- Cao, W.; Zhou, X.H.; McCallum, N.C.; Hu, Z.Y.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, X.T.; Jin, Z.K.; Guo, W.C.; Duan, G.G.; Liu, X.H.; Li, Y.W. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, G.W.; Yan, J.K.; Gong, D.M. Inhibitory effect of morin on tyrosinase: Insights from spectroscopic and molecular docking studies. Food Chem. 2014, 163, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef]
- Franco, D.C.Z.; de Carvalho, G.S.G.; Rocha, P.R.; Teixeira, R.D.; da Silva, A.D.; Raposo, N.R.B. Inhibitory Effects of Resveratrol Analogs on Mushroom Tyrosinase Activity. Molecules 2012, 17, 11816–11825. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.K.; Park, M.H.; Hyun, S.S.; Kahn, S.Y.; Joo, K.S.; Kang, H.C.; Kwon, W.T. In vivo anti-melanogenesis activity and in vitro skin permeability of niacinamide-loaded flexible liposomes (Bounsphere (TM)). J. Drug Delivery Sci. Technol. 2016, 31, 147–152. [Google Scholar] [CrossRef]
- Ephrem, E.; Elaissari, H.; Greige-Gerges, H. Improvement of skin whitening agents efficiency through encapsulation: Current state of knowledge. Int. J. Pharm. 2017, 526, 50–68. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Shin, S.; Kum, H.; Ryu, D.; Kim, M.; Jung, E.; Park, D. Protective Effects of a New Phloretin Derivative against UVB-Induced Damage in Skin Cell Model and Human Volunteers. Int. J. Mol. Sci. 2014, 15, 18919–18940. [Google Scholar] [CrossRef]
- Chen, J.M.; Li, Q.L.; Ye, Y.L.; Huang, Z.Y.; Ruan, Z.P.; Jin, N. Phloretin as both a substrate and inhibitor of tyrosinase: Inhibitory activity and mechanism. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117642. [Google Scholar] [CrossRef]
- Wei, L.N.; Zhao, J.; Meng, Y.H.; Guo, Y.R.; Luo, C.X. Antibacterial activity, safety and preservative effect of aminoethyl-phloretin on the quality parameters of salmon fillets. Lwt Food Sci. Technol. 2020, 118, 108874. [Google Scholar] [CrossRef]
- Hu, X.N.; Zhou, Z.R.; Han, L.J.; Li, S.J.; Zhou, W. Preparation and characterization of phloretin by complexation with cyclodextrins. New J. Chem. 2020, 44, 5218–5223. [Google Scholar] [CrossRef]
- Gu, L.Y.; Sun, R.; Wang, W.J.; Xia, Q. Nanostructured lipid carriers for the encapsulation of phloretin: Preparation and in vitro characterization studies. Chem. Phys. Lipids 2022, 242, 105150. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.J.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.H.; Ko, S.H.; Shim, J.S.; Kim, D.D.; Cho, H.J. Mussel-Inspired Hyaluronic Acid Derivative Nanostructures for Improved Tumor Targeting and Penetration. ACS Appl. Mater. Interfaces 2017, 9, 22308–22320. [Google Scholar] [CrossRef]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical nanocrystals: A promising approach for improved topical drug delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef]
- Park, K.C.; Huh, S.Y.; Choi, H.R.; Kim, D.S. Biology of melanogenesis and the search for hypopigmenting agents. Dermatol. Sin. 2010, 28, 53–58. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Ma, T.X.; Sha, X.M.; Zhang, L.; Tu, Z.C. Improving tyrosinase inhibitory activity of grass carp fish scale gelatin hydrolysate by gastrointestinal digestion: Purification, identification and action mechanism. Lwt Food Sci. Technol. 2022, 159, 113205. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigm. Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, Y.X.; Lei, X.L.; Liao, L.K.; Fu, T.K.; Yuan, Y.; Huang, X.B.; Zou, L.Q.; Liu, Y.H.; Ruan, R.; et al. Chemical composition and evaluation of antioxidant activities, antimicrobial, and anti-melanogenesis effect of the essential oils extracted from Dalbergia pinnata (Lour.) Prain. J. Ethnopharmacol. 2020, 254, 112731. [Google Scholar] [CrossRef]
- den Hertog, J. Chemical genetics: Drug screens in zebrafish. Biosci. Rep. 2005, 25, 289–297. [Google Scholar] [CrossRef]
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.R.; Love, D.R. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.W.; Hu, Y.T.; Hu, J.Y.; Chang, W.C.; Huang, Q.R.; Hsieh, S.C. Nanoemulsified adlay bran oil reduces tyrosinase activity and melanin synthesis in B16F10 cells and zebrafish. Food Sci. Nutr. 2019, 7, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Calienni, M.N.; Temprana, C.F.; Prieto, M.J.; Paolino, D.; Fresta, M.; Tekinay, A.B.; Alonso, S.D.; Montanari, J. Nano-formulation for topical treatment of precancerous lesions: Skin penetration, in vitro, and in vivo toxicological evaluation. Drug Deliv. Transl. Res. 2018, 8, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Chen, Q.X.; Wang, Q.; Song, K.K.; Wang, J.; Sha, L.; Guan, X. Inhibition of the activity of mushroom tyrosinase by alkylbenzoic acids. Food Chem. 2006, 94, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.M.; Su, W.C.; Li, C.; Shi, Y.; Chen, Q.X.; Zheng, J.; Tang, D.L.; Chen, S.M.; Wang, Q. Anti-melanogenesis of novel kojic acid derivatives in B16F10 cells and zebrafish. Int. J. Biol. Macromol. 2019, 123, 723–731. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, I.H.; Huang, X.Z.; Chen, H.J.; Chang, S.T.; Chang, M.L.; Ho, Y.T.; Chang, H.T. Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model. Pharmaceutics 2021, 13, 1059. [Google Scholar] [CrossRef]
- Tayier, N.; Qin, N.Y.; Zhao, L.N.; Zeng, Y.; Wang, Y.; Hu, G.; Wang, Y.Q. Theoretical Exploring of a Molecular Mechanism for Melanin Inhibitory Activity of Calycosin in Zebrafish. Molecules 2021, 26, 6998. [Google Scholar] [CrossRef]
- Park, J.J.; Hwang, S.J.; Kang, Y.S.; Jung, J.; Park, S.; Hong, J.E.; Park, Y.; Lee, H.J. Synthesis of arbutin-gold nanoparticle complexes and their enhanced performance for whitening. Arch. Pharm. Res. 2019, 42, 977–989. [Google Scholar] [CrossRef]
- Xu, H.X.; Li, X.F.; Xin, X.; Mo, L.; Zou, Y.C.; Zhao, G.L.; Yu, Y.G.; Chen, K.B. Antityrosinase Mechanism and Antimelanogenic Effect of Arbutin Esters Synthesis Catalyzed by Whole-Cell Biocatalyst. J. Agric. Food Chem. 2021, 69, 4243–4252. [Google Scholar] [CrossRef]
- Gao, W.; Lee, D.; Meng, Z.J.; Li, T.L. Exploring intracellular fate of drug nanocrystals with crystal-integrated and environment-sensitive fluorophores. J. Control. Release 2017, 267, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Teramura, T.; Terada, K. Detection of Cocrystal Formation Based on Binary Phase Diagrams Using Thermal Analysis. Pharm. Res. 2013, 30, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Govindammal, M.; Prasath, M.; Selvapandiyan, M. Spectroscopic (FT-IR, FT-Raman) investigations, quantum chemical calculations, ADMET and molecular docking studies of phloretin with B-RAF inhibitor. Chem. Pap. 2021, 75, 3771–3785. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Megharbel, S.M.; Hussien, M.A.; Hamza, R.Z.; Al-Omar, M.A.; Naglah, A.M.; Afifi, W.M.; Kobeasy, M.I. Spectroscopic, structural characterizations and antioxidant capacity of the chromium (III) niacinamide compound as a diabetes mellitus drug model. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 122–131. [Google Scholar] [CrossRef]
- Han, L.; Ma, S.; Jiang, S.; Zhang, Y.; He, Y.; Du, Z.; Zhang, K. Preparation and Characterization of Phloretin Solid Dispersions. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 10–13. [Google Scholar]
- Guo, M.R.; Dong, Y.J.; Wang, Y.Z.; Ma, M.C.; He, Z.G.; Fu, Q. Fabrication, characterization, stability and in vitro evaluation of nitrendipine nanocrystals by media milling. Powder Technol. 2019, 358, 20–28. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Peng, Y.Y.; Guo, M.S.; Cai, T. Facile Tuning of the Photoluminescence and Dissolution Properties of Phloretin through Cocrystallization. Cryst. Growth Des. 2019, 19, 6837–6844. [Google Scholar] [CrossRef]
- Li, Z.; Matzger, A.J. Influence of Coformer Stoichiometric Ratio on Pharmaceutical Cocrystal Dissolution: Three Cocrystals of Carbamazepine/4-Aminobenzoic Acid. Mol. Pharm. 2016, 13, 990–995. [Google Scholar] [CrossRef]
- Kaur, R.; Cavanagh, K.L.; Rodriguez-Hornedo, N.; Matzger, A.J. Multidrug Cocrystal of Anticonvulsants: Influence of Strong Intermolecular Interactions on Physiochemical Properties. Cryst. Growth Des. 2017, 17, 5012–5016. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Amano, Y.; Ohuchi, A.; Kitahara, T.; Takema, Y. The Essential Role of p53 in Hyperpigmentation of the Skin via Regulation of Paracrine Melanogenic Cytokine Receptor Signaling. J. Biol. Chem. 2009, 284, 4343–4353. [Google Scholar] [CrossRef]
- Chalupa, A.; Koshoffer, A.; Galan, E.; Yu, L.; Liu, F.T.; Boissy, R.E. Melanocytic Galectin-3 Is Associated with Tyrosinase-Related Protein-1 and Pigment Biosynthesis. J. Investig. Dermatol. 2015, 135, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Patamu, M.I.; Aerziguli, T.; Reshalaiti, A.W. Effects of Nicotinamide on Melanin Transportation in Human Skin Melanocytes. J. Environ. Health 2009, 26, 680–683. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiang, H.; Xue, X.; Chen, Y.; He, Z.; Yu, Z.; Zhang, L.; Miao, X. Dual Antimelanogenic Effect of Nicotinamide-Stabilized Phloretin Nanocrystals in Larval Zebrafish. Pharmaceutics 2022, 14, 1825. https://doi.org/10.3390/pharmaceutics14091825
Li Y, Xiang H, Xue X, Chen Y, He Z, Yu Z, Zhang L, Miao X. Dual Antimelanogenic Effect of Nicotinamide-Stabilized Phloretin Nanocrystals in Larval Zebrafish. Pharmaceutics. 2022; 14(9):1825. https://doi.org/10.3390/pharmaceutics14091825
Chicago/Turabian StyleLi, Yixuan, Hong Xiang, Xinyue Xue, Yilan Chen, Zhiyuan He, Zhongrui Yu, Li Zhang, and Xiaoqing Miao. 2022. "Dual Antimelanogenic Effect of Nicotinamide-Stabilized Phloretin Nanocrystals in Larval Zebrafish" Pharmaceutics 14, no. 9: 1825. https://doi.org/10.3390/pharmaceutics14091825









