Molecular Imaging of Steroid-Induced Osteonecrosis of the Femoral Head through iRGD-Targeted Microbubbles
Abstract
:1. Introduction
2. Materials and Methods
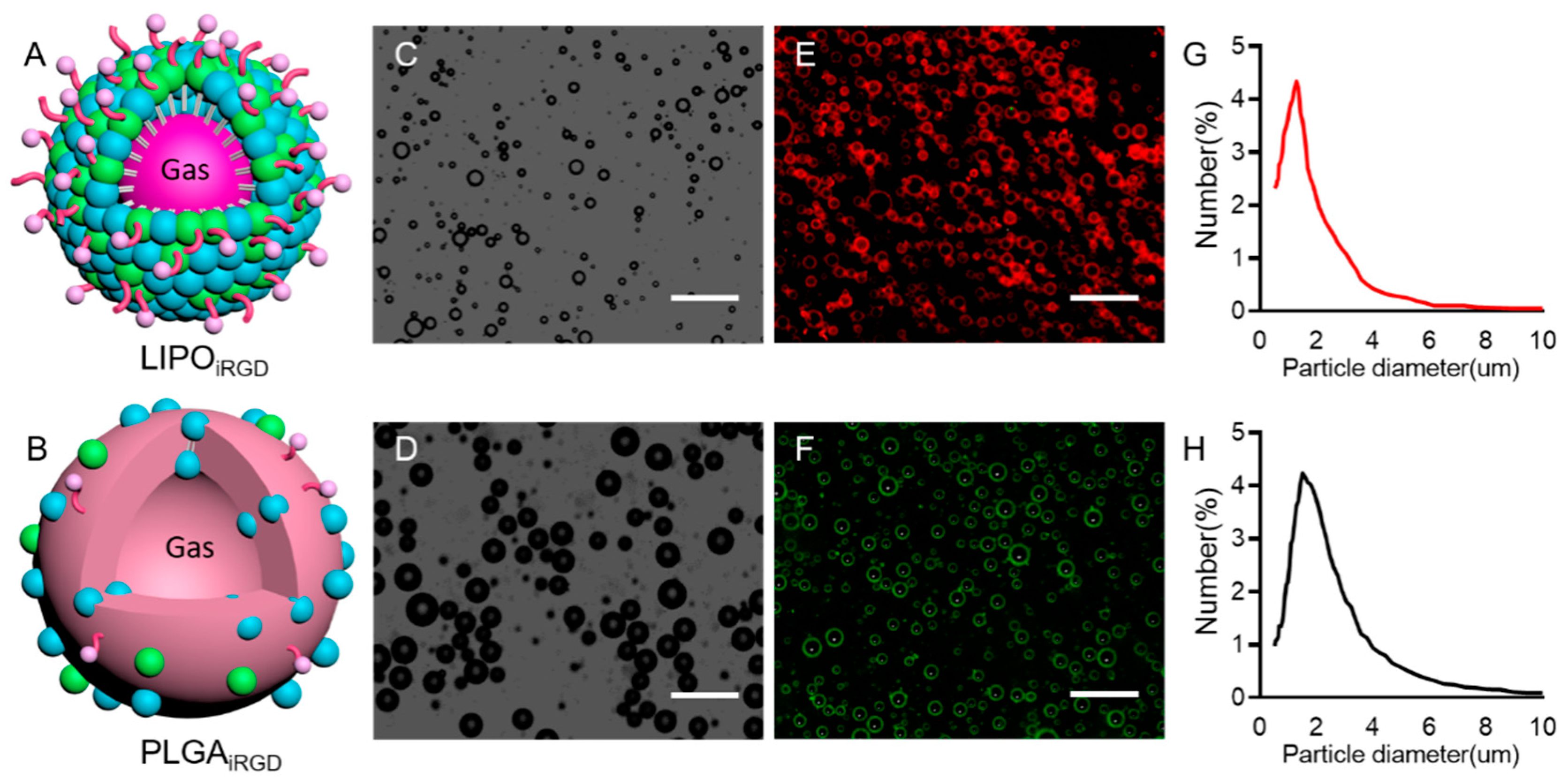
2.1. Preparation of Targeted LIPOiRGD and PLGAiRGD
2.2. Characterization of LIPOiRGD and PLGAiRGD
2.3. Cell Culture
2.4. Establishment of ONFH Model
2.5. In Vivo Ultrasound Molecular Imaging
2.6. Image Data Analysis
2.7. Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
3.1. Preparation and Characterization of iRGD-Targeted and Control MBs
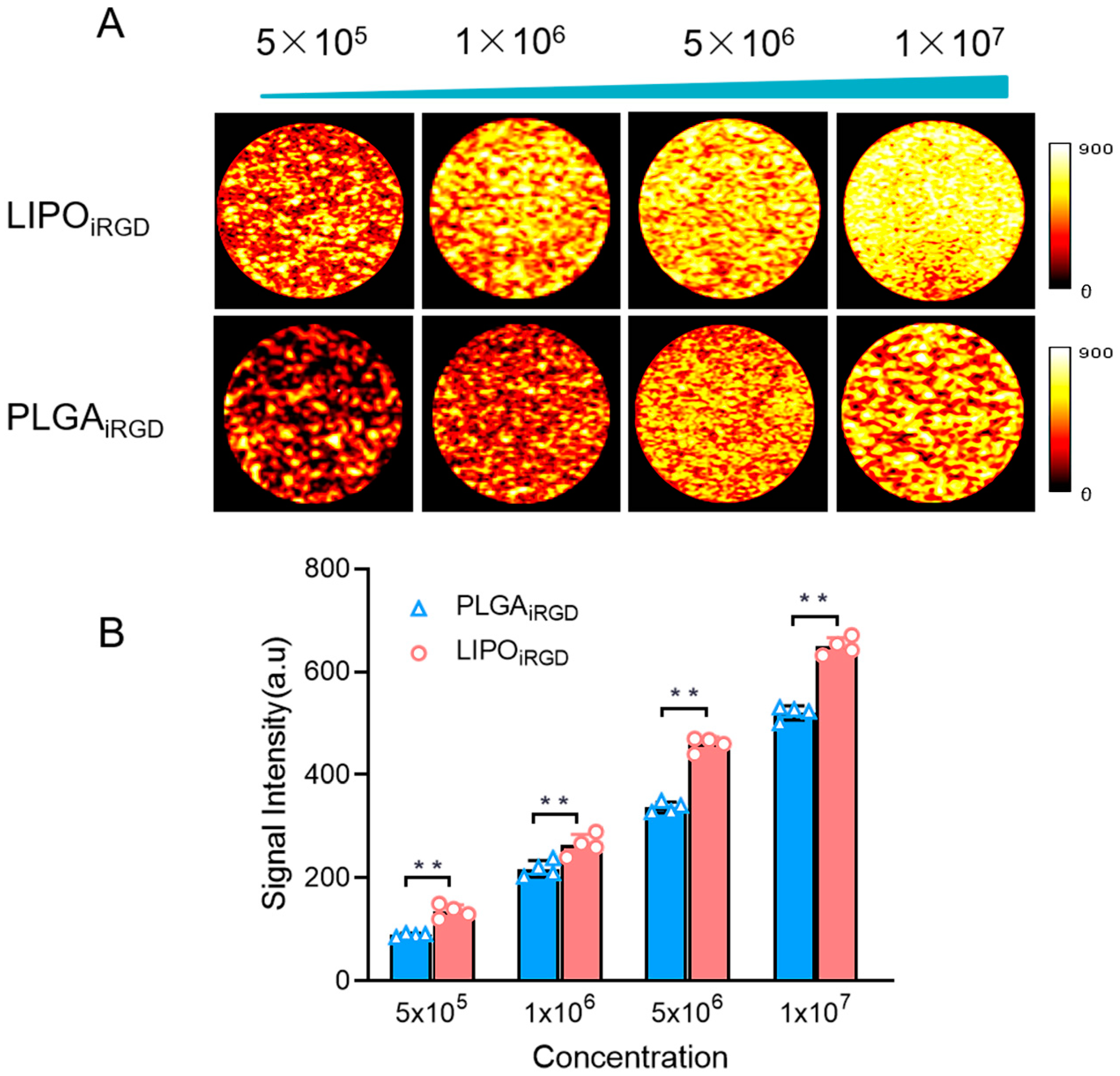
3.2. In Vitro Imaging Performance of the Targeted MBs
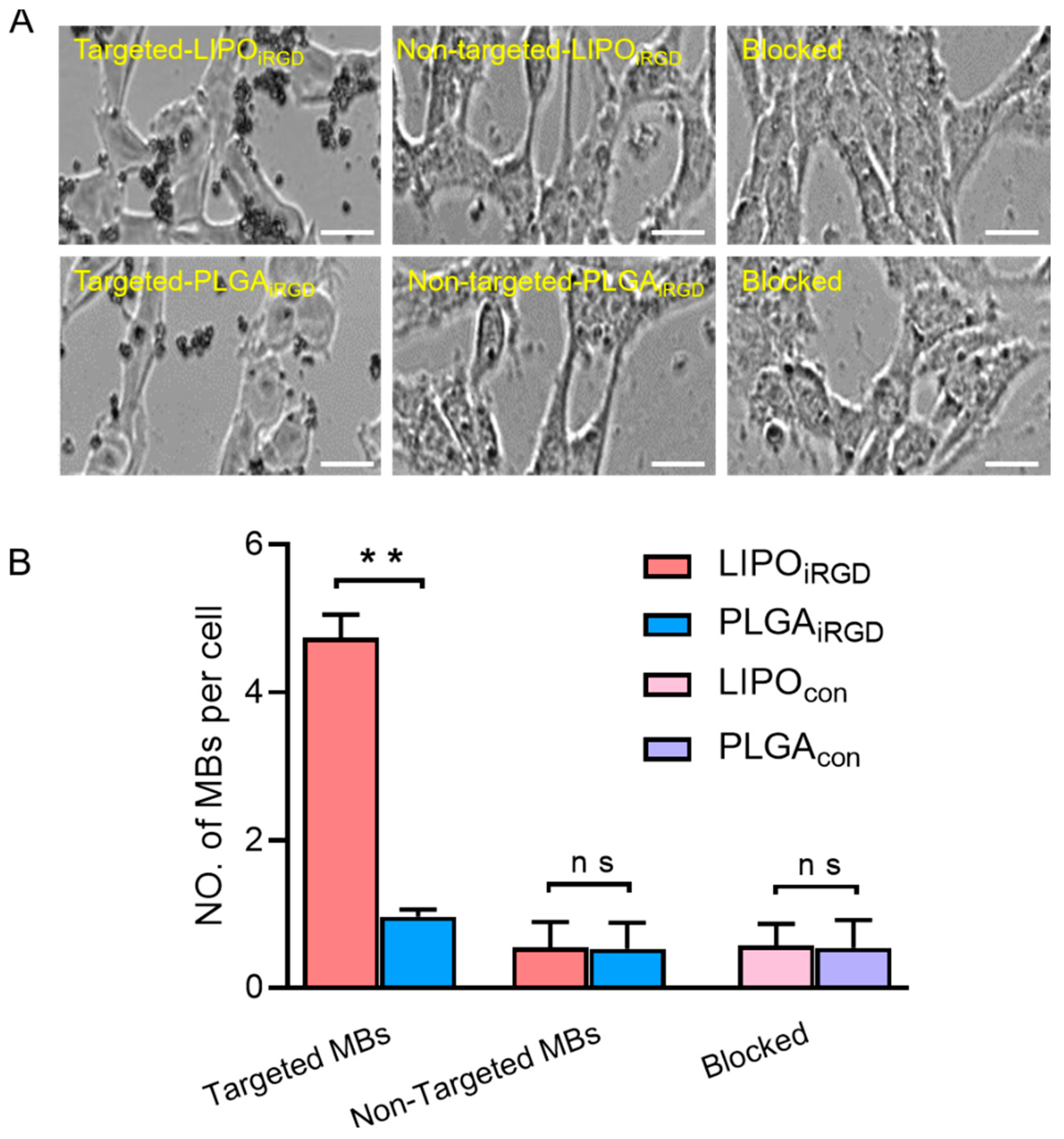
3.3. Binding Affinity of the Two Targeted UCAs to bEnd.3 Cells
3.4. Magnetic Resonance Imaging Detection
3.5. In Vivo Ultrasound Molecular Imaging
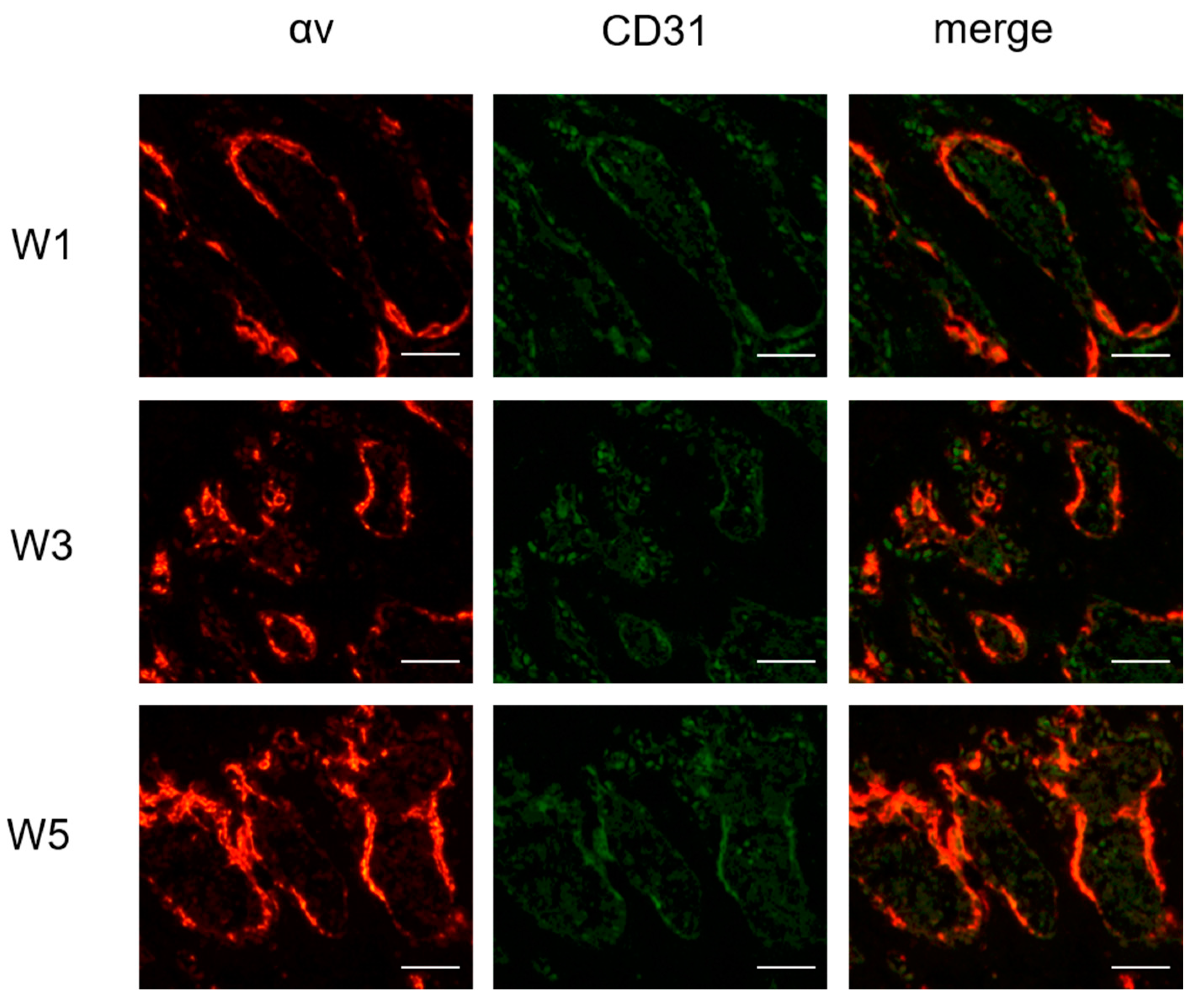
3.6. Immunofluorescence Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauzeur, J.-P.; Malaise, M.; De Maertelaer, V. A prospective cohort study of the clinical presentation of non-traumatic osteonecrosis of the femoral head: Spine and knee symptoms as clinical presentation of hip osteonecrosis. Int. Orthop. 2016, 40, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Mont, M.A.; Koo, K.H.; Chen, C.H.; Cheng, E.Y.; Cui, Q.; Drescher, W.; Gangji, V.; Goodman, S.B.; Ha, Y.C.; et al. The 2019 Revised Version of Association Research Circulation Osseous Staging System of Osteonecrosis of the Femoral Head. J. Arthroplast. 2020, 35, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, Y.; He, J.; Pavlos, N.; Wang, C.; Kenny, J.; Yuan, J.; Zhang, Q.; Xu, J.; He, W. Steroid-induced osteonecrosis of the femoral head reveals enhanced reactive oxygen species and hyperactive osteoclasts. Int. J. Biol. Sci. 2020, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Mahmodi, G.; Manouchehri, S.; Mashhadzadeh, A.; Khodadadi, M.; Servatan, M.; Ganjali, M.; Azambre, B.; Kim, S.; Ramsey, J.; et al. Zeolite in tissue engineering: Opportunities and challenges. Med. Comm. 2020, 1, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, F.; Wang, B.; Liu, B.; Li, L.; Kim, S.; Goodman, S.; Hernigou, P.; Cui, Q.; Lineaweaver, W.; et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J. Orthop. Transl. 2020, 21, 100–110. [Google Scholar] [CrossRef]
- Liu, L.H.; Zhang, Q.Y.; Sun, W.; Li, Z.R.; Gao, F.Q. Corticosteroid-induced Osteonecrosis of the Femoral Head: Detection, Diagnosis, and Treatment in Earlier Stages. Chin. Med. J. 2017, 130, 2601–2607. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, Y.; Wang, Y.; Zhang, C. Circulating exosome levels in the diagnosis of steroid-induced osteonecrosis of the femoral head. Bone Jt. Res. 2016, 5, 276–279. [Google Scholar] [CrossRef]
- Fu, W.; Liu, B.; Wang, B.; Zhao, D. Early diagnosis and treatment of steroid-induced osteonecrosis of the femoral head. Int. Orthop. 2019, 43, 1083–1087. [Google Scholar] [CrossRef]
- Trueta, J.; Harrison, M. The normal vascular anatomy of the femoral head in adult man. J. Bone Jt. Surg. Br. 1953, 35, 442–461. [Google Scholar] [CrossRef]
- Atsumi, T.; Yamano, K.; Muraki, M.; Yoshihara, S.; Kajihara, T. The blood supply of the lateral epiphyseal arteries in Perthes’ disease. J. Bone Jt. Surg. Br. 2000, 82, 392–398. [Google Scholar] [CrossRef]
- Cohen-Rosenblum, A.; Cui, Q. Osteonecrosis of the Femoral Head. Orthop. Clin. N. Am. 2019, 50, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Akahane, M. Non-contrast enhanced MR angiography: Established techniques. J. Magn. Reson. Imaging 2012, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.; Leary, C.J.; Ohki, S.K. Magnetic resonance angiography: The nuts and bolts. Tech. Vasc. Interv. Radiol. 2001, 4, 27–44. [Google Scholar] [CrossRef]
- Lim, R.P.; Koktzoglou, I. Noncontrast magnetic resonance angiography: Concepts and clinical applications. Radiol. Clin. N. Am. 2015, 53, 457–476. [Google Scholar] [CrossRef]
- Insko, E.K.; Carpenter, J.P. Magnetic resonance angiography. Semin. Vasc. Surg. 2004, 17, 83–101. [Google Scholar] [CrossRef]
- Faggioni, L.; Gabelloni, M. Iodine Concentration and Optimization in Computed Tomography Angiography: Current Issues. Investig. Radiol. 2016, 51, 816–822. [Google Scholar] [CrossRef]
- Burton, C.; Mayo, J.; Cunningham, I. Energy subtraction angiography is comparable to digital subtraction angiography in terms of iodine Rose SNR. Med. Phys. 2016, 43, 5925. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Wang, Y.; Wang, Z.; Li, C.; Fu, W.; Qiu, X.; Zhao, D. Digital Subtraction Angiography and Magnetic Resonance Imaging-Based Staging of Circulatory Obstruction in the Femoral Head during Osteonecrosis of the Femoral Head Development. Ann. Plast. Surg. 2020, 85, 677–684. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, S.; Zhao, D.; Wang, B. Evaluating the Blood Supply of the Femoral Head during Different Stages of Necrosis Using Digital Subtraction Angiography. Orthopedics 2019, 42, e210–e215. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Sennoga, C.; Kanbar, E.; Auboire, L.; Dujardin, P.; Fouan, D.; Escoffre, J.; Bouakaz, A. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017, 14, 1031–1043. [Google Scholar] [CrossRef]
- Moestue, S.; Gribbestad, I.; Hansen, R. Intravascular targets for molecular contrast-enhanced ultrasound imaging. Int. J. Mol. Sci. 2012, 13, 6679–6697. [Google Scholar] [CrossRef]
- Wen, Q.; Wan, S.; Liu, Z.; Xu, S.; Wang, H.; Yang, B. Ultrasound contrast agents and ultrasound molecular imaging. J. Nanosci. Nanotechnol. 2014, 14, 190–209. [Google Scholar] [CrossRef]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef]
- Huang, B.; Wang, W.; Li, Q.; Wang, Z.; Yan, B.; Zhang, Z.; Wang, L.; Huang, M.; Jia, C.; Lu, J. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat. Commun. 2016, 7, 13885. [Google Scholar] [CrossRef]
- Sakai, T.; Sugano, N.; Nishii, T.; Haraguchi, K.; Ochi, T.; Ohzono, K. MR findings of necrotic lesions and the extralesional area of osteonecrosis of the femoral head. Skelet. Radiol. 2000, 29, 133–141. [Google Scholar] [CrossRef]
- Radke, S.; Battmann, A.; Jatzke, S.; Eulert, J.; Jakob, F.; Schütze, N. Expression of the angiomatrix and angiogenic proteins CYR61, CTGF, and VEGF in osteonecrosis of the femoral head. J. Orthop. Res. 2006, 24, 945–952. [Google Scholar] [CrossRef]
- Wang, Q.; Onuma, K.; Liu, C.; Wong, H.; Bloom, M.S.; Elliott, E.E.; Cao, R.R.; Hu, N.; Lingampalli, N.; Sharpe, O.; et al. Dysregulated integrin alphaVbeta3 and CD47 signaling promotes joint inflammation, cartilage breakdown, and progression of osteoarthritis. JCI Insight 2019, 4, e128616. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.B.; Zaharias, R.; Stanford, C. Osteoblast integrin adhesion and signaling regulate mineralization. J. Dent. Res. 2001, 80, 1540–1544. [Google Scholar] [CrossRef]
- Min, S.K.; Kang, H.K.; Jung, S.Y.; Jang, D.H.; Min, B.M. A vitronectin-derived peptide reverses ovariectomy-induced bone loss via regulation of osteoblast and osteoclast differentiation. Cell Death Differ. 2018, 25, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodivala-Dilke, K. alphavbeta3 integrin and angiogenesis: A moody integrin in a changing environment. Curr. Opin. Cell Biol. 2008, 20, 514–519. [Google Scholar] [CrossRef]
- Yan, F.; Xu, X.; Chen, Y.; Deng, Z.; Liu, H.; Xu, J.; Zhou, J.; Tan, G.; Wu, J.; Zheng, H. A Lipopeptide-Based alphavbeta(3) Integrin-Targeted Ultrasound Contrast Agent for Molecular Imaging of Tumor Angiogenesis. Ultrasound Med. Biol. 2015, 41, 2765–2773. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Jiang, P.; Li, F.; Yu, B.; Yan, F. Lipid/PLGA Hybrid Microbubbles as a Versatile Platform for Noninvasive Image-Guided Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 41842–41852. [Google Scholar] [CrossRef]
- Zuo, R.; Kong, L.; Wang, M.; Wang, W.; Xu, J.; Chai, Y.; Guan, J.; Kang, Q. Exosomes derived from human CD34 stem cells transfected with miR-26a prevent glucocorticoid-induced osteonecrosis of the femoral head by promoting angiogenesis and osteogenesis. Stem Cell Res. Ther. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Nie, Z.; Sun, F.; Peng, H. Glucocorticoids induce femoral head necrosis in rats through the ROS/JNK/c-Jun pathway. FEBS Open Bio 2021, 11, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-R.; Steinberg, M.E.; Cheng, E.Y. Osteonecrosis of the femoral head: Diagnosis and classification systems. Curr. Rev. Musculoskelet. Med. 2015, 8, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Zlitni, A.; Gambhir, S.S. Molecular imaging agents for ultrasound. Curr. Opin. Chem. Biol. 2018, 45, 113–120. [Google Scholar] [CrossRef]
- Whitman, G.J.; Hortobagyi, G.N. Ultrasound Molecular Imaging: A Good Start. J. Clin. Oncol. 2017, 35, 2101–2102. [Google Scholar] [CrossRef]
- Brown, E.; Lindner, J.R. Ultrasound Molecular Imaging: Principles and Applications in Cardiovascular Medicine. Curr. Cardiol. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Li, W.; Sakai, T.; Nishii, T.; Nakamura, N.; Takao, M.; Yoshikawa, H.; Sugano, N. Distribution of TRAP-positive cells and expression of HIF-1alpha, VEGF, and FGF-2 in the reparative reaction in patients with osteonecrosis of the femoral head. J. Orthop. Res. 2009, 27, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; D’Amico, G.; Hodivala-Dilke, K.; Reynolds, L. Integrins: The keys to unlocking angiogenesis. Arter. Thromb. Vasc. Biol. 2008, 28, 1703–1713. [Google Scholar] [CrossRef]
- Somanath, P.; Ciocea, A.; Byzova, T. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem. Biophys. 2009, 53, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Sun, J.; Zhu, S.; He, J.; Hao, L.; Xiao, L.; Zhu, Y.; Wang, Q.; Pan, X.; Wang, Z.; et al. Ultrasound-mediated destruction of oxygen and paclitaxel loaded dual-targeting microbubbles for intraperitoneal treatment of ovarian cancer xenografts. Cancer Lett. 2017, 391, 1–11. [Google Scholar] [CrossRef]
- Borden, M.A. Intermolecular forces model for lipid microbubble shells. Langmuir 2018, 35, 10042–10051. [Google Scholar] [CrossRef]
- Chan, W.P.; Liu, Y.J.; Huang, G.S.; Lin, M.F.; Huang, S.; Chang, Y.C.; Jiang, C.C. Relationship of idiopathic osteonecrosis of the femoral head to perfusion changes in the proximal femur by dynamic contrast-enhanced MRI. AJR Am. J. Roentgenol. 2011, 196, 637–643. [Google Scholar] [CrossRef]
- Kim, H.; Sanders, M.; Athavale, S.; Bian, H.; Bauss, F. Local bioavailability and distribution of systemically (parenterally) administered ibandronate in the infarcted femoral head. Bone 2006, 39, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Lutz, A.M.; Paulmurugan, R.; Patel, M.R.; Chu, P.; Rosenberg, J.; Gambhir, S.S. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 2008, 248, 936–944. [Google Scholar] [CrossRef]
- Nan, X.; Zhang, X.; Liu, Y.; Zhou, M.; Chen, X.; Zhang, X. Dual-Targeted Multifunctional Nanoparticles for Magnetic Resonance Imaging Guided Cancer Diagnosis and Therapy. ACS Appl. Mater. Interfaces 2017, 9, 9986–9995. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Zhao, S.; Zhang, J.; Lai, M.; Sun, L.; Yan, F. Molecular Imaging of Steroid-Induced Osteonecrosis of the Femoral Head through iRGD-Targeted Microbubbles. Pharmaceutics 2022, 14, 1898. https://doi.org/10.3390/pharmaceutics14091898
Zhao P, Zhao S, Zhang J, Lai M, Sun L, Yan F. Molecular Imaging of Steroid-Induced Osteonecrosis of the Femoral Head through iRGD-Targeted Microbubbles. Pharmaceutics. 2022; 14(9):1898. https://doi.org/10.3390/pharmaceutics14091898
Chicago/Turabian StyleZhao, Ping, Shuai Zhao, Jiaqi Zhang, Manlin Lai, Litao Sun, and Fei Yan. 2022. "Molecular Imaging of Steroid-Induced Osteonecrosis of the Femoral Head through iRGD-Targeted Microbubbles" Pharmaceutics 14, no. 9: 1898. https://doi.org/10.3390/pharmaceutics14091898




