Promising Strategies for Transdermal Delivery of Arthritis Drugs: Microneedle Systems
Abstract
:1. Introduction
2. Types of Arthritis
2.1. Rheumatoid Arthritis
2.2. Osteoarthritis
2.3. Gouty Arthritis
2.4. Other Arthritis
3. Drug Delivery Strategies for Arthritis
3.1. Oral Drugs
3.2. Injections
3.3. Transdermal Application
4. MN Drug Delivery System
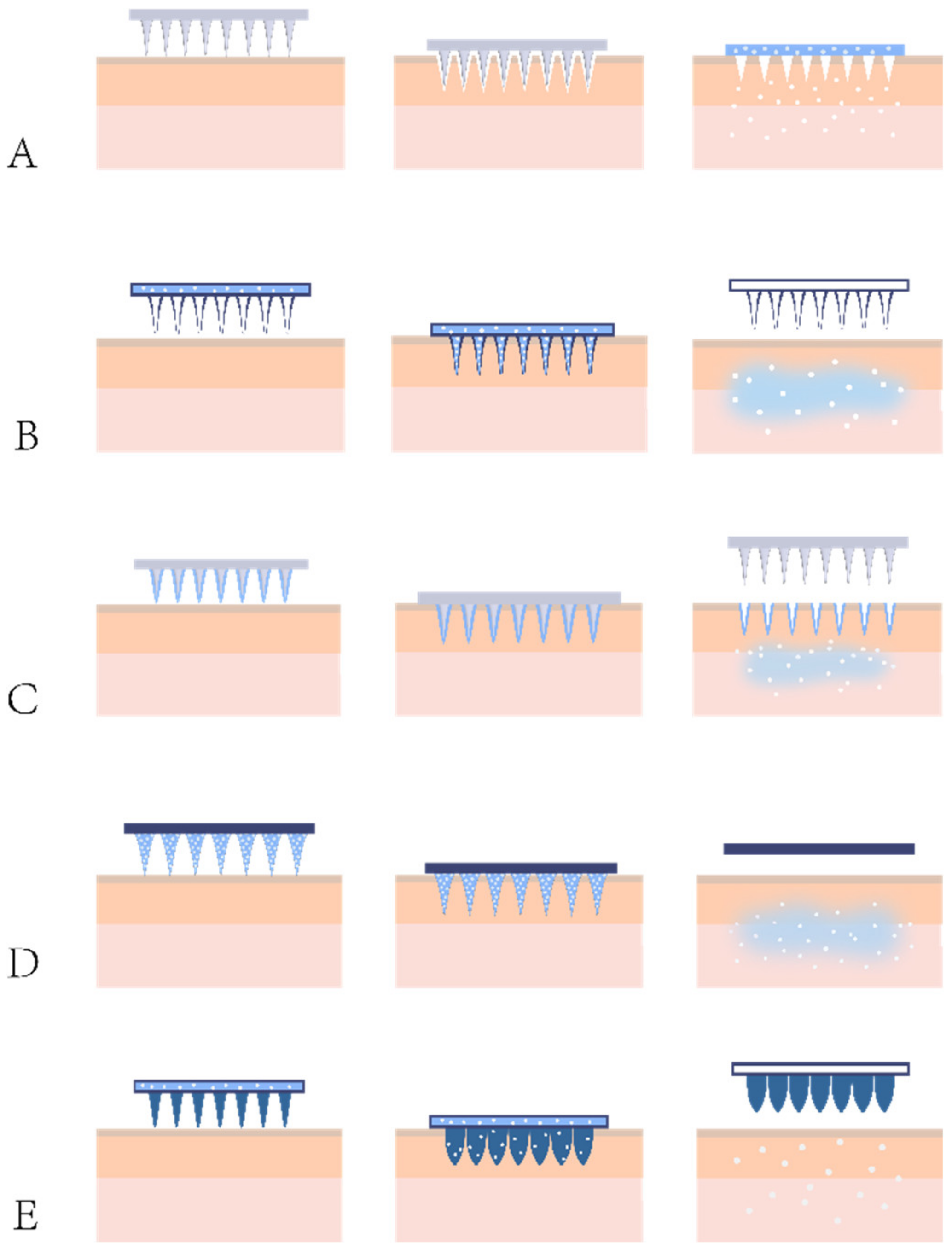
4.1. Types of MNs
4.1.1. Solid MNs
4.1.2. Hollow MNs
4.1.3. Coated MNs
4.1.4. Dissolving MNs
4.1.5. Hydrogel-Forming MNs
4.1.6. Other Novel MNs
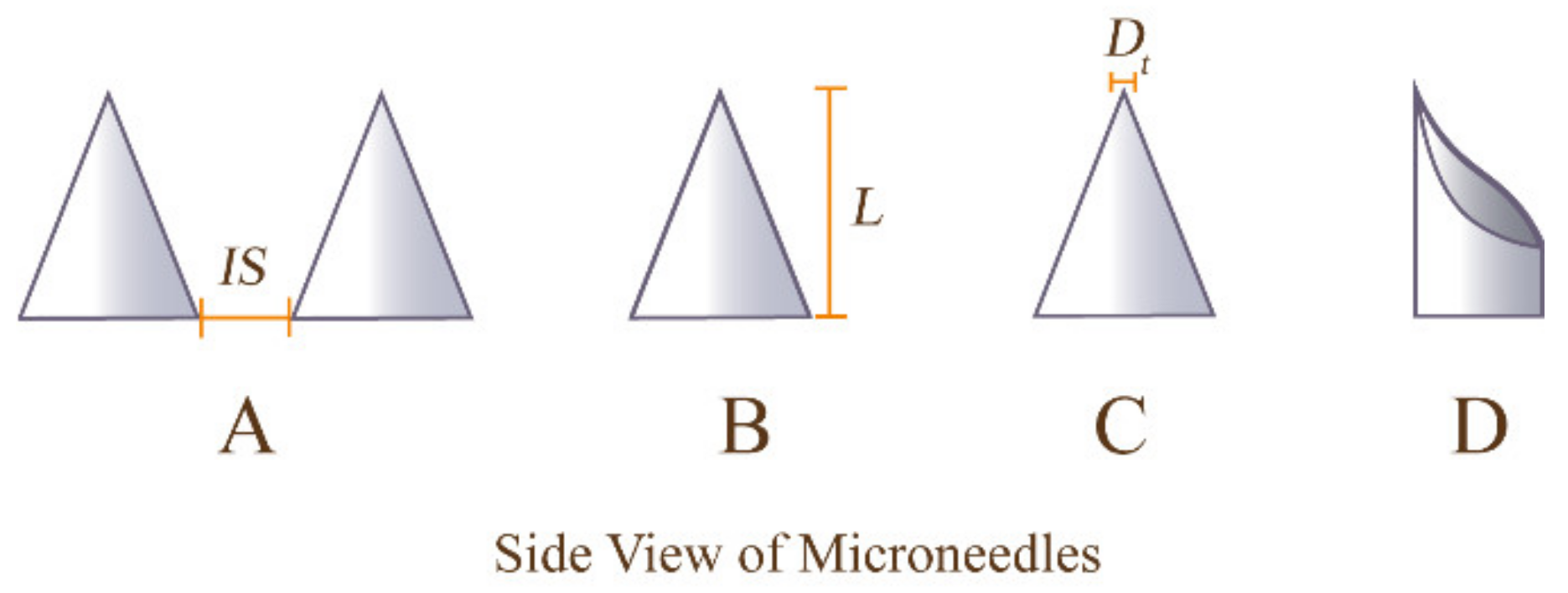
4.2. Requirements and Design of Geometry and Mechanical Strength of MNs
4.2.1. Geometry of MNs
4.2.2. Mechanical Strength of MNs
5. Recent Advancements of MNs in Arthritis Treatment
5.1. Solid MNs
5.2. Hollow MNs
5.3. Coated MNs
5.4. Dissolving MNs
5.5. Hydrogel-Forming MNs
6. Translation of MNs from Laboratory to Clinic and Market
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senthelal, S.; Li, J.; Goyal, A.; Bansal, P.; Thomas, M.A. Arthritis; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017; Institute for Health Metrics and Evaluation: Seattle, WA, USA, 2018. [Google Scholar]
- Park, J.; Mendy, A.; Vieira, E.R. Various Types of Arthritis in the United States: Prevalence and Age-Related Trends From 1999 to 2014. Am. J. Public Health 2018, 108, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Genovese, M.C.; Fleischmann, R.; Kivitz, A.; Lee, E.-B.; Van Hoogstraten, H.; Kimura, T.; St John, G.; Mangan, E.K.; Burmester, G.R. Efficacy and safety of sarilumab in combination with csDMARDs or as monotherapy in subpopulations of patients with moderately to severely active rheumatoid arthritis in three phase III randomized, controlled studies. Arthritis Res. Ther. 2020, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.-E.; Morel, J.; Perrodeau, E.; Bardin, T.; Combe, B.; Dougados, M.; Flipo, R.-M.; Saraux, A.; Schaeverbeke, T.; Sibilia, J.; et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: Prospective cohort study. BMJ 2019, 364, l67. [Google Scholar] [CrossRef]
- Papadopoulos, C.G.; Gartzonikas, I.K.; Pappa, T.K.; Markatseli, T.E.; Migkos, M.P.; Voulgari, P.V.; Drosos, A.A. Eight-year survival study of first-line tumour necrosis factor α inhibitors in rheumatoid arthritis: Real-world data from a university centre registry. Rheumatol. Adv. Pract. 2019, 3, rkz007. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Mahmood, A.; Waghule, T.; Gorantla, S.; Kumar Dubey, S.; Alexander, A.; Singhvi, G. Revisiting techniques to evaluate drug permeation through skin. Expert Opin. Drug Deliv. 2021, 18, 1829–1842. [Google Scholar] [CrossRef]
- Vanić, Z.; Holæter, A.M.; Skalko-Basnet, N. (Phospho)lipid-based Nanosystems for Skin Administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef]
- Shang, H.; Younas, A.; Zhang, N. Recent advances on transdermal delivery systems for the treatment of arthritic injuries: From classical treatment to nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1778. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Li, C.; Zhang, J.; Qin, L.; Lai, Y. Recent Advances of Microneedles and Their Application in Disease Treatment. Int. J. Mol. Sci. 2022, 23, 2401. [Google Scholar] [CrossRef]
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, M.J.; Das, D.B. Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, M.; Hutton, A.R.J.; Donnelly, R.F. Microneedle Mediated Transdermal Delivery of Protein, Peptide and Antibody Based Therapeutics: Current Status and Future Considerations. Pharm. Res. 2020, 37, 117. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Aghabegi Moghanjoughi, A.; Khoshnevis, D.; Zarrabi, A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Petlin, D.G.; Tverdokhlebov, S.I.; Anissimov, Y.G. Plasma treatment as an efficient tool for controlled drug release from polymeric materials: A review. J. Control. Release 2017, 266, 57–74. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef]
- Ross, R.F. Device for Delivery of Rheumatoid Arthritis Medication. U.S. Patent 9,522,263, 20 December 2016. [Google Scholar]
- Joint Disease: Arthritis in Patient Populations. Available online: https://www.boneandjointburden.org/fourth-edition/iiib0/joint-disease-arthritis-patient-populations (accessed on 5 April 2022).
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, itc1–itc16. [Google Scholar] [CrossRef]
- Almutairi, K.B.; Nossent, J.C.; Preen, D.B.; Keen, H.I.; Inderjeeth, C.A. The Prevalence of Rheumatoid Arthritis: A Systematic Review of Population-based Studies. J. Rheumatol. 2021, 48, 669–676. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Danks, L.; Komatsu, N.; Guerrini, M.M.; Sawa, S.; Armaka, M.; Kollias, G.; Nakashima, T.; Takayanagi, H. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 2016, 75, 1187–1195. [Google Scholar] [CrossRef]
- Wang, X.; Xia, S.; Fu, B. RNA-seq analysis of synovial fibroblasts in human rheumatoid arthritis. Mol. Med. Rep. 2014, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yoshida, K.; Nishizawa, T.; Otani, K.; Yamashita, Y.; Okabe, H.; Hadano, Y.; Kayama, T.; Kurosaka, D.; Saito, M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022, 23, 2871. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Keller, S.F.; Mandell, B.F. Management and Cure of Gouty Arthritis. Med. Clin. N. Am. 2021, 105, 297–310. [Google Scholar] [CrossRef]
- Punzi, L.; Scanu, A.; Galozzi, P.; Luisetto, R.; Spinella, P.; Scirè, C.A.; Oliviero, F. One year in review 2020: Gout. Clin. Exp. Rheumatol. 2020, 38, 807–821. [Google Scholar]
- Galozzi, P.; Bindoli, S.; Doria, A.; Oliviero, F.; Sfriso, P. Autoinflammatory Features in Gouty Arthritis. J. Clin. Med. 2021, 10, 1880. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Prakken, B.; Albani, S.; Martini, A. Juvenile idiopathic arthritis. Lancet 2011, 377, 2138–2149. [Google Scholar] [CrossRef]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef]
- Dougados, M.; Baeten, D. Spondyloarthritis. Lancet 2011, 377, 2127–2137. [Google Scholar] [CrossRef]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.K. Reactive Arthritis. Infect. Dis. Clin. N. Am. 2017, 31, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Gershwin, M.E. Diagnosis and classification of reactive arthritis. Autoimmun. Rev. 2014, 13, 546–549. [Google Scholar] [CrossRef]
- Cheeti, A.; Chakraborty, R.K.; Ramphul, K. Reactive Arthritis; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Salvo, F.; Fourrier-Réglat, A.; Bazin, F.; Robinson, P.; Riera-Guardia, N.; Haag, M.; Caputi, A.P.; Moore, N.; Sturkenboom, M.C.; Pariente, A.; et al. Cardiovascular and gastrointestinal safety of NSAIDs: A systematic review of meta-analyses of randomized clinical trials. Clin. Pharmacol. Ther. 2011, 89, 855–866. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Katturajan, R.; Vijayalakshmi, S.; Rasool, M.; Evan Prince, S. Molecular toxicity of methotrexate in rheumatoid arthritis treatment: A novel perspective and therapeutic implications. Toxicology 2021, 461, 152909. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Perpétuo, I.P.; Caetano-Lopes, J.; Rodrigues, A.M.; Campanilho-Marques, R.; Ponte, C.; Canhão, H.; Ainola, M.; Fonseca, J.E. Effect of Tumor Necrosis Factor Inhibitor Therapy on Osteoclasts Precursors in Rheumatoid Arthritis. Biomed. Res. Int. 2017, 2017, 2690402. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.M.; Chen, D.Y. Infection risk in patients undergoing treatment for inflammatory arthritis: Non-biologics versus biologics. Expert Rev. Clin. Immunol. 2020, 16, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D. New guidelines for topical NSAIDs in the osteoarthritis treatment paradigm. Curr. Med. Res. Opin. 2010, 26, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, X.; Li, W.; Chen, W.; Wang, X.; Ma, Z.; Lin, N. Tripterygium wilfordii: An inspiring resource for rheumatoid arthritis treatment. Med. Res. Rev. 2021, 41, 1337–1374. [Google Scholar] [CrossRef]
- Jiang, Q.; Tang, X.-P.; Chen, X.-C.; Xiao, H.; Liu, P.; Jiao, J. Will Chinese external therapy with compound Tripterygium wilfordii hook F gel safely control disease activity in patients with rheumatoid arthritis: Design of a double-blinded randomized controlled trial. BMC Complementary Altern. Med. 2017, 17, 444. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.; Lee, W.; Ren, L.; Liu, B.; Liang, L.; Wang, Z.; Jiang, L. Additive Manufacturing of Honeybee-Inspired Microneedle for Easy Skin Insertion and Difficult Removal. ACS Appl. Mater. Interfaces 2018, 10, 29338–29346. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Batra, U.; Puppala, E.R.; Waghule, T.; Naidu, V.G.M.; Singhvi, G. Emerging trends in microneedle-based drug delivery strategies for the treatment of rheumatoid arthritis. Expert Opin. Drug Deliv. 2022, 19, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Yang, G.; Gao, Y. Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles: rhIFNα-1b example. Asian J. Pharm. Sci. 2021, 16, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef]
- Singh, V.; Kesharwani, P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. J. Control. Release 2021, 338, 394–409. [Google Scholar] [CrossRef]
- Shende, P.; Salunke, M. Transepidermal microneedles for co-administration of folic acid with methotrexate in the treatment of rheumatoid arthritis. Biomed. Phys. Eng. Express 2019, 5, 025023. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Zhang, P.; Du, J.; Wang, Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J. Control. Release 2018, 286, 201–209. [Google Scholar] [CrossRef]
- Tas, C.; Joyce, J.C.; Nguyen, H.X.; Eangoor, P.; Knaack, J.S.; Banga, A.K.; Prausnitz, M.R. Dihydroergotamine mesylate-loaded dissolving microneedle patch made of polyvinylpyrrolidone for management of acute migraine therapy. J. Control. Release 2017, 268, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; He, P.; Zhao, J.; He, C.; Jiang, M.; Zhang, Z.; Zhang, Z.; Sun, X. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J. Control. Release 2021, 336, 537–548. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Niu, Y.; Li, Z.; Li, A.; Yang, H.; Xu, F.; Li, F. A Hydrogel Microneedle Patch for Point-of-Care Testing Based on Skin Interstitial Fluid. Adv. Healthc. Mater. 2020, 9, 1901201. [Google Scholar] [CrossRef]
- Al-Kasasbeh, R.; Brady, A.J.; Courtenay, A.J.; Larrañeta, E.; McCrudden, M.T.C.; O’Kane, D.; Liggett, S.; Donnelly, R.F. Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches. Drug Deliv. Transl. Res. 2020, 10, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Xu, S.-H.; Zhou, H.; Wang, X.; Dong, B.; Gao, H.; Tang, J.; Yang, Y.-W. pH and Glutathione Dual-Responsive Dynamic Cross-Linked Supramolecular Network on Mesoporous Silica Nanoparticles for Controlled Anticancer Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 28656–28664. [Google Scholar] [CrossRef]
- Di, J.; Yao, S.; Ye, Y.; Cui, Z.; Yu, J.; Ghosh, T.K.; Zhu, Y.; Gu, Z. Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots. ACS Nano 2015, 9, 9407–9415. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lin, Z.-W.; Ling, M.-H. Near-Infrared Light-Activatable Microneedle System for Treating Superficial Tumors by Combination of Chemotherapy and Photothermal Therapy. ACS Nano 2016, 10, 93–101. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, T.; Aoyagi, S. 3D laser lithographic fabrication of hollow microneedle mimicking mosquitos and its characterisation. Int. J. Nanotechnol. 2018, 15, 157–173. [Google Scholar] [CrossRef]
- Cho, W.K.; Ankrum, J.A.; Guo, D.; Chester, S.A.; Yang, S.Y.; Kashyap, A.; Campbell, G.A.; Wood, R.J.; Rijal, R.K.; Karnik, R.; et al. Microstructured barbs on the North American porcupine quill enable easy tissue penetration and difficult removal. Proc. Natl. Acad. Sci. USA 2012, 109, 21289–21294. [Google Scholar] [CrossRef]
- Finnin, B.C.; Morgan, T.M. Transdermal penetration enhancers: Applications, limitations, and potential. J. Pharm. Sci. 1999, 88, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, T.; Chujo, K.; Miyamoto, K.; Okida, M.; Nakamura, K.; Aoki, N.; Morita, R. Metal microneedle fabrication using twisted light with spin. Opt. Express 2010, 18, 17967–17973. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.G.; Lee, T.W.; Oh, D.J.; Hwang, S.J.; Jung, I.; Lee, S.; Ko, J. Nickel microneedles fabricated by sequential copper and nickel electroless plating and copper chemical wet etching. Sens. Mater 2008, 20, 45–53. [Google Scholar]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C 2019, 103, 109717. [Google Scholar] [CrossRef]
- Lee, W.-J.; Han, M.-R.; Kim, J.-S.; Park, J.-H. A tearable dissolving microneedle system for shortening application time. Expert Opin. Drug Deliv. 2019, 16, 199–206. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally Invasive Protein Delivery with Rapidly Dissolving Polymer Microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Kahkoska, A.R.; Gu, Z. Bioresponsive transcutaneous patches. Curr. Opin. Biotechnol. 2017, 48, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Yonghang, C.; Xinfang, L.; Weijiang, Y.; Youxiang, W. Stimuli-Responsive Polymeric Microneedles for Transdermal Drug Delivery. Prog. Chem. 2021, 33, 1152. [Google Scholar] [CrossRef]
- Shoffstall, A.J.; Srinivasan, S.; Willis, M.; Stiller, A.M.; Ecker, M.; Voit, W.E.; Pancrazio, J.J.; Capadona, J.R. A Mosquito Inspired Strategy to Implant Microprobes into the Brain. Sci. Rep. 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.J.; Shi, L.T.; Wu, C.W. Biomechanical Property of a Natural Microneedle: The Caterpillar Spine. J. Med. Devices 2011, 5, 034502. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Yue, X.; Zhang, J.; Huang, C.; Zhao, S.; Wu, A.; Li, X.; Qu, Y.; Zhang, C. Strategy for osteoarthritis therapy: Improved the delivery of triptolide using liposome-loaded dissolving microneedle arrays. Int. J. Pharm. 2021, 609, 121211. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Dong, Z.; Chen, Y.; Zhao, W.; Wang, Y.; Zhang, L.; Chen, M.; Wu, C.; Wang, Q. Dissolving Microneedle Arrays with Optimized Needle Geometry for Transcutaneous Immunization. Eur. J. Pharm. Sci. 2020, 151, 105361. [Google Scholar] [CrossRef]
- Khann, P.; Silv, H.; Bhansali, S. Variation in microneedle geometry to increase shear strength. Procedia Eng. 2010, 5, 977–980. [Google Scholar] [CrossRef]
- Sabri, A.H.; Kim, Y.; Marlow, M.; Scurr, D.J.; Segal, J.; Banga, A.K.; Kagan, L.; Lee, J.B. Intradermal and transdermal drug delivery using microneedles–Fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 2020, 153, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Dijksman, J.A.; van Hecke, M.; Verpoorten, H.; van den Berg, A.; Luttge, R.; Bouwstra, J.A. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J. Control. Release 2008, 128, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Mansor, N.H.A.; Markom, M.A.; Tan, E.S.M.M.; Adom, A.H. Design and Fabrication of Biodegradable Microneedle Using 3D Rapid Prototyping Printer. J. Phys. Conf. Ser. 2019, 1372, 012053. [Google Scholar] [CrossRef]
- Martanto, W.; Moore, J.S.; Couse, T.; Prausnitz, M.R. Mechanism of fluid infusion during microneedle insertion and retraction. J. Control. Release 2006, 112, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.J.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, Optimization and Characterisation of Polymeric Microneedle Arrays Prepared by a Novel Laser-Based Micromoulding Technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef]
- Żuber, Z.; Owczarek, A.; Sobczyk, M.; Migas-Majoch, A.; Turowska-Heydel, D.; Sternal, A.; Michalczak, J.; Chudek, J. Establishing percentile charts for hip joint capsule and synovial cavity thickness in apparently healthy children. Pediatric Rheumatol. 2017, 15, 8. [Google Scholar] [CrossRef]
- Sharma, M. Chapter 18-Transdermal and Intravenous Nano Drug Delivery Systems: Present and Future. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–550. [Google Scholar]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Yung, K.L.; Xu, Y.; Kang, C.; Liu, H.; Tam, K.; Ko, S.; Kwan, F.; Lee, T.M. Sharp tipped plastic hollow microneedle array by microinjection moulding. J. Micromech. Microeng. 2011, 22, 015016. [Google Scholar] [CrossRef]
- Wang, P.; Wester, B.A.; Rajaraman, S.; Paik, S.; Kim, S.; Allen, M.G. Hollow polymer microneedle array fabricated by photolithography process combined with micromolding technique. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 7026–7029. [Google Scholar]
- Yadav, V.; Sharma, P.K.; Murty, U.S.; Mohan, N.H.; Thomas, R.; Dwivedy, S.K.; Banerjee, S. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int. J. Pharm. 2021, 605, 120815. [Google Scholar] [CrossRef]
- Römgens, A.M.; Bader, D.L.; Bouwstra, J.A.; Baaijens, F.P.T.; Oomens, C.W.J. Monitoring the penetration process of single microneedles with varying tip diameters. J. Mech. Behav. Biomed. Mater. 2014, 40, 397–405. [Google Scholar] [CrossRef]
- Xenikakis, I.; Tsongas, K.; Tzimtzimis, E.K.; Zacharis, C.K.; Theodoroula, N.; Kalogianni, E.P.; Demiri, E.; Vizirianakis, I.S.; Tzetzis, D.; Fatouros, D.G. Fabrication of hollow microneedles using liquid crystal display (LCD) vat polymerization 3D printing technology for transdermal macromolecular delivery. Int. J. Pharm. 2021, 597, 120303. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The Effects of Geometry on Skin Penetration and Failure of Polymer Microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Tahk, D.; Yu, J.; Min, D.-H.; Jeon, N.L. Design rules for a tunable merged-tip microneedle. Microsyst. Nanoeng. 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef]
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotechnol. 2021, 12, 1034–1046. [Google Scholar] [CrossRef]
- Cao, J.; Su, J.; An, M.; Yang, Y.; Zhang, Y.; Zuo, J.; Zhang, N.; Zhao, Y. Novel DEK-Targeting Aptamer Delivered by a Hydrogel Microneedle Attenuates Collagen-Induced Arthritis. Mol. Pharm. 2021, 18, 305–316. [Google Scholar] [CrossRef]
- Kathuria, H.; Lim, D.; Cai, J.; Chung, B.G.; Kang, L. Microneedles with Tunable Dissolution Rate. ACS Biomater. Sci. Eng. 2020, 6, 5061–5068. [Google Scholar] [CrossRef]
- Chew, S.W.T.; Zeng, Y.; Cui, M.; Chang, H.; Zheng, M.; Wei, S.; Zhao, W.; Xu, C. In Situ Generation of Zinc Oxide Nanobushes on Microneedles as Antibacterial Coating. SLAS Technol. 2019, 24, 181–187. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Wei, S.; Zhang, L.; Zong, S. Development and evaluation of tofacitinib transdermal system for the treatment of rheumatoid arthritis in rats. Drug Dev. Ind. Pharm. 2021, 47, 878–886. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Chen, W.; Wang, C.; Yan, L.; Huang, L.; Zhu, X.; Chen, B.; Sant, H.J.; Niu, X.; Zhu, G.; Yu, K.N.; et al. Improved polyvinylpyrrolidone microneedle arrays with non-stoichiometric cyclodextrin. J. Mater. Chem. B 2014, 2, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Wang, M.; Yang, X.; Tang, Y.; Pang, M.; Wang, W.; Chen, L.; Wu, C.; Xu, Y. Microneedles mediated bioinspired lipid nanocarriers for targeted treatment of alopecia. J. Control. Release 2021, 329, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fakhraei Lahiji, S.; Jang, Y.; Huh, I.; Yang, H.; Jang, M.; Jung, H. Exendin-4–encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 2018, 8, 1170. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, F.; Liu, J.; Fan, G.; Welsh, W.; Zhu, H.; Jin, T. Phase-Transition Microneedle Patches for Efficient and Accurate Transdermal Delivery of Insulin. Adv. Funct. Mater. 2015, 25, 4633–4641. [Google Scholar] [CrossRef]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar] [CrossRef]
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.; Vora, L.; Larrañeta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef]
- So, J.-W.; Park, H.-H.; Lee, S.S.; Kim, D.-C.; Shin, S.-C.; Cho, C.-W. Effect of microneedle on the pharmacokinetics of ketoprofen from its transdermal formulations. Drug Deliv. 2009, 16, 52–56. [Google Scholar] [CrossRef]
- Abla, M.J.; Chaturvedula, A.; O’Mahony, C.; Banga, A.K. Transdermal delivery of methotrexate for pediatrics using silicon microneedles. Ther. Deliv. 2013, 4, 543–551. [Google Scholar] [CrossRef]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bai, J.; Lu, Y.; Du, S.; Shang, K.; Li, P.; Yang, L.; Dong, B.; Tan, N. Anti-arthritic effects of microneedling with bee venom gel. J. Tradit. Chin. Med. Sci. 2016, 3, 256–262. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Y.; Li, Z.; Zhao, J.; Feng, N. Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Mo, Y.; Zhang, Q.; Tian, W.; Xue, Y.; Bai, J.; Du, S. Microneedle-Assisted Percutaneous Delivery of Paeoniflorin-Loaded Ethosomes. Molecules 2018, 23, 3371. [Google Scholar] [CrossRef]
- Huang, J.; Cui, Y.; Yang, Y.; Li, H.; Zhang, Y.; Yang, H.; Du, S.; Bai, J. Optical Coherence Tomography and Microdialysis for Microneedle-Mediated Penetration Enhancement Study of Paeoniflorin-Loaded Ethosomes. Skin Pharmacol. Phys. 2021, 34, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bushra, F. Delivery of Denosumab via Hollow Microneedle. Doctoral Dissertation, Brac University, Dhaka, Bangladesh, 2020. [Google Scholar]
- Cárcamo-Martínez, Á.; Mallon, B.; Anjani, Q.K.; Domínguez-Robles, J.; Utomo, E.; Vora, L.K.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Enhancing intradermal delivery of tofacitinib citrate: Comparison between powder-loaded hollow microneedle arrays and dissolving microneedle arrays. Int. J. Pharm. 2021, 593, 120152. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Yang, Y.; Friden, P.M.; Banga, A.K. Synergistic effect of iontophoresis and soluble microneedles for transdermal delivery of methotrexate. J. Pharm. Pharmacol. 2010, 60, 27–33. [Google Scholar] [CrossRef]
- Korkmaz, E.; Friedrich, E.E.; Ramadan, M.H.; Erdos, G.; Mathers, A.R.; Burak Ozdoganlar, O.; Washburn, N.R.; Falo, L.D. Therapeutic intradermal delivery of tumor necrosis factor-alpha antibodies using tip-loaded dissolvable microneedle arrays. Acta Biomater. 2015, 24, 96–105. [Google Scholar] [CrossRef]
- Dangol, M.; Yang, H.; Li, C.G.; Lahiji, S.F.; Kim, S.; Ma, Y.; Jung, H. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J. Control. Release 2016, 223, 118–125. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, C.; Zhang, S.; Yang, G.; He, M.; Gao, Y. Systemic delivery of artemether by dissolving microneedles. Int. J. Pharm. 2016, 508, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Gui, S.; Wu, X.; Chen, L.; Cao, Y.; Yin, D.; Ma, P. Sinomenine hydrochloride-loaded dissolving microneedles enhanced its absorption in rabbits. Pharm. Dev. Technol. 2016, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Amodwala, S.; Kumar, P.; Thakkar, H.P. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: A patient friendly approach to manage arthritis. Eur. J. Pharm. Sci. 2017, 104, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, N.; Wang, Z.; Su, J.; Yang, J.; Han, J.; Zhao, Y. Microneedle-Assisted Transdermal Delivery of Etanercept for Rheumatoid Arthritis Treatment. Pharmaceutics 2019, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Tao, C.; Zou, J.; Zheng, H.; Zhu, J.; Zhu, Z.; Zhu, J.; Liu, L.; Li, F.; Song, X. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: A biocomfortable attempt to treat Rheumatoid Arthritis. Int. J. Pharm. 2019, 563, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Cheng, N.; Zhao, J.; Hou, X.; Zhang, Y.; Feng, N. Novel nanostructured lipid carriers-loaded dissolving microneedles for controlled local administration of aconitine. Int. J. Pharm. 2019, 572, 118741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, B.; Liao, L.; Hu, X.; Hu, Q.; Gao, Y.; Qiu, Y. Enhanced transdermal delivery of polydatin via a combination of inclusion complexes and dissolving microneedles for treatment of acute gout arthritis. J. Drug Deliv. Sci. Technol. 2020, 55, 101487. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, J.; Li, W.; Yang, L.; Dong, H.; Zhang, X. Programmable Polymeric Microneedles for Combined Chemotherapy and Antioxidative Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 55559–55568. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Chen, H.; Jin, Y.; Wang, Z.; Lu, Y.; Wang, Y. Dosage-efficacy relationship and pharmacodynamics validation of brucine dissolving microneedles against rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2021, 63, 102537. [Google Scholar] [CrossRef]
- Yu, K.; Yu, X.; Cao, S.; Wang, Y.; Zhai, Y.; Yang, F.; Yang, X.; Lu, Y.; Wu, C.; Xu, Y. Layered dissolving microneedles as a need-based delivery system to simultaneously alleviate skin and joint lesions in psoriatic arthritis. Acta Pharm. Sin. B 2021, 11, 505–519. [Google Scholar] [CrossRef]
- Hu, H.; Ruan, H.; Ruan, S.; Pei, L.; Jing, Q.; Wu, T.; Hou, X.; Xu, H.; Wang, Y.; Feng, N.; et al. Acid-responsive PEGylated branching PLGA nanoparticles integrated into dissolving microneedles enhance local treatment of arthritis. Chem. Eng. J. 2022, 431, 134196. [Google Scholar] [CrossRef]
- Liao, L.; Hu, Y.; Liao, S.; Chen, Z.; Hu, Q.; Guo, B.; Qiu, Y. Mixed Micelles Loaded Dissolving Microneedles for Enhanced and Sustained Transdermal Delivery of Indomethacin. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Cao, Y.; Tao, Y.; Zhou, Y.; Gui, S. Development of sinomenine hydrochloride-loaded polyvinylalcohol/maltose microneedle for transdermal delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Tekko, I.; Donnelly, R.; McCarthy, H.; McElnay, J.; Taggart, C.; Rooney, M. Delivering methotrexate transdermally for treatment of Juvenile Idiopathic Arthritis employing novel PVA-based hydrogel-forming microneedles: In-vitro studies. Rheumatology 2018, 56, kex390.008. [Google Scholar] [CrossRef]
- Tekko, I.A.; Chen, G.; Donnelly, R.F.; McElnay, J.; McCarthy, H.; Rooney, M. P40 Novel transdermal delivery system for methotrexate to treat juvenile idiopathic arthritis: No pain, only gain. Rheumatology 2018, 57, key273.042. [Google Scholar] [CrossRef]
- He, Y.; Majid, K.; Maqbool, M.; Hussain, T.; Yousaf, A.M.; Khan, I.U.; Mehmood, Y.; Aleem, A.; Arshad, M.S.; Younus, A.; et al. Formulation and characterization of lornoxicam-loaded cellulosic-microsponge gel for possible applications in arthritis. Saudi. Pharm. J. 2020, 28, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Vučen, S.R.; Vuleta, G.; Crean, A.M.; Moore, A.C.; Ignjatović, N.; Uskoković, D. Improved percutaneous delivery of ketoprofen using combined application of nanocarriers and silicon microneedles. J. Pharm. Pharmacol. 2013, 65, 1451–1462. [Google Scholar] [CrossRef]
- Hu, Q.; Zhong, X.; Tian, H.; Liao, P. The Efficacy of Denosumab in Patients With Rheumatoid Arthritis: A Systematic Review and Pooled Analysis of Randomized or Matched Data. Front. Immunol. 2021, 12, 799575. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Abdalla, H.B.; Jain, A.K.; Napimoga, M.H.; Clemente-Napimoga, J.T.; Gill, H.S. Microneedles Coated with Tramadol Exhibit Antinociceptive Effect in a Rat Model of Temporomandibular Hypernociception. J. Pharmacol. Exp. Ther. 2019, 370, 834–842. [Google Scholar] [CrossRef]
- Saha, I.; Rai, V.K. Hyaluronic acid based microneedle array: Recent applications in drug delivery and cosmetology. Carbohydr. Polym. 2021, 267, 118168. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Banga, A.K. Electrically Assisted Transdermal and Topical Drug Delivery; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Donnelly, R.F.; Singh, T.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; McAlister, E.; Courtenay, A.J.; González-Vázquez, P.; Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.L.B.; Shanmugam, S.; Santos, L.N.S.; Campos, C.d.A.; Santos, A.M.; Batista, M.S.; Araujo, A.A.d.S.; Serafini, M.R. Microneedles as an alternative technology for transdermal drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2020, 30, 433–452. [Google Scholar] [CrossRef]
- Kim, S.; Yang, H.; Eum, J.; Ma, Y.; Fakhraei Lahiji, S.; Jung, H. Implantable powder-carrying microneedles for transdermal delivery of high-dose insulin with enhanced activity. Biomaterials 2020, 232, 119733. [Google Scholar] [CrossRef]
- Ripolin, A.; Quinn, J.; Larrañeta, E.; Vicente-Perez, E.M.; Barry, J.; Donnelly, R.F. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 2017, 521, 92–101. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Soon, W.J.; Choi, J.; Zou, S.; Kang, L. Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J. Pharm. Sci. 2013, 102, 4100–4108. [Google Scholar] [CrossRef]
- Jacobse, J.; Ten Voorde, W.; Tandon, A.; Romeijn, S.G.; Grievink, H.W.; van der Maaden, K.; van Esdonk, M.J.; Moes, D.J.A.R.; Loeff, F.; Bloem, K.; et al. Comprehensive evaluation of microneedle-based intradermal adalimumab delivery vs. subcutaneous administration: Results of a randomized controlled clinical trial. Br. J. Clin. Pharmacol. 2021, 87, 3162–3176. [Google Scholar] [CrossRef]
- Creelman, B.; Frivold, C.; Jessup, S.; Saxon, G.; Jarrahian, C. Manufacturing readiness assessment for evaluation of the microneedle array patch industry: An exploration of barriers to full-scale manufacturing. Drug Deliv. Transl. Res. 2022, 12, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Spierings, E.L.; Brandes, J.L.; Kudrow, D.B.; Weintraub, J.; Schmidt, P.C.; Kellerman, D.J.; Tepper, S.J. Randomized, double-blind, placebo-controlled, parallel-group, multi-center study of the safety and efficacy of ADAM zolmitriptan for the acute treatment of migraine. Cephalalgia 2018, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
| Type of Arthritis | Patient Age Group | Cause |
|---|---|---|
| RA | All ages | Synovitis caused by immune system diseases |
| OA | Over 60 years | Degenerative lesions with articular cartilage damage |
| GA | Over 40 years | Hyperuricemia and the deposition of MSU crystals in the joint capsule, bursa, cartilage, bone, and other tissues |
| JIA | Under 16 | Unknown |
| PSA | All ages | Psoriasis |
| AS | 10 to 40 years | Heredity (the abnormality of HLA-B27) |
| ReA | 20 to 40 years | Gastrointestinal or genitourinary tract infection |
| Drug Classification | Active Pharmaceutical Ingredient | Type | Indications | Route |
|---|---|---|---|---|
| NSAIDs | Acetylsalicylic acid, Celecoxib, Choline magnesium trisalicylate, Diflunisal, Etodolac, Etoricoxib, Fenbufen, Nabumetone, Oxaprozin, Sulindac, Tiaprofenic acid, Tolmetin, Valdecoxib | Small-molecule | Arthritis | PO |
| Benzydamine, Bufexamac, Etofenamate, Flufenamic acid, Salicylic acid | Topical | |||
| Dexketoprofen, Flurbiprofen, Ibuprofen, Ketoprofen, Meclofenamic acid, Niflumic acid | PO/Topical | |||
| Aceclofenac, Diclofenac, Indomethacin, Naproxen, Piroxicam | PO/IM/Topical | |||
| Lornoxicam, Tenoxicam | PO/IM/IV | |||
| Meloxicam | PO/IM/IV/Topical | |||
| Corticosteroid | Prednisone, Cortisone acetate | PO | ||
| Betamethasone, Hydrocortisone | Topical | |||
| Prednisolone | PO/Topical | |||
| Hydrocortisone succinate | IM/IV | |||
| Methylprednisolone | PO/IV/SC/Intra-articular | |||
| Dexamethasone | PO/IV/IM/Intra-articular/Topical | |||
| Triamcinolone | PO/Intra-articular/IM/Topical | |||
| Analgesic drug | Fenoprofen | PO | ||
| Capsaicin | Topical | |||
| Acetaminophen | PO/IV | |||
| Thiocolchicoside | PO/IM/Topical | |||
| Conventional synthetic DMARDs | Auranofin, Chloroquine, Hydroxychloroquine, Mycophenolate mofetil, Penicillamine | RA | PO | |
| Azathioprine, Cyclosporine | RA/PSA | PO/IV | ||
| Methotrexate | RA/JIA/ PSA | PO/IV/IM/SC/Intra-articular | ||
| Sulfasalazine | RA/JIA/ PSA | PO | ||
| Leflunomide | RA/GA/ JIA/PSA | PO | ||
| Sodium aurothiomalate | RA/OA/ JIA/PSA | IM | ||
| Calcineurin inhibitor | Tacrolimus | RA | PO/Topical | |
| JAK inhibitor | Tofacitinib | RA/PSA | PO | |
| Baricitinib | RA | PO | ||
| Upadacitinib | RA/PSA/AS | PO | ||
| Supplements | Chondroitin sulfate | OA | PO | |
| Glucosamine | OA | PO/IM | ||
| Hyaluronic acid | OA | Intra-articular | ||
| Curcumin | Arthritis | PO/Topical | ||
| TNF inhibitor | Adalimumab | Protein | RA/JIA/ PSA/AS | SC |
| Certolizumab pegol | RA/JIA/ PSA/AS | SC | ||
| Etanercept | RA/OA/ JIA/PSA/AS | SC | ||
| Golimumab | RA/PSA/AS | SC/IV | ||
| Infliximab | RA/OA/ PSA/JIA/AS | IV/Intra-articular | ||
| T-cell inhibitor | Abatacept | RA/PSA/ JIA | SC/IV | |
| B-cell inhibitor | Rituximab | RA | SC/IV | |
| IL 1 inhibitor | Sarilumab | RA | SC | |
| Anakinra | RA/OA/ GA /JIA | SC/Intra-articular | ||
| Canakinumab | RA/GA/ JIA | Intra-articular | ||
| IL 6R inhibitor | Tocilizumab | RA/JIA | SC/IV | |
| IL 12\23 inhibitor | Ustekinumab | PSA | SC | |
| IL-17A inhibitor | Secukinumab | PSA | SC | |
| IL 23 inhibitor | Risankizumab | PSA | SC | |
| Ixekizumab | PSA | SC | ||
| RANKL inhibitor | Denosumab | OA | SC |
| Types of MNs | Advantages | Disadvantages | Research Stages | Ref. |
|---|---|---|---|---|
| Solid MNs | Simple to manufacture. High mechanical strength. | It may break when inserted into the skin, resulting in part of the MNs remaining on the skin after removing the MNs, causing invisible damage. Two-step dosing, slightly cumbersome steps, and prone to germ infection before dosing and after insertion. | It is mainly used for pretreatment of drug administration. Leiden University Medical Center developed a solid MN skin patch vaccine for the treatment of COVID-19 in April and is now in interventional clinical trials (data from ClinicalTrails.gov website). | [12,18,56] |
| Hollow MNs | Controlled dose of drug delivery. Adjustable drug delivery rate. No restriction on the type of drug administered. | It may break when inserted into the skin, resulting in part of the MNs remaining on the skin after removing the MNs, causing invisible damage. The skin hole caused by the insertion increases the risk of skin infection. High manufacturing requirements and preparation cost. | Accelovance Inc developed hollow MNs for intradermal delivery of normal saline in 2013, which has completed clinical trials and has not yet been listed (data from ClinicalTrails.gov website, Yaozhi data). | [12,57,58] |
| Coated MNs | Simple manufacturing. Rapid drug release. | The maximum drug dose that can be loaded is only 1 mg, so it is only suitable for the administration of drugs with high efficacy or small required doses. The frictional part will remain on the skin surface, resulting in a difference between the actual dose and the theoretical dose. The coating itself will affect the sharpness of the needle, and there is a barrier to penetration. Used needles need to be discarded, causing waste and producing sharp waste that is not easy to dispose of. The skin hole caused by the insertion increases the risk of skin infection. | Coated MNs currently under experimental research include: insulin-coated MNs for the treatment of hyperglycemia, desmopressin-coated MNs for the treatment of enuresis in children, and MNs for the treatment of hepatitis C. DNA vaccine-coated MNs et al. | [18,58,59,60,61,62] |
| Dissolving MNs | Dissolution rate can be adjusted by changing the material and shape of the needle body. One-step drug delivery, simple process. A wide selection of needle materials with good biocompatibility. Needle parts are completely dissolving, leaving no sharp waste after use. | Uncontrollable drug release. Lower mechanical strength than other types of MNs. | Methotrexate combined with PLGA-dissolving MNs for the treatment of arthritis has controlled release and targeting effects, and is currently under experimental research. HA-dissolving MNs of 5-aminolevulinic acid for the treatment of cancer and DHE-dissolving MNs for the treatment of acute migraines are also under experimental research. | [12,63,64,65,66] |
| Hydrogel-forming MNs | Good biocompatibility Needle mechanical strength and drug delivery rate can be adjusted by changing the density of polymer cross-linking. The drug can be wrapped in the entire MN patch, suitable for high-dose administration. The drug will not be released suddenly, but will pass through the channel continuously at a certain speed, which can prolong the administration time. | Small doses of drugs are easily lost during encapsulation or absorption. Incomplete and uncontrolled drug release. | Hydrogel-forming MNs are widely used in the treatment of arthritis. Melittin-modified HA hydrogel-forming MNs with better effect in the treatment of arthritis can prevent hemolysis and pain caused by injection of purified melittin. The MNs are under experimental research. In addition, hydrogel-forming MNs for the detection of plasma glucose, lactic acid, or chlorine levels, and those for the treatment of non-melanoma skin cancers are also under experimental research. | [18,67,68,69,70] |
| Stimulus-responsive MNs | Good mechanical properties. Excellent biocompatibility. Effectively improving the specificity of drug delivery and reducing toxicity and side effects. | Poor controllability. Difficult to control the dosage precisely. | The stimuli-responsive MNs currently under experimental research include: hyaluronidase stimuli-responsive MNs for the treatment of tumors and hypoxia-responsive MNs for the treatment of diabetes. | [71,72,73,74] |
| Bionic MNs | High mechanical strength. Good biocompatibility. Painless insertion. | Complicated machining process and expensive equipment. More difficult to produce. | The invention of bionic MNs plays a role in promoting and inspiring the application of MNs in the fields of biosignal recording, tissue adhesion, and transdermal drug delivery. | [55,75,76,77] |
| Type of MN | Materials | Fabrication Process | Single MN Base Width × Height (μm) | Array Area/cm2 | Array Number | API | API Classification | Drug Delivery Enhancement Technology | Animal Models | Result | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solid MNs | Polycarbonate | ×500 | Ketoprofen | NSAIDs | When MN and ketoprofen gel were coupled, the AUC and Cmax of ketoprofen dramatically increased and the relative bioavailability was higher. | [127] | |||||
| Silicon | ×200 | 0.25 | 4 × 4 | Methotrexate | Conventional synthetic DMARDs | The plasma concentration of methotrexate would increase linearly with increasing number of MNs. | [128] | ||||
| ×200 | Triptolide | Herbal extracts | Liposome hydrogel patch | CIA | The drug delivered by MN could promote transdermal absorption effectively. | [129] | |||||
| ×250/750 | Bee venom | Bio-Drugs | Sodium urate-induced acute gouty inflammation, Lipopolysaccharide (LPS)-induced acute inflammation | MN can promote the percutaneous absorption of the active macromolecules: bee venom gel. | [130] | ||||||
| 100 × 250 | 0.25 | Alkaloids from Aconitum sinomontanum | Herbal extracts | Nanostructured lipid carriers | AIA | MN led to deeper permeation and combination of MN and NLCs; could improve the therapeutic efficacy. | [131] | ||||
| ×250/500/750/1000 | Paeoniflorin | Herbal extracts | Ethosomes | Both ethosome and MN can enhance the penetration of paeoniflorin, and MN shows a more dramatic effect. | [132] | ||||||
| Paeoniflorin | Herbal extracts | Ethosomes | MN could promote the entry of the ethosomes into the skin and greatly improved the possibility of deep penetration of the water-soluble paeoniflorin. | [133] | |||||||
| Coated MNs | Medical-Grade liquid crystalline polymer | micromolding/solvent casting method | 139 ± 17 × 1160 ± 43 | 1 | 6 × 6 | Lidocaine | Analgesic drug | MNs show faster release of drug than TS and can be used for instant supply of the same drug. | [118] | ||
| Hollow MNs | 3D printing method | Denosumab | RANKL inhibitor | In comparison to the subcutaneous group, similar rate of release was observed with the 3D printed hollow MN without inducing any stimuli of pain. | [134] | ||||||
| PVP, PVA | micromolding/spin-casting method | 460 × 1200 | 0.35 | 9 × 9 | Tofacitinib | JAK inhibitor | The amount of drug permeated using MNs is superior to other approaches and dissolving MN shows better ability to promote penetration. | [135] | |||
| Dissolving MNs | MT | micromolding/spin-casting method | 210 × 700 | 10 × 10 | Methotrexate | Conventional synthetic DMARDs | Iontophoretic delivery | MNA-delivered anti-TNF-α Ab treatment had a therapeutic effect in an animal model of psoriasiform dermatitis and effectively reduced key biomarkers of psoriasiform inflammation including epidermal thickness and IL-1b expression. | [136] | ||
| CMC | drawing lithography | ×600 | 5 × 5/9 × 9 | TNF-α antibodies | TNF inhibitor | Imiquimod-induced psoriasiform inflammation | IPS-based DMN-mediated delivery of CAP was able to significantly modulate macrophages for the production of TNF-α, IL-1β, and IL-6 compared to topical application. | [137] | |||
| HA, PVP | micromolding method | 380 × 680 | 13 × 13 | Capsaicin | Lipophilic drugs | Innovative polymeric system | CIA | DMNs resulted in lower peak plasma levels but higher plasma ARM concentration at 8 h after administration and could reverse paw edema, similar to ARM intramuscular injection. | [138] | ||
| Oligo-HA | micromolding/solvent casting method | 300 × 800 | 7 × 10 | Artemether | Lipophilic drugs | CIA | SH-DM significantly enhanced the permeation rate of drug compared to the control of SH-G and AUC, and RBA value of SH-DM was 1.99 times higher than that of SH-G. | [139] | |||
| MT, PLGA | micromolding/spin-casting method | ×1500 | 35 | Sinomenine | Herbal extracts | The MN patch showed a significant drug deposition within skin (63.37%) and an improved transdermal flux (1.60 μg/cm2/h) with a 2.58-fold enhancement in permeation compared to plain drug solution. | [140] | ||||
| PVP, PVA | micromolding method | 55.42 ± 8.66 × 508.46 ± 9.32 | 28 | Meloxicam | NSAIDs | Carrageenan-induced arthritis | A synergistic 25-fold enhancement of delivery was observed in vivo when a combination of MNs and iontophoresis was used compared with either modality alone. | [141] | |||
| Acrylate-modified HA | micromolding/spin-casting method | 300 × 800 | 15 × 15 | Etanercept | TNF inhibitor | AIA | MN showed good bioequivalence to the classical subcutaneous injection administration. | [142] | |||
| PVP, CS, CMC | micromolding/spin-casting method | 300 × 500 | 12 × 12 | Neurotoxin | Analgesic drug | CIA | DMNs-NT showed favorable biocompatibility and the skin penetration depth and the cumulative of NT in DMNs-NT was much higher than the NT solution. | [143] | |||
| PVP | micromolding method | Methotrexate | Conventional synthetic DMARDs | Multiple emulsion (w/o/w type) system | AIA | The MN patch significantly suppressed paw swelling compared to positive control. | [64] | ||||
| PVP | micromolding method | 300 × 350 | aconitine | Herbal extracts | Nanostructured lipid carriers | AIA | DMNs showed a higher AUC by enhancing the transdermal delivery efficiency of the ACO-NLCs. | [144] | |||
| PVP-K30 | micromolding/vacuum method | 300 × 550 | 1 | 20 × 20 | polydatin | Herbal extracts | Hydroxypropyl-β-cyclodextrin inclusion complexes | Monosodium urate-induced acute gouty arthritis | The complex-loaded DMNs showed better therapeutic effects on the arthritic mice and lower toxicity. | [145] | |
| HA, Methacrylate-modified HA | micromolding/two-step filling method | ×700 | 0.81 | 10 × 10 | Melittin | Bio-Drugs | AIA | HA-based MN could be as effective as SC injection in inhibiting the progression of RA, and simply modified HAMN with cross-linkable groups showed slow-release properties. | [67] | ||
| PVP | micromolding/vacuum method | 200 × 650 | 12 × 12 | Methotrexate | Conventional synthetic DMARDs | AIA | The drug-loaded MN treatment showed better and faster therapeutics compared with the oral groups because of the avoidance of the first-pass effect and sustained release effect. | [146] | |||
| PVP K30, CS, PVA | micromolding/spin-casting method | 300 × 600 | Brucine | Herbal extracts | AIA | Bru-MN indicated an effective role in inhibiting toe swelling in RA rats, achieving the same effects as methotrexate. | [147] | ||||
| HA, PVA | micromolding/spin-casting method | 350 × 800 | 11 × 11 | Triptolide | Lipophilic drugs | Liposome | Monosodium iodoacetate-induced osteoarthritis | TP-Lipo@DMNs had a slow-release effect compared with intra-articular injection and significantly reduced knee joint swelling and the level of inflammatory cytokines. | [94] | ||
| HA, Dextran, PVP K17 | micromolding/spin-casting method | 200 × 600 | 12 × 12 | Tacrolimus, Diclofenac | Calcineurin inhibitor, NSAIDs | Carrageenan/kaolin-induced arthritis | The layered MNs had stronger effects on inhibiting disease development than the other MN groups and injection groups. | [148] | |||
| HA, PVA, Polysaccharides | micromolding/vacuum method | 600 × 500 | 15 × 15 | Tetrandrine | Herbal extracts | Calcium carbonate-hybridized PLGA nanocarrier | AIA | Tet-6 s-NP (CaCO3)/GP-MN strongly reduced synovial inflammation and angiogenesis, exerting a most obvious anti-inflammatory effect on rats with AA. | [149] | ||
| PVP/VA | micromolding/vacuum method | 260 × 504 | 1 | 20 × 20 | Indomethacin | NSAIDs | Mixed micelles | Mixed micelle-loaded DMNs showed much shorter lag time and higher bioavailability compared to the commercial patch. | [150] | ||
| Hydrogel-forming MNs | PVA, MT | micromolding/freezing and thawing method | ×500 | Sinomenine hydrochloride | Herbal extracts | The sinomenine hydrochloride (SH) in SH-loaded MT/ PVA MN exhibited lower clearance, longer retention time, higher bioavailability and stability versus SH-loaded hydrogel. | [151] | ||||
| PVA | micromolding method | 300 × 729.5 ± 11.2 | 11 × 11 | Methotrexate | Conventional synthetic DMARDs | PVA-based HFMNs delivered variable doses of drug through skin more efficiently compared with the previous HF-MNs and could be removed without leaving any measurable residues. | [152] | ||||
| PVA | micromolding/solvent casting method | 300 × 729.5 ± 50 | 11 × 11 | Methotrexate | Conventional synthetic DMARDs | The HFMN patch was able to deliver MTX (around 40% of the applied dose) in a controlled and sustained manner. | [153] | ||||
| Methacrylate-modified HA | micromolding/vacuum method | 300 × 800 | 1 | 15 × 15 | DTA6 | DEK protein inhibitors | CIA | HMN had similar or better efficacy than intravenous injection and would efficiently alleviate arthritis and profoundly improve the compliance of patients. | [115] | ||
| PVA, PVP K90, HPMC, PEG4000, PEG10000, Glycerol | micromolding/spin-casting method | 300 × 800 | 0.5 | 11 × 11 | Methotrexate | Conventional synthetic DMARDs | HFMN could deliver MTX in a sustained manner over 24 h, with significantly lower Cmax, while maintaining the same or even better delivered dose than that achieved by the oral administration route. | [126] |
| NCT number | Title | Status | Interventions | Population | Date | Locations | |
|---|---|---|---|---|---|---|---|
| 1 | NCT03607903 | Adalimumab Microneedles in Healthy Volunteers | Phase 1\2 Completed | Adalimumab ID (microneedle: MicronJet600)\SC | Enrollment: 24 Age: 18 to 45 years Sex: All | 11 July 2018 to 30 October 2018 | Centre for Human Drug Research, Leiden, Netherlands |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zeng, J.; Liu, Z.; Zhou, Q.; Wang, X.; Zhao, F.; Zhang, Y.; Wang, J.; Liu, M.; Du, R. Promising Strategies for Transdermal Delivery of Arthritis Drugs: Microneedle Systems. Pharmaceutics 2022, 14, 1736. https://doi.org/10.3390/pharmaceutics14081736
Wang J, Zeng J, Liu Z, Zhou Q, Wang X, Zhao F, Zhang Y, Wang J, Liu M, Du R. Promising Strategies for Transdermal Delivery of Arthritis Drugs: Microneedle Systems. Pharmaceutics. 2022; 14(8):1736. https://doi.org/10.3390/pharmaceutics14081736
Chicago/Turabian StyleWang, Jitong, Jia Zeng, Zhidan Liu, Qin Zhou, Xin Wang, Fan Zhao, Yu Zhang, Jiamiao Wang, Minchen Liu, and Ruofei Du. 2022. "Promising Strategies for Transdermal Delivery of Arthritis Drugs: Microneedle Systems" Pharmaceutics 14, no. 8: 1736. https://doi.org/10.3390/pharmaceutics14081736
APA StyleWang, J., Zeng, J., Liu, Z., Zhou, Q., Wang, X., Zhao, F., Zhang, Y., Wang, J., Liu, M., & Du, R. (2022). Promising Strategies for Transdermal Delivery of Arthritis Drugs: Microneedle Systems. Pharmaceutics, 14(8), 1736. https://doi.org/10.3390/pharmaceutics14081736






