Diclofenac Loaded Biodegradable Nanoparticles as Antitumoral and Antiangiogenic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticles Preparation
2.3. Design of Experiments
2.4. Physicochemical Characterization
2.5. Morphological Characterization
2.6. Interaction Studies
2.7. γ-Irradiation Sterilization
2.8. In Vitro Drug Release
2.9. Short-Term Stability
2.10. Antiangiogenic Capacity
2.11. In Vitro Cytotoxicity Assay
2.11.1. Cell Lines
2.11.2. Determination of Antiproliferative Activity
2.12. Statistical Analysis
3. Results
3.1. Design of Experiments
3.2. Morphological Characterization
3.3. Interaction Studies
3.4. In Vitro Drug Release
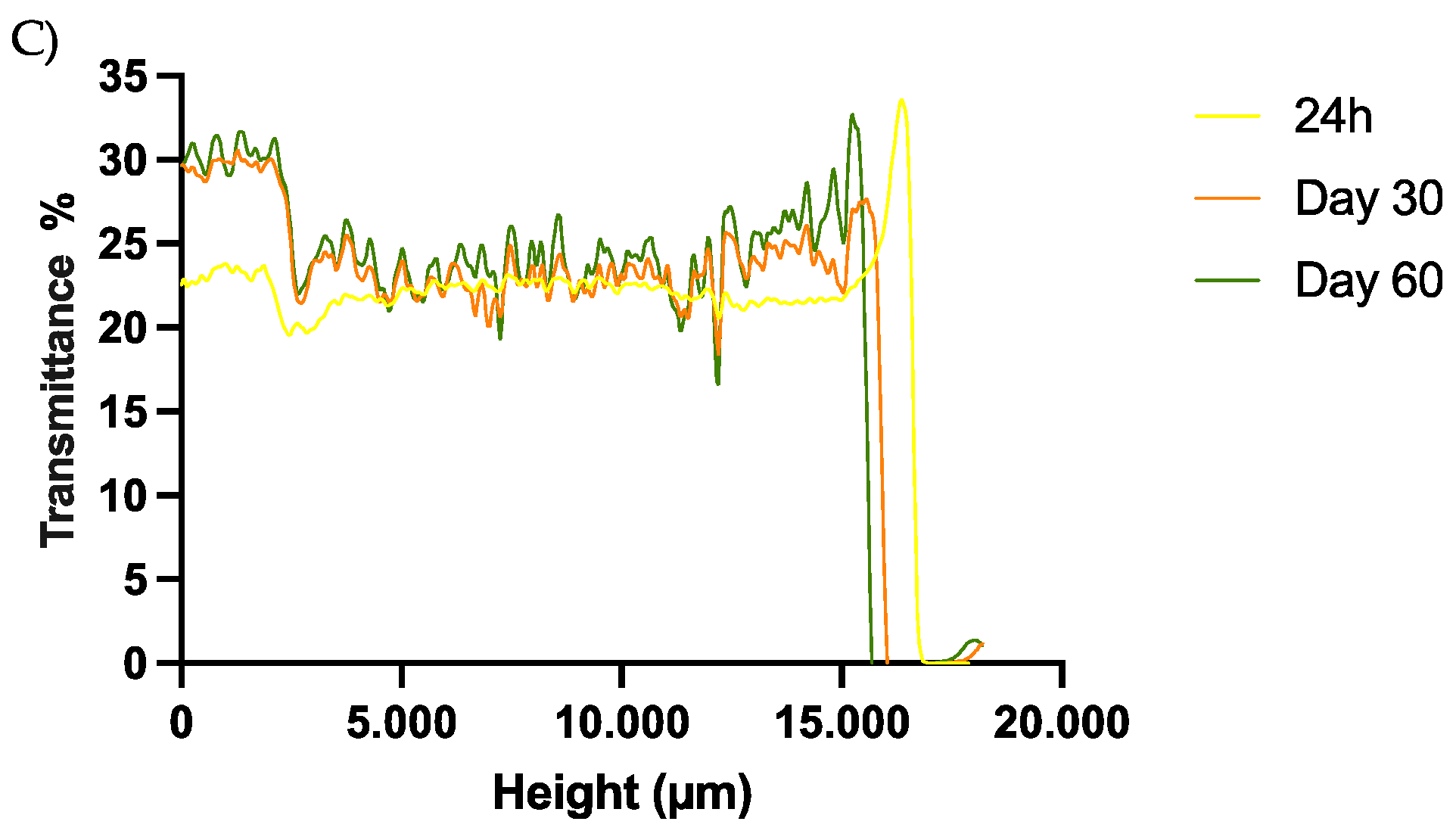
3.5. Short-Term Stability
3.6. Sterilization Using γ-Irradiation
3.7. Antiangiogenic Properties
3.8. Cytotoxicity of Fabricated Formulation towards Selected Cancer Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Thiruchenthooran, V.; Świtalska, M.; Bonilla, L.; Espina, M.; García, M.L.; Wietrzyk, J.; Sánchez-López, E.; Gliszczyńska, A. Novel Strategies against Cancer: Dexibuprofen-Loaded Nanostructured Lipid Carriers. Int. J. Mol. Sci. 2022, 23, 11310. [Google Scholar] [CrossRef] [PubMed]
- Parra-Nieto, J.; del Cid, M.A.G.; de Cárcer, I.A.; Baeza, A. Inorganic Porous Nanoparticles for Drug Delivery in Antitumoral Therapy. Biotechnol. J. 2021, 16, e2000150. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Molinaro, R.; Fresta, M.; Duranti, A.; Cosco, D. α-Acylamino-β-lactone N-Acylethanolamine-hydrolyzing Acid Amidase Inhibitors Encapsulated in PLGA Nanoparticles: Improvement of the Physical Stability and Protection of Human Cells from Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants 2022, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Mare, R.; Paolino, D.; Salvatici, M.C.; Cilurzo, F.; Fresta, M. Sclareol-loaded hyaluronan-coated PLGA nanoparticles: Physico-chemical properties and in vitro anticancer features. Int. J. Biol. Macromol. 2019, 132, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Diclofenac as an anti-cancer agent. Ecancermedicalscience 2016, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Phadke, S.; Kanekar, T.; Gumaste, S.; Parikh, V. Application of simplex lattice design for the development of extended release tablets of model drug diclofenac sodium. Ther. Deliv. 2019, 10, 515–525. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Namlı, İ.; Güleç, K.; Kıyan, H.T. Diclofenac sodium loaded PLGA nanoparticles for inflammatory diseases with high anti-inflammatory properties at low dose: Formulation, characterization and in vivo HET-CAM analysis. Microvasc. Res. 2020, 130, 103991. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Singh, S.K.; Kim, S.K. Preparations and Applications of Alginate Nanoparticles; Elsevier Inc.: San Diego, CA, USA, 2017; ISBN 9780128098172. [Google Scholar]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.L.; Harirforoosh, S. Design and optimization of PLGA-based diclofenac loaded nanoparticles. PLoS ONE 2014, 9, e87326. [Google Scholar] [CrossRef] [PubMed]
- da Feltrin, F.S.; Agner, T.; Sayer, C.; Lona, L.M.F. Curcumin encapsulation in functional PLGA nanoparticles: A promising strategy for cancer therapies. Adv. Colloid Interface Sci. 2022, 300, 102582. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC-Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Esim, O.; Bakirhan, N.K.; Sarper, M.; Savaser, A.; Ozkan, S.A.; Ozkan, Y. Influence of emulsifiers on the formation and in vitro anticancer activity of epirubicin loaded PLGA nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102027. [Google Scholar] [CrossRef]
- Khanal, S.; Adhikari, U.; Rijal, N.; Bhattarai, S.; Sankar, J.; Bhattarai, N. pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. J. Funct. Biomater. 2016, 7, 21. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Amir, M.; Sarafroz, M. Quantification and evaluations of catechin hydrate polymeric nanoparticles used in brain targeting for the treatment of epilepsy. Pharmaceutics 2020, 12, 203. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnology 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Esteruelas, G.; Halbaut, L.; García-Torra, V.; Espina, M.; Cano, A.; Ettcheto, M.; Camins, A.; Souto, E.B.; Luisa García, M.; Sánchez-López, E. Development and optimization of Riluzole-loaded biodegradable nanoparticles incorporated in a mucoadhesive in situ gel for the posterior eye segment. Int. J. Pharm. 2022, 612, 121379. [Google Scholar] [CrossRef]
- González-Fernández, F.M.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Calpena, A.C.; Folch, J.; Barenys, M.; Sánchez-López, E.; Camins, A.; García, M.L. Epigallocatechin-3-gallate loaded PEGylated-PLGA nanoparticles: A new anti-seizure strategy for temporal lobe epilepsy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1073–1085. [Google Scholar] [CrossRef]
- Yang, D.H.; Kim, H.J.; Park, K.; Kim, J.K.; Chun, H.J. Preparation of poly-l-lysine-based nanoparticles with ph-sensitive release of Curcumin for targeted imaging and therapy of liver cancer in vitro and in vivo. Drug Deliv. 2018, 25, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Carvajal-Vidal, P.; Halbault Bellowa, L.; Calpena, A.C.; Espina, M.; García, M.L. In-situ forming gels containing fluorometholone-loaded polymeric nanoparticles for ocular inflammatory conditions. Colloids Surf. B Biointerfaces 2019, 175, 365–374. [Google Scholar] [CrossRef]
- Carvajal-Vidal, P.; Fábrega, M.J.; Espina, M.; Calpena, A.C.; García, M.L. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: Optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102026. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Banerjee, S.; Ghosh, A.K.; Chattopadhyay, P.; Verma, A.; Ghosh, A. A RP-HPLC method for quantification of diclofenac sodium released from biological macromolecules. Int. J. Biol. Macromol. 2013, 58, 354–359. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Azhari, H.; Strauss, M.; Hook, S.; Boyd, B.J.; Rizwan, S.B. Stabilising cubosomes with Tween 80 as a step towards targeting lipid nanocarriers to the blood-brain barrier. Eur. J. Pharm. Biopharm. 2016, 104, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Article dexibuprofen biodegradable nanoparticles: One step closer towards a better ocular interaction study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef]
- Granja, P.L.; Silva, A.M.; Garcia, M.L.; Souto, E.B. Customized cationic nanoemulsions loading triamcinolone acetonide for corneal neovascularization secondary to inflammatory processes. Int. J. Pharm. 2022, 623, 121938. [Google Scholar] [CrossRef]
- West, D.C.; Thompson, W.D.; Sells, P.G.; Burbridge, M.F. Angiogenesis Assays Using Chick Chorioallantoic Membrane. Methods Mol. Med. 2003, 46, 107–129. [Google Scholar]
- Cho, J.; Doshi, A.; Rosenthal, P.; Beppu, A.; Miller, M.; Aceves, S.; Broide, D. Smad3 deficient mice have reduced esophageal fibrosis and angiogenesis in a mouse model of egg induced eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2014, 23, 1–7. [Google Scholar] [CrossRef]
- Elfarnawany, M.H. Signal Processing Methods for Quantitative Power Doppler Microvascular Angiography. Electron. Thesis Diss. 2015, 3106. [Google Scholar]
- Kim, J.H.; Bae, C.; Kim, M.J.; Song, I.H.; Ryu, J.H.; Choi, J.H.; Lee, C.J.; Nam, J.S.; Kim, J. Il A novel nucleolin-binding peptide for Cancer Theranostics. Theranostics 2020, 10, 9153–9171. [Google Scholar] [CrossRef]
- Liang, B.; Jiang, D.; Pan, L.; Xiong, F.; Feng, S.; Wu, S.; Ye, H.; Yu, Z.; Shi, C.; Gao, S. Lipase-triggered drug release from BCL2 inhibitor ABT-199-loaded nanoparticles to elevate anti-leukemic activity through enhanced drug targeting on the mitochondrial membrane. Acta Biomater. 2022, 145, 246–259. [Google Scholar] [CrossRef]
- Stompor, M.; Podgórski, R.; Marta, Ś.; Wietrzyk, J. Biotinylated xanthohumol: Synthesis and in vitro biological evaluation for anticancer therapy. Preprint 2018, 1–14. [Google Scholar] [CrossRef]
- Czarnecka, M.; Switalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis and biological evaluation of phosphatidylcholines with cinnamic and 3-methoxycinnamic acids with potent antiproliferative activity. RSC Adv. 2018, 8, 35744–35752. [Google Scholar] [CrossRef]
- Nevozhay, D. Cheburator software for automatically calculating drug inhibitory concentrations from in vitroscreening assays. PLoS ONE 2014, 9, e106186. [Google Scholar] [CrossRef]
- Kumar, N.; Khar, R.K.; Kumar Jain, G.; Kumar, P.; Malik, A. Nanosuspension approach of acyclovir using het-cam method. Int. J. Drug Discov. Med. Res. 2020, 9, 454–458. [Google Scholar]
- Bonilla, L.; Espina, M.; Severino, P.; Cano, A.; Ettcheto, M.; Camins, A.; García, M.L.; Souto, E.B.; Sánchez-López, E. Lipid Nanoparticles for the Posterior Eye Segment. Pharmaceutics 2022, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Elmsmari, F.; González Sánchez, J.A.; Duran-Sindreu, F.; Belkadi, R.; Espina, M.; García, M.L.; Sánchez-López, E. Calcium hydroxide-loaded PLGA biodegradable nanoparticles as an intracanal medicament. Int. Endod. J. 2021, 54, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef] [PubMed]
- Durga, D.H.N.; Lohithasu, D.; Murthy, K.V.R. Development and evaluation of diclofenac sodium controlled release dosage forms using natural, hydrophilic and hydrophobic polymers and its comparative studies. Indian J. Pharm. Educ. Res. 2017, 51, 116–127. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. RVG29-functionalized lipid nanoparticles for Quercetin brain delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; García, M.L.; Calpena, A.C. Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: Ocular anti-inflammatory applications. Colloids Surf. B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef]
- Forna, N.; Damir, D.; Duceac, L.D.; Dabija, M.G.; Calin, G.; Ichim, D.L.; Gutu, C.; Grierosu, C.; Eva, L.; Ciuhodaru, M.I.; et al. Nano-Architectonics of Antibiotic-Loaded Polymer Particles as Vehicles for Active Molecules. Appl. Sci. 2022, 12, 1998. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Shen, W.; Du, B.; Yang, J.; Zhang, Q. A polycationic brush mediated co-delivery of doxorubicin and gene for combination therapy. Polymers 2019, 11, 60. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A. The effect of diclofenac on proliferation and production of growth factors by endothelial cells (HMEC-1) under hypoxia and inflammatory conditions. Acta Pharm. 2014, 64, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mayorek, N.; Naftali-Shani, N.; Grunewald, M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS ONE 2010, 5, e12715. [Google Scholar] [CrossRef] [PubMed]
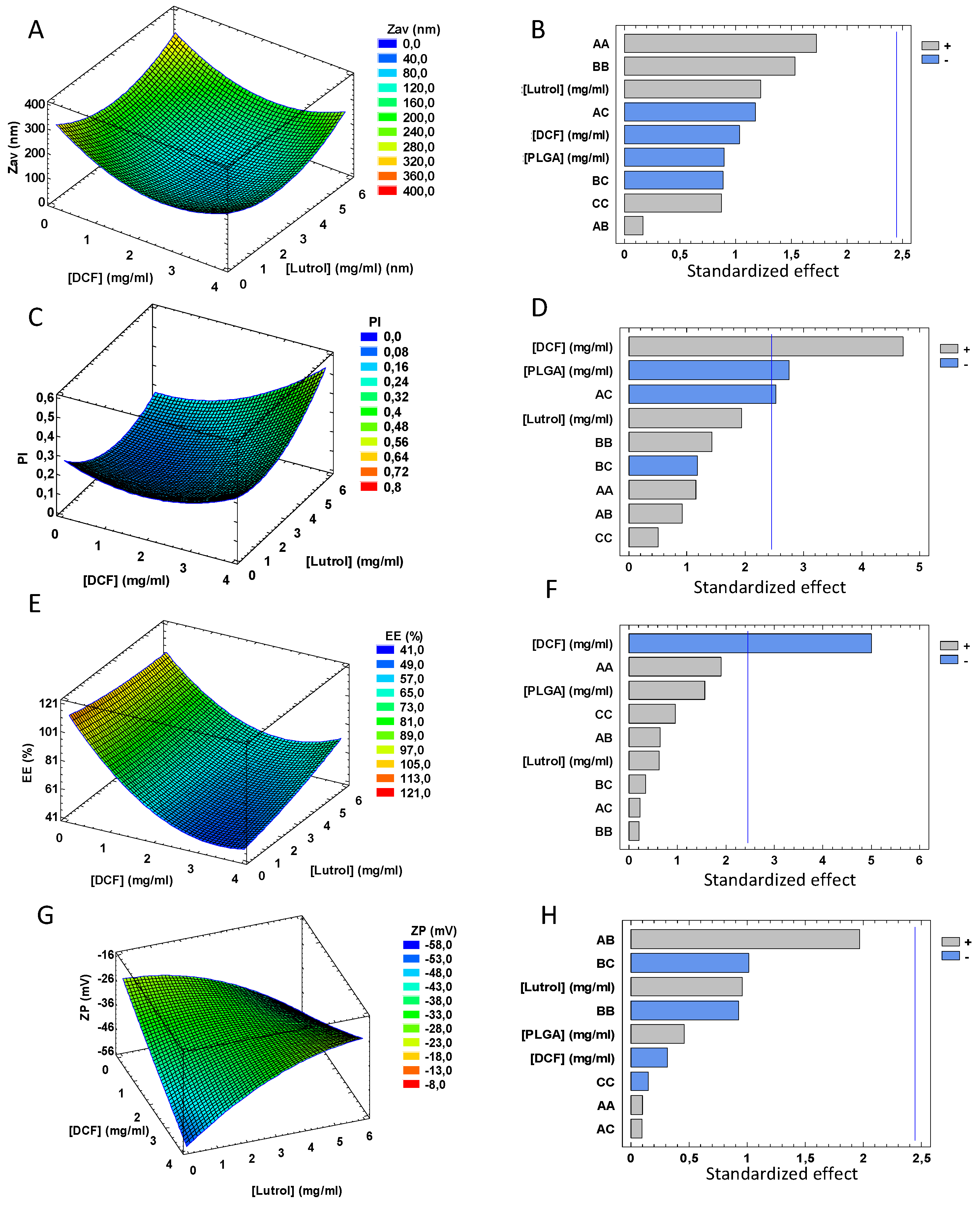





| CDCF | CLutrol | CPLGA | ZAV ± SD (nm) | PI ± SD | ZP ± SD (mV) | EE (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code level | mg/mL | Code level | mg/mL | Code level | mg/mL | |||||
| Factorial points | ||||||||||
| F1 | −1 | 1 | −1 | 1.5 | −1 | 10 | 165.8 ± 0.4 | 0.091 ± 0.044 | −36.9 ± 0.8 | 73.80 |
| F2 | +1 | 3 | −1 | 1.5 | −1 | 10 | 149.7 ± 5.4 | 0.330 ± 0.001 | −47.2 ± 3.3 | 49.19 |
| F3 | −1 | 1 | +1 | 4.5 | −1 | 10 | 192.8 ± 0.7 | 0.109 ± 0.021 | −37.1 ± 1.9 | 78.48 |
| F4 | +1 | 3 | +1 | 4.5 | −1 | 10 | 249.4 ± 1.1 | 0.510 ± 0.170 | −25.2 ± 9.4 | 56.91 |
| F5 | −1 | 1 | −1 | 1.5 | +1 | 18 | 150.1 ± 1.7 | 0.102 ± 0.043 | −34.8 ± 0.5 | 80.53 |
| F6 | +1 | 3 | −1 | 1.5 | +1 | 18 | 122.5 ± 5.3 | 0.194 ± 0.016 | −36.6 ± 2.1 | 53.60 |
| F7 | −1 | 1 | +1 | 4.5 | +1 | 18 | 183.9 ± 3.8 | 0.094 ± 0.024 | −34.6 ± 0.6 | 84.43 |
| F8 | +1 | 3 | +1 | 4.5 | +1 | 18 | 104.6 ± 1.4 | 0.189 ± 0.042 | −29.8 ± 1.4 | 71.15 |
| Axial points | ||||||||||
| F9 | 1.68 | 3.68 | 0 | 3 | 0 | 14 | 105.2 ± 1.8 | 0.267 ± 0.057 | −37.2 ± 1.4 | 41.50 |
| F10 | −1.68 | 0.32 | 0 | 3 | 0 | 14 | 166.0 ± 3.9 | 0.101 ± 0.025 | −30.9 ± 0.3 | 90.79 |
| F11 | 0 | 2 | 1.68 | 5.52 | 0 | 14 | 144.8 ± 3.1 | 0.281 ± 0.015 | −42.1 ± 0.9 | 47.98 |
| F12 | 0 | 2 | −1.68 | 0.48 | 0 | 14 | 110.8 ± 2.6 | 0.120 ± 0.048 | −35.9 ± 0.9 | 55.60 |
| F13 | 0 | 2 | 0 | 3 | 1.68 | 20.72 | 115.5 ± 1.2 | 0.091 ± 0.017 | −35.8 ± 0.1 | 64.57 |
| F14 | 0 | 2 | 0 | 3 | −1.68 | 7.28 | 85.5 ± 2.4 | 0.201 ± 0.039 | −34.7 ± 2.8 | 51.58 |
| Center points | ||||||||||
| F15 | 0 | 2 | 0 | 3 | 0 | 14 | 90.2 ± 1.2 | 0.078 ± 0.039 | −33.6 ± 1.6 | 56.64 |
| F16 | 0 | 2 | 0 | 3 | 0 | 14 | 94.4 ± 4.1 | 0.184 ± 0.123 | −34.2 ± 2.6 | 56.07 |
| Factor | Concentration (mg/mL) |
|---|---|
| [DCF] | 1.2 |
| [Lutrol] | 3.0 |
| [PLGA] | 16.0 |
| Physicochemical Parameter | Before γ-Radiation | After γ-Radiation |
|---|---|---|
| Zav (nm) | 149.0 ± 1.4 | 149.2 ± 0.8 |
| PI | 0.060 ± 0.013 | 0.077 ±0.005 |
| EE (%) | 82.4 ± 0.3 | 80.9 ± 1.8 |
| ZP (mV) | −39.3 ± 1.6 | −38.6 ± 2.2 |
| Compound | Cell Lines IC50 [μg/mL] | ||||
|---|---|---|---|---|---|
| MV4-11 | A-549 | MDA-MB-468 | MCF-7 | MCF-10A | |
| DCF | 23.95 ± 5.9 | 31.02±6.4 | 33.4 ± 1.8 | 30.46 ± 7 | 37.26 ± 1.08 |
| Empty NPs | n.a. | n.a. | n.a. | n.a. | n.a. |
| DCF NPs | 25.74 ± 4.41 | 69.75 ± 9.4 | n.a. | 80.6 ± 10 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteruelas, G.; Souto, E.B.; Espina, M.; García, M.L.; Świtalska, M.; Wietrzyk, J.; Gliszczyńska, A.; Sánchez-López, E. Diclofenac Loaded Biodegradable Nanoparticles as Antitumoral and Antiangiogenic Therapy. Pharmaceutics 2023, 15, 102. https://doi.org/10.3390/pharmaceutics15010102
Esteruelas G, Souto EB, Espina M, García ML, Świtalska M, Wietrzyk J, Gliszczyńska A, Sánchez-López E. Diclofenac Loaded Biodegradable Nanoparticles as Antitumoral and Antiangiogenic Therapy. Pharmaceutics. 2023; 15(1):102. https://doi.org/10.3390/pharmaceutics15010102
Chicago/Turabian StyleEsteruelas, Gerard, Eliana B. Souto, Marta Espina, María Luisa García, Marta Świtalska, Joanna Wietrzyk, Anna Gliszczyńska, and Elena Sánchez-López. 2023. "Diclofenac Loaded Biodegradable Nanoparticles as Antitumoral and Antiangiogenic Therapy" Pharmaceutics 15, no. 1: 102. https://doi.org/10.3390/pharmaceutics15010102
APA StyleEsteruelas, G., Souto, E. B., Espina, M., García, M. L., Świtalska, M., Wietrzyk, J., Gliszczyńska, A., & Sánchez-López, E. (2023). Diclofenac Loaded Biodegradable Nanoparticles as Antitumoral and Antiangiogenic Therapy. Pharmaceutics, 15(1), 102. https://doi.org/10.3390/pharmaceutics15010102









