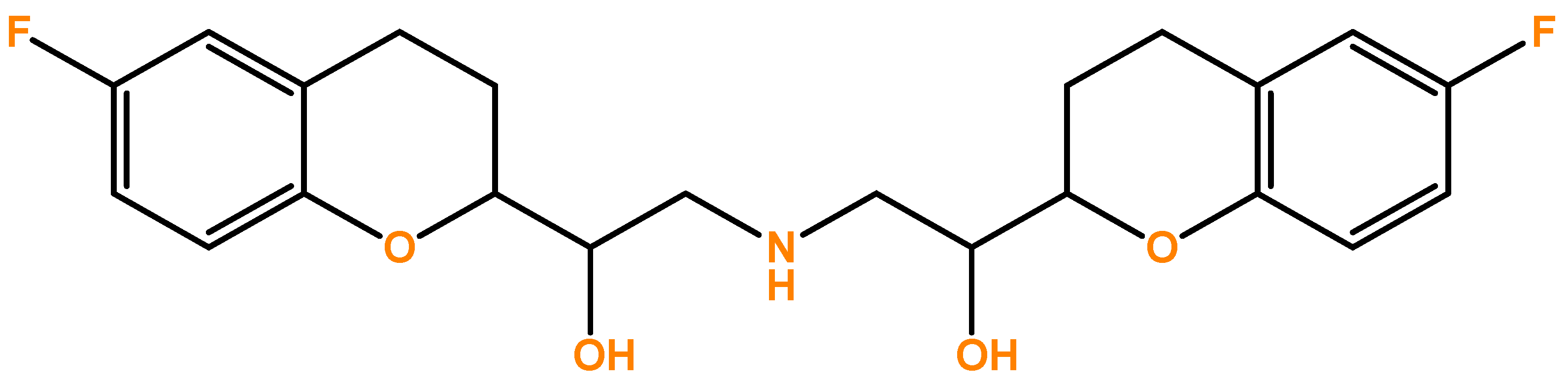
New Data for Nebivolol after In Silico PK Study: Focus on Young Patients and Dosage Regimen
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Data Collection
2.2. Population Pharmacokinetic Modelling
2.3. Model Evaluation and Validation
2.4. Monte Carlo Simulation and Optimization of NEB Dose Regimen
3. Results
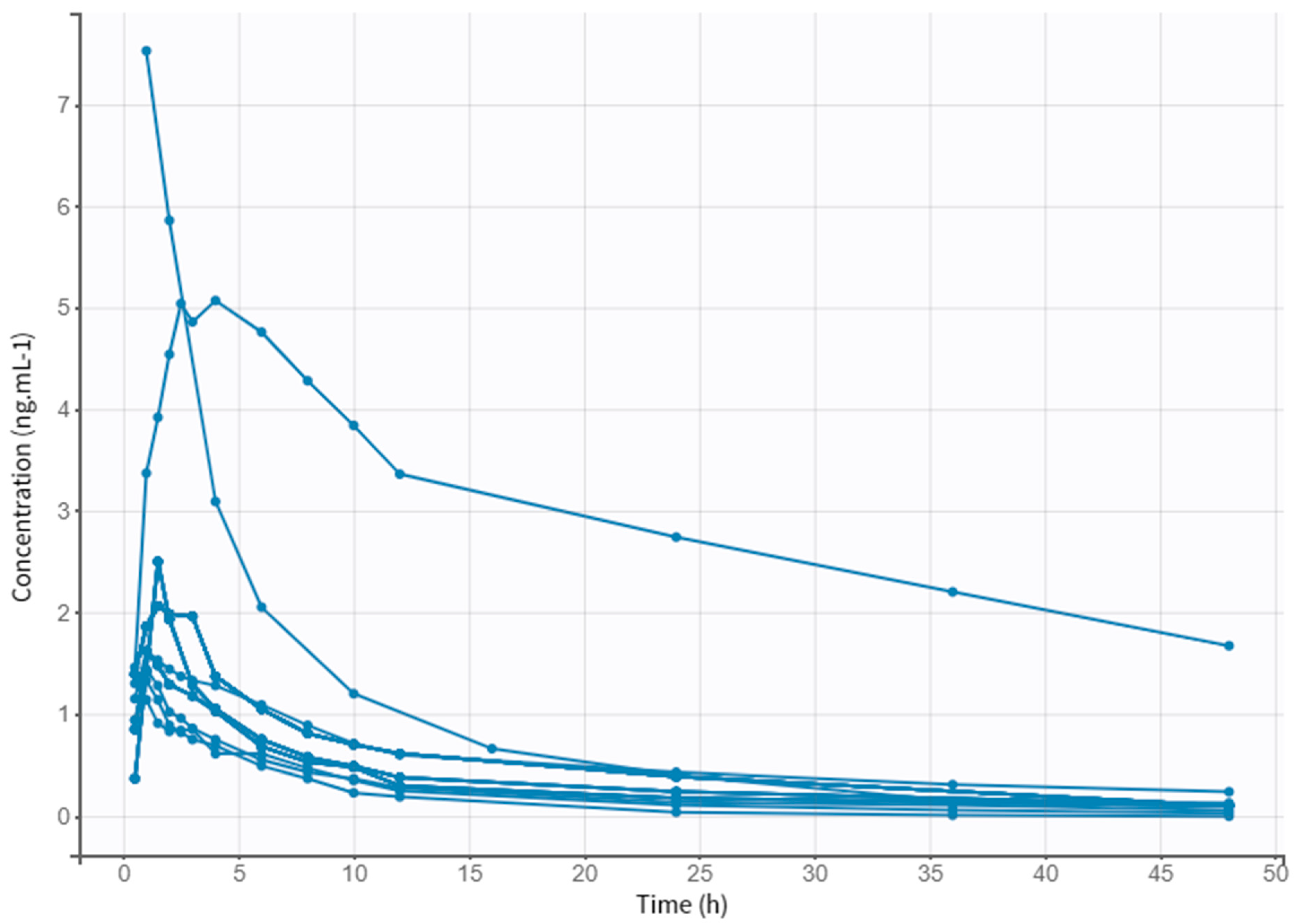
3.1. Collection of the Literature Data
3.2. Subject Characteristics
3.3. Model Building Process
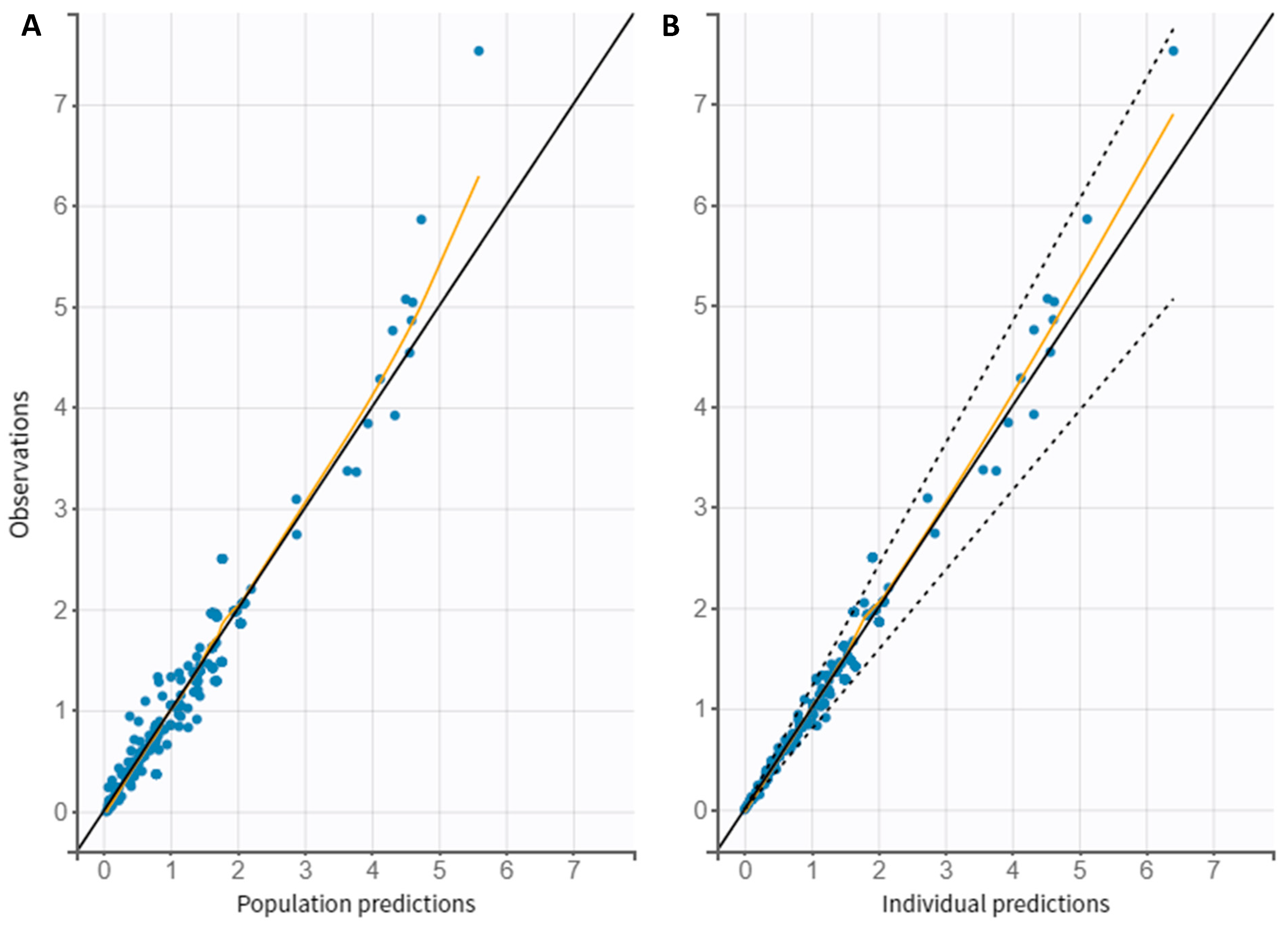
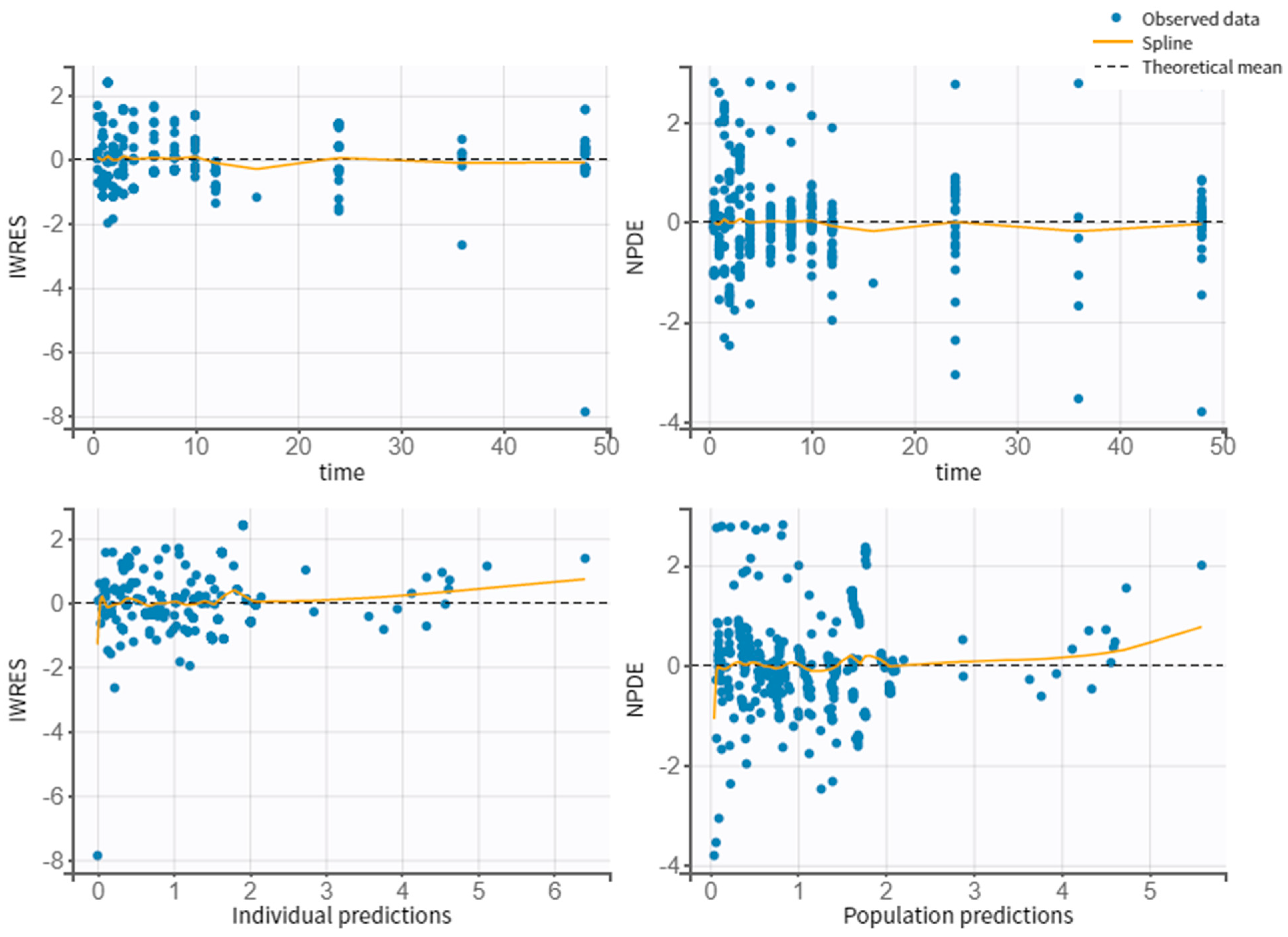
3.4. Model Evaluation
3.5. Dose Regimen Optimization
3.5.1. Multiple-Dose Treatment of 2.5 mg
3.5.2. Proposed Treatment of 6 mg
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fongemie, J.; Felix-Getzik, E. A Review of Nebivolol Pharmacology and Clinical Evidence. Drugs 2015, 75, 1349–1371. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, J. Nebivolol: A Review. Expert Opin. Pharmacother. 2004, 5, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Mangrella, M.; Rossi, F.; Fici, F.; Rossi, F. Pharmacology of Nebivolol. Pharmacol. Res. 1998, 38, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Prisant, L.M. Nebivolol: Pharmacologic Profile of an Ultraselective, Vasodilatory Β1-Blocker. J. Clin. Pharmacol. 2008, 48, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Olawi, N.; Krüger, M.; Grimm, D.; Infanger, M.; Wehland, M. Nebivolol in the Treatment of Arterial Hypertension. Basic Clin. Pharmacol. Toxicol. 2019, 125, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Drug Approval Package: Nebivolol NDA #021742. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021742s000TOC.cfm (accessed on 26 June 2022).
- Colquitt, R.B.; Colquhoun, D.A.; Thiele, R.H. In Silico Modelling of Physiologic Systems. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer—Extract Data from Plots, Images, and Maps. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 11 April 2022).
- Chen, C.L.; Desai-Krieger, D.; Ortiz, S.; Kerolous, M.; Wright, H.M.; Ghahramani, P. A Single-Center, Open-Label, 3-Way Crossover Trial to Determine the Pharmacokinetic and Pharmacodynamic Interaction between Nebivolol and Valsartan in Healthy Volunteers at Steady State. Am. J. Ther. 2015, 22, e130–e140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Briciu, C.; Neag, M.; Muntean, D.; Vlase, L.; Bocsan, C.; Buzoianu, A.; Gheldiu, A.M.; Achim, M.; Popa, A. A Pharmacokinetic Drug Interaction Study between Nebivolol and Paroxetine in Healthy Volunteers. J. Clin. Pharm. Ther. 2014, 39, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Briciu, C.; Neag, M.; Muntean, D.; Bocsan, C.; Buzoianu, A.; Antonescu, O.; Gheldiu, A.M.; Achim, M.; Popa, A.; Vlase, L. Phenotypic Differences in Nebivolol Metabolism and Bioavailability in Healthy Volunteers. Clujul Med. 2015, 88, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Gheldiu, A.M.; Popa, A.; Neag, M.; Muntean, D.; Bocsan, C.; Buzoianu, A.; Vlase, L.; Tomuta, I.; Briciu, C. Assessment of a Potential Pharmacokinetic Interaction between Nebivolol and Bupropion in Healthy Volunteers. Pharmacology 2016, 98, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gheldiu, A.M.; Vlase, L.; Popa, A.; Briciu, C.; Muntean, D.; Bocsan, C.; Buzoianu, A.; Achim, M.; Tomuta, I.; Todor, I.; et al. Investigation of a Potential Pharmacokinetic Interaction between Nebivolol and Fluvoxamine in Healthy Volunteers. J. Pharm. Pharm. Sci. 2017, 20, 68–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, X.; Lei, Y.; He, L.; Liu, W.; Li, M.; Ran, L.; Yu, M.; Guo, X.; Yu, P.; Liu, Z.; et al. No Influence of CYP2D6∗10 Genotype and Phenotype on the Pharmacokinetics of Nebivolol in Healthy Chinese Subjects. J. Clin. Pharm. Ther. 2015, 40, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.C.; Adams, Z.H.; Baker, R.P.; Hope, K.A.; Paton, J.F.R.; Hart, E.C.; Nightingale, A.K. Investigation and Treatment of High Blood Pressure in Young People: Too Much Medicine or Appropriate Risk Reduction? Hypertens 2020, 75, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, S.; Wan, Z.; Ni, S.; Xu, B.; Zhao, X.; Liu, L. Influence of CYP2D6*5 and *10 Polymorphism on the Pharmacokinetics of Nebivolol in Healthy Chinese Subjects. J. Clin. Pharm. Ther. 2020, 45, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Khan, B.V.; Lato, J.; Brener, L.; Ma, Y.; Lukic, T. Nebivolol Monotherapy in Younger Adults (Younger than 55 Years) with Hypertension: A Randomized, Placebo-Controlled Trial. J. Clin. Hypertens. 2013, 15, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Jeon, D.W.; Ahn, H.S.; Jin, D.K.; Lee, H.S.; Lee, J.-Y.; Lim, H.-S.; Manolis, A.J.; Rha, S.-W.; Park, S.W. Efficacy and Safety of Nebivolol in Korean Patients with Hypertension by Age and Sex: A Subanalysis from the BENEFIT-KOREA Study. Clin. Hypertens 2021, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Kotecha, D.; Van Veldhuisen, D.J.; Babalis, D.; Böhm, M.; Coats, A.J.; Roughton, M.; Poole-Wilson, P.; Tavazzi, L.; Flather, M. Efficacy and Safety of Nebivolol in Elderly Heart Failure Patients with Impaired Renal Function: Insights from the SENIORS Trial. Eur. J. Heart Fail. 2009, 11, 872–880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manrique, C.; Whaley-Connell, A.; Sowers, J.R. Nebivolol in Obese and Non-Obese Hypertensive Patients. J. Clin. Hypertens 2009, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Germino, F.W.; Lin, Y.; Bowen, L.; Pejović, V. Efficacy and Tolerability of Nebivolol: Does Age Matter? A Retrospective Analysis of Three Randomized, Placebo-Controlled Trials in Stage I–II Hypertension. Ther. Adv. Cardiovasc. Dis. 2012, 6, 185–199. [Google Scholar] [CrossRef] [PubMed]
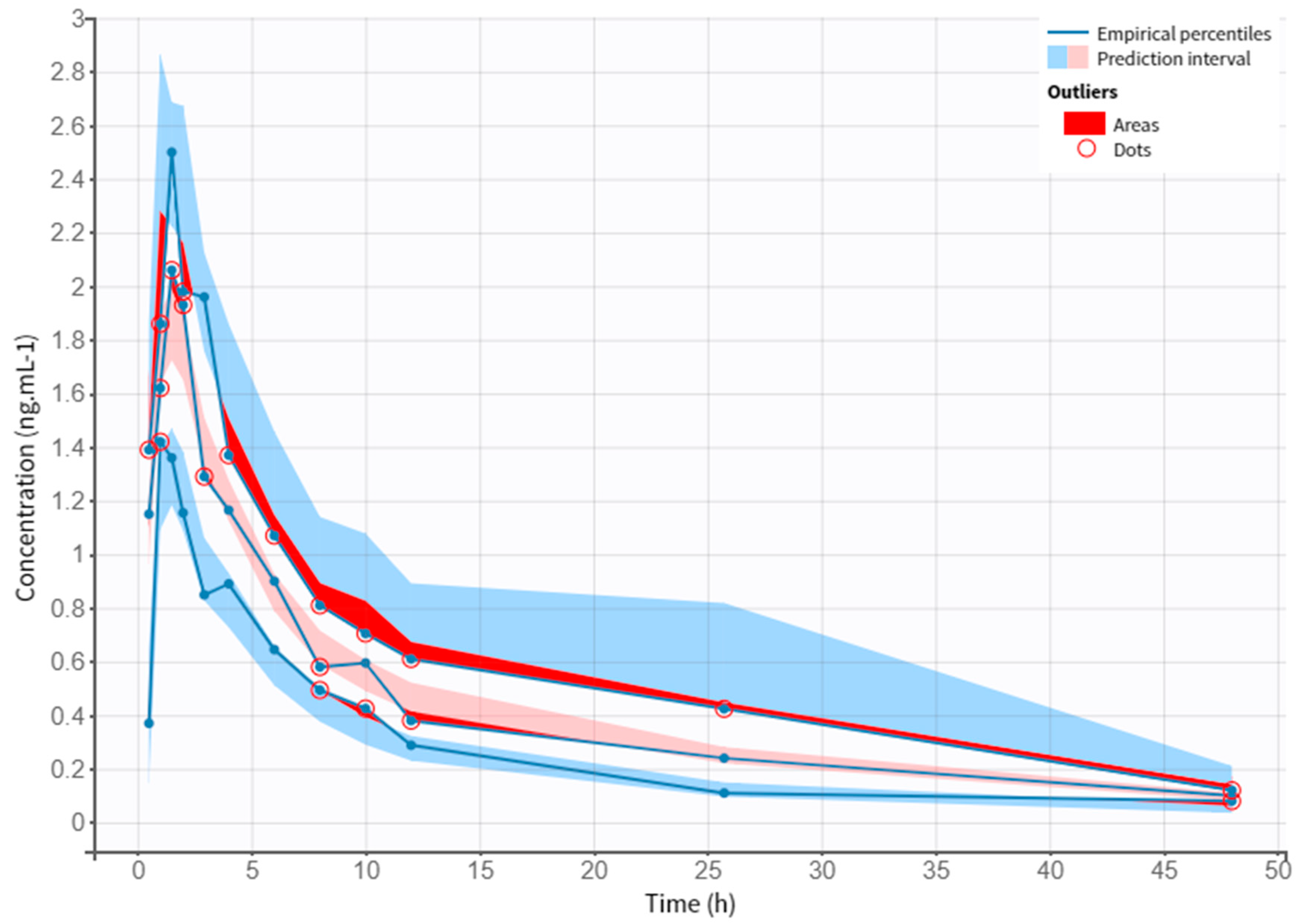
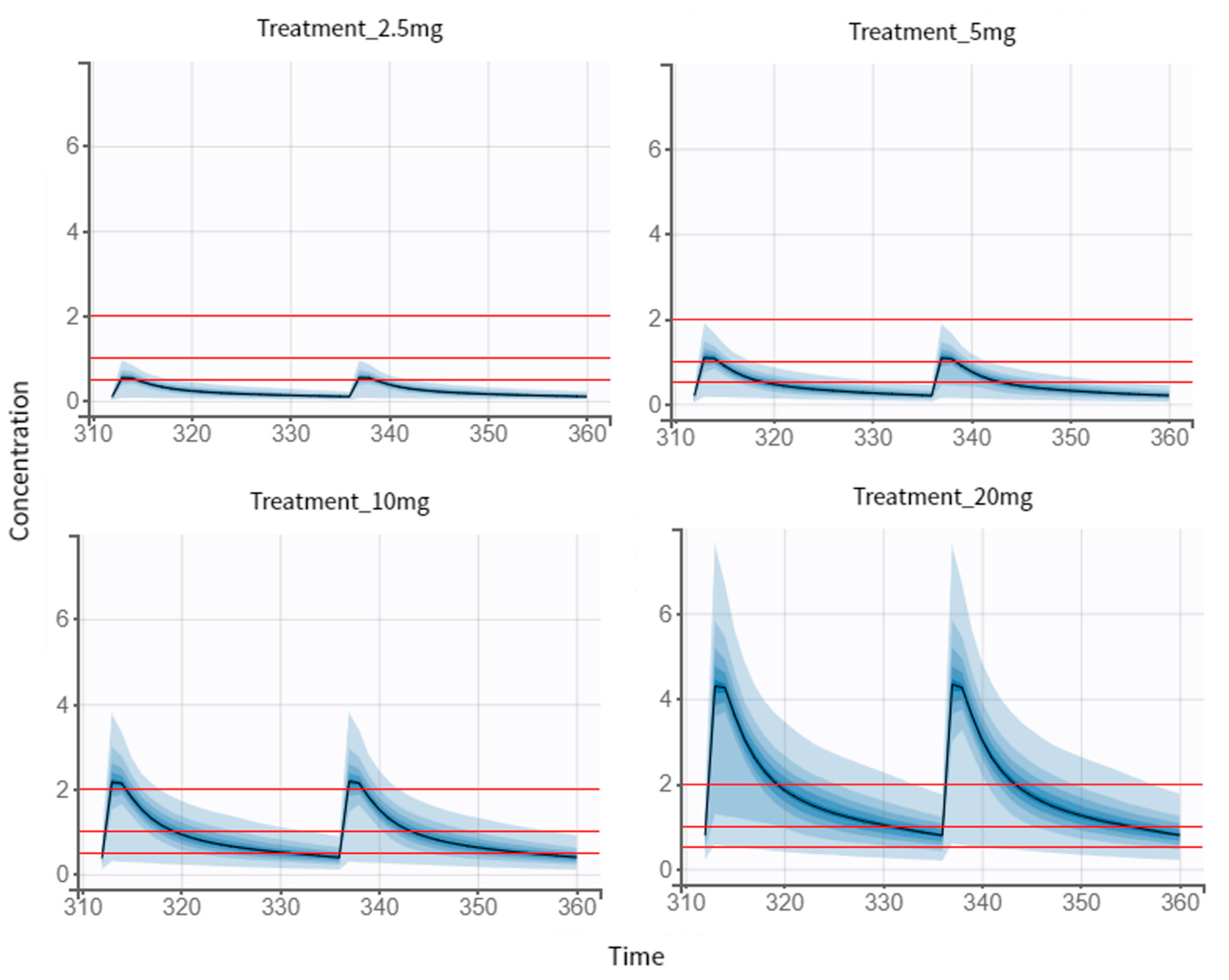

| No. Treatment | Dose Regimen for NEB |
|---|---|
| 1 | 5 mg once a day for 15 days |
| 2 | 10 mg once a day for 15 days |
| 3 | 20 mg once a day for 15 days |
| 4 | 2.5 mg once a day for 15 days |
| 5 * | 2.5 mg BID for 15 days |
| 6 * | 2.5 mg q6 h for 15 days |
| 7 * | 6 mg q36 h for 15 days |
| 8 * | 6 mg q48 h for 15 days |
| Study Number | Reference | Studied Population | NEB Doses | Period of Treatments | Number of Individuals Generated |
|---|---|---|---|---|---|
| 1 | [9] | Healthy adults (n = 30) | 20 mg | Oral daily dose for 27 days | 1 |
| 2 | [10] | Healthy adults (n = 23) | 5 mg | Single oral dose | 1 |
| 3 | [11] | Healthy adults (n = 43) | 5 mg | Single oral dose | 2 |
| 4 | [12] | Healthy adults (n = 18) | 5 mg | Single oral dose | 1 |
| 5 | [13] | Healthy adults (n = 18) | 5 mg | Single oral dose | 1 |
| 6 | [14] | Healthy adults (n = 24) | 10 mg | Single oral dose | 24 |
| Total | 30 | ||||
| Parameter | NEB (n = 30) |
|---|---|
| Age (years) | 26.70 ± 1.40 (20–34 years) |
| Body-mass index (kg/m2) | 25.00 ± 1.00 |
| Genotype (n, %) | |
| Extensive Metabolizers (EMs) | 17, 56.7 |
| Intermediate Metabolizers (IMs) | 12, 40 |
| Poor Metabolizers (PMs) | 1, 3.3 |
| Ethnic (n, %) | |
| Caucasian | 6, 20 |
| Asian | 24, 80 |
| Parameter | Mean | RSE (%) |
|---|---|---|
| 5 mg | ||
| Cmax (ng·mL−1) | 2.40 | 0.78 |
| AUC (ng·h·mL−1) | 37.29 | 1.09 |
| Tmax (h) | 1.66 | 18.77 |
| Kel (L/h) | 0.17 | 0.16 |
| t1/2 (h) | 6.92 | 5.31 |
| 10 mg | ||
| Cmax (ng·mL−1) | 2.65 | ND |
| AUC (ng·h·mL−1) | 1.51 | ND |
| Tmax (h) | 21.56 | ND |
| Kel (L/h) | 2.72 | ND |
| t1/2 (h) | 12.52 | ND |
| 20 mg | ||
| Cmax (ng·mL−1) | 8.02 | 3.47 |
| AUC (ng·h·mL−1) | 41.50 | 0.67 |
| Tmax (h) | 1.32 | 29.76 |
| Kel (L/h) | ND | ND |
| t1/2 (h) | 11.03 | 2.12 |
| Parameter | Estimate | RSE (%) |
|---|---|---|
| Tlag pop | 0.30 | 10.8 |
| Ka pop | 2.06 | 10.4 |
| Cl pop | 0.22 | 36.7 |
| V1 pop | 4.21 | 4.96 |
| Q pop | 0.59 | 5.73 |
| V2 pop | 7.12 | 11.2 |
| Error model parameters | ||
| b | 0.13 | 4.63 |
| Target | 2.5 mg | 5 mg | 10 mg | 20 mg |
|---|---|---|---|---|
| Efficacy Target | 1% | 72.2% | 22.6% | 0.5% |
| Safety Target | 97% | 95.5% | 69.6% | 19.1% |
| Target | 2.5 mg BID | 2.5 mg q6 h | 6 mg q36 h | 6 mg q48 h |
|---|---|---|---|---|
| Efficacy Target | 0.9% | 0% | 75.2% | 72.6% |
| Safety Target | 99.1% | 100% | 97% | 96.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, L.; Costa, B.; Vale, N. New Data for Nebivolol after In Silico PK Study: Focus on Young Patients and Dosage Regimen. Pharmaceutics 2022, 14, 1911. https://doi.org/10.3390/pharmaceutics14091911
Marques L, Costa B, Vale N. New Data for Nebivolol after In Silico PK Study: Focus on Young Patients and Dosage Regimen. Pharmaceutics. 2022; 14(9):1911. https://doi.org/10.3390/pharmaceutics14091911
Chicago/Turabian StyleMarques, Lara, Bárbara Costa, and Nuno Vale. 2022. "New Data for Nebivolol after In Silico PK Study: Focus on Young Patients and Dosage Regimen" Pharmaceutics 14, no. 9: 1911. https://doi.org/10.3390/pharmaceutics14091911
APA StyleMarques, L., Costa, B., & Vale, N. (2022). New Data for Nebivolol after In Silico PK Study: Focus on Young Patients and Dosage Regimen. Pharmaceutics, 14(9), 1911. https://doi.org/10.3390/pharmaceutics14091911







