A Comparative Study between A Protein Based Amorphous Formulation and Other Dissolution Rate Enhancing Approaches: A Case Study with Rifaximin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Amorphous Solid Dispersions by Spray Drying
2.3. Preparation of the Nanocrystalline Formulation by Wet Milling
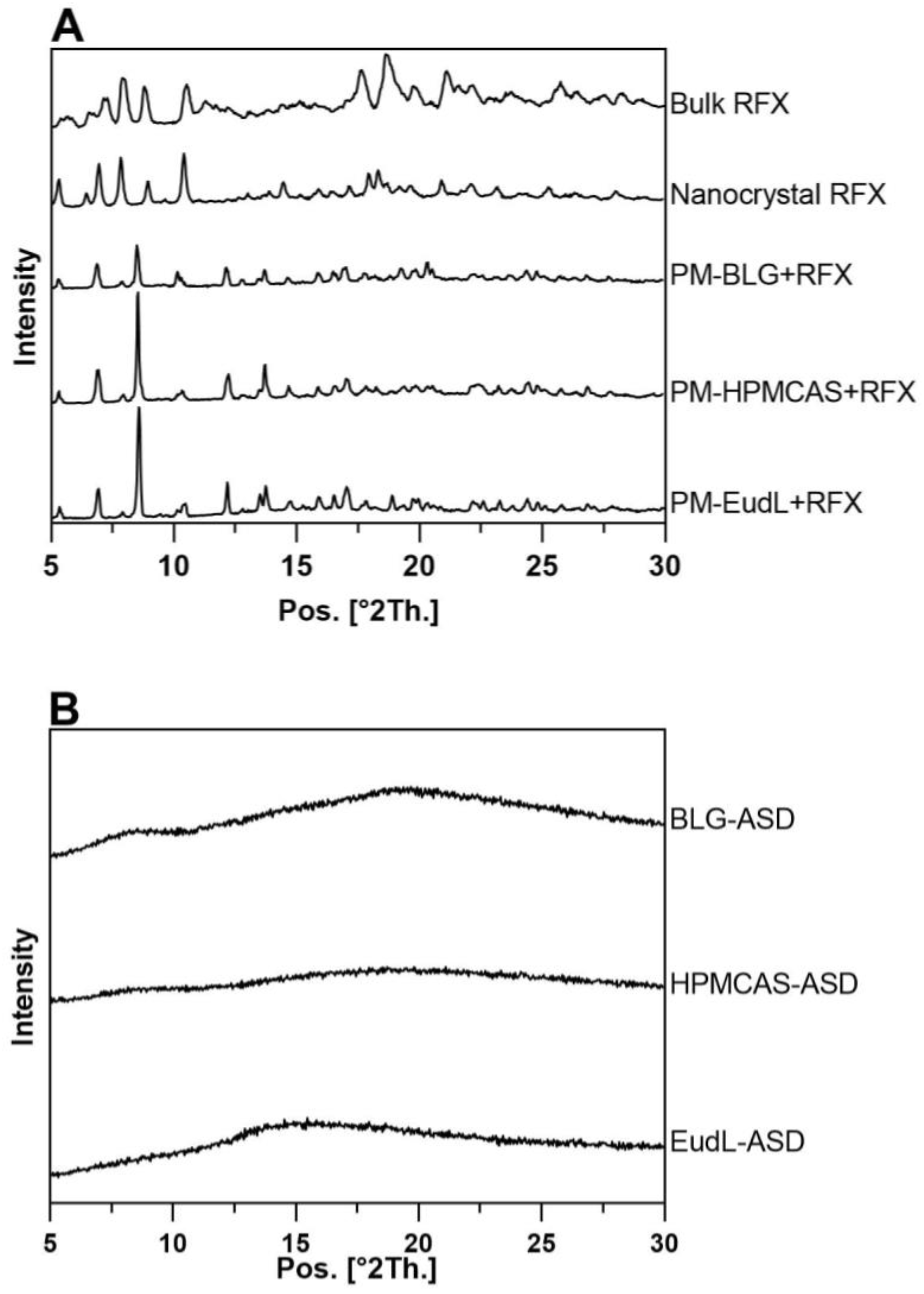
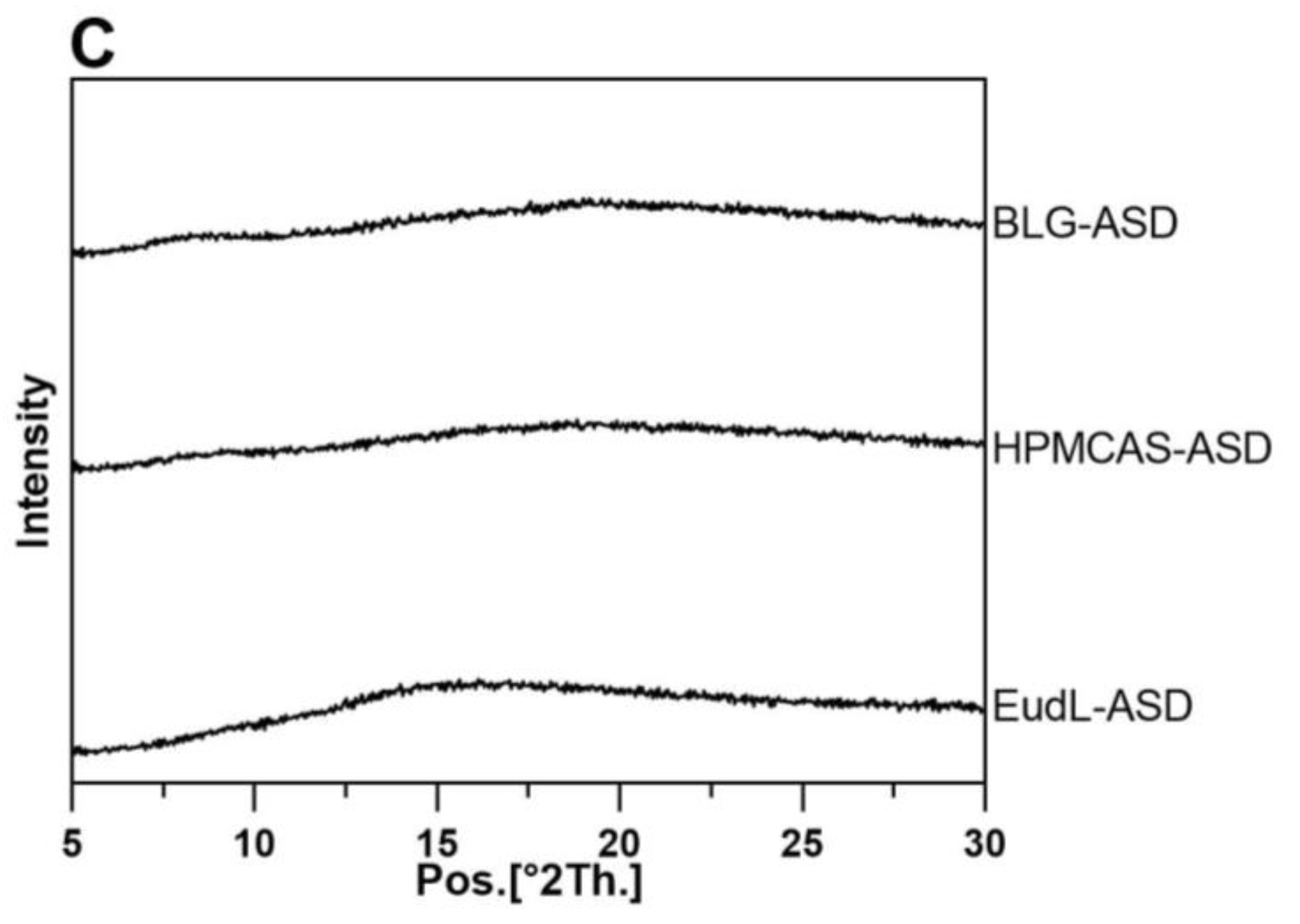
2.4. X-ray Powder Diffraction (XRPD)
2.5. Modulated Differential Scanning Calorimetry (mDSC)
2.6. Thermogravimetric Analysis (TGA)
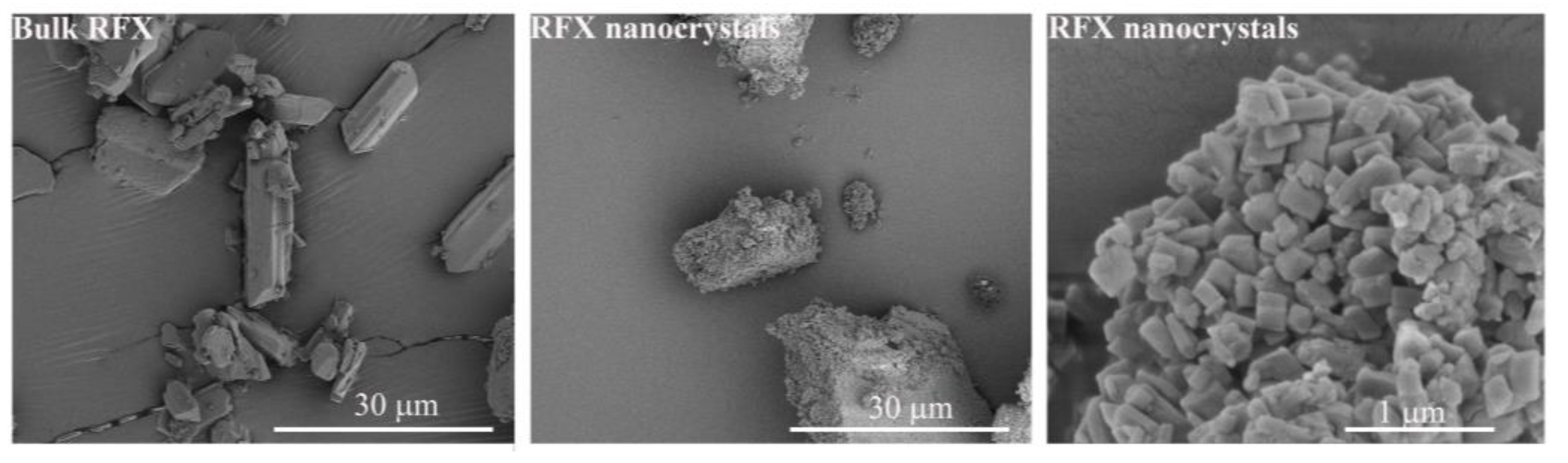
2.7. Scanning Electron Microscopy (SEM)
2.8. Physical Stability
2.9. Powder Dissolution
2.10. High-Performance Liquid Chromatography (HPLC)
3. Results
3.1. Solid-State Characterization and Physical Stability
3.2. Morphology
3.3. Powder Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Sarnes, A.; Ostergaard, J.; Jensen, S.S.; Aaltonen, J.; Rantanen, J.; Hirvonen, J.; Peltonen, L. Dissolution study of nanocrystal powders of a poorly soluble drug by UV imaging and channel flow methods. Eur. J. Pharm. Sci. 2013, 50, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.S.; Zhang, G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug. Deliv. Rev. 2016, 101, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.D.; Lee, P.I. Probing the mechanisms of drug release from amorphous solid dispersions in medium-soluble and medium-insoluble carriers. J. Control Release 2015, 211, 85–93. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [Green Version]
- Kapourani, A.; Andriotis, E.G.; Chachlioutaki, K.; Kontogiannopoulos, K.N.; Klonos, P.A.; Kyritsis, A.; Pavlidou, E.; Bikiaris, D.N.; Fatouros, D.G.; Barmpalexis, P. High-Drug-Loading Amorphous Solid Dispersions via In Situ Thermal Cross-Linking: Unraveling the Mechanisms of Stabilization. Mol. Pharm. 2021, 18, 4393–4414. [Google Scholar] [CrossRef]
- Knopp, M.M.; Tajber, L.; Tian, Y.; Olesen, N.E.; Jones, D.S.; Kozyra, A.; Lobmann, K.; Paluch, K.; Brennan, C.M.; Holm, R.; et al. Comparative Study of Different Methods for the Prediction of Drug-Polymer Solubility. Mol. Pharm. 2015, 12, 3408–3419. [Google Scholar] [CrossRef] [Green Version]
- Pas, T.; Vergauwen, B.; Van den Mooter, G. Exploring the feasibility of the use of biopolymers as a carrier in the formulation of amorphous solid dispersions—Part I: Gelatin. Int. J. Pharmaceut. 2018, 535, 47–58. [Google Scholar] [CrossRef]
- Khoder, M.; Abdelkader, H.; ElShaer, A.; Karam, A.; Najlah, M.; Alany, R.G. Efficient approach to enhance drug solubility by particle engineering of bovine serum albumin. Int. J. Pharm. 2016, 515, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Bohr, A.; Rades, T.; Grohganz, H.; Lobmann, K. Whey proteins as stabilizers in amorphous solid dispersions. Eur. J. Pharm. Sci. 2019, 128, 144–151. [Google Scholar] [CrossRef]
- Descombe, J.; Dubourg, D.; Picard, M.; Palazzini, E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int. J. Clin. Pharmacol. Res. 1994, 14, 51–56. [Google Scholar] [PubMed]
- Beig, A.; Fine-Shamir, N.; Lindley, D.; Miller, J.M.; Dahan, A. Advantageous solubility-permeability interplay when using amorphous solid dispersion (ASD) formulation for the BCS class IV P-gp substrate rifaximin: Simultaneous increase of both the solubility and the permeability. AAPS J. 2017, 19, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Dalal, S.K.; Jahagirdar, H.A. Solid Dispersion of Rifaximin. Patent EP2493456A2, 5 September 2012. [Google Scholar]
- Kogawa, A.C.; Salgado, H.R.N. Status of rifaximin: A review of characteristics, uses and analytical methods. Crit. Rev. Anal. Chem. 2018, 48, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraceni, P.; Vargas, V.; Solà, E.; Alessandria, C.; de Wit, K.; Trebicka, J.; Angeli, P.; Mookerjee, R.P.; Durand, F.; Pose, E. The use of rifaximin in patients with cirrhosis. Hepatology 2021, 74, 1660–1673. [Google Scholar] [CrossRef]
- Tai, C.S.; Chen, Y.Y.; Chen, W.L. β-Lactoglobulin influences human immunity and promotes cell proliferation. BioMed. Res. Int. 2016, 2016, 7123587. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.D.; Calvo, M. Interaction of Beta-Lactoglobulin with Retinol and Fatty-Acids and Its Role as a Possible Biological Function for This Protein—A Review. J. Dairy Sci. 1995, 78, 978–988. [Google Scholar] [CrossRef]
- Viscomi, G.C.; Campana, M.; Barbanti, M.; Grepioni, F.; Polito, M.; Confortini, D.; Rosini, G.; Righi, P.; Cannata, V.; Braga, D. Crystal forms of rifaximin and their effect on pharmaceutical properties. Crystengcomm 2008, 10, 1074–1081. [Google Scholar] [CrossRef]
- Wu, W.Q.; Ueda, H.; Lobmann, K.; Rades, T.; Grohganz, H. Organic acids as co-formers for co-amorphous systems—Influence of variation in molar ratio on the physicochemical properties of the co-amorphous systems. Eur. J. Pharm. Biopharm. 2018, 131, 25–32. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Vaka, S.R.K.; Bommana, M.M.; Desai, D.; Djordjevic, J.; Phuapradit, W.; Shah, N. Excipients for Amorphous Solid Dispersions. In Amorphous Solid Dispersions; Springer: New York, NY, USA, 2014; pp. 123–161. [Google Scholar]
- Hiew, T.N.; Zemlyanov, D.Y.; Taylor, L.S. Balancing Solid-State Stability and Dissolution Performance of Lumefantrine Amorphous Solid Dispersions: The Role of Polymer Choice and Drug-Polymer Interactions. Mol. Pharmaceut. 2022, 19, 392–413. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug. Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Materials | Tgs (°C) | Moisture Content (%) | |
|---|---|---|---|
| Fresh | 5 Weeks | ||
| Bulk RFX | 197.8 | ||
| BLG | 240.4 | ||
| HPMCAS | 121.6 | ||
| EudL | 187.0 | ||
| BLG-ASD | 200.1 | 3.9 | 5.7 |
| HPMCAS-ASD | 153.1 | 1.5 | 1.7 |
| EudL-ASD | 193.2 | 4.1 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, X.; Margrethe Brekstad Kjellin, M.; Schaal, Z.; Zhang, T.; Löbmann, K.; Leng, D. A Comparative Study between A Protein Based Amorphous Formulation and Other Dissolution Rate Enhancing Approaches: A Case Study with Rifaximin. Pharmaceutics 2023, 15, 126. https://doi.org/10.3390/pharmaceutics15010126
Zhuo X, Margrethe Brekstad Kjellin M, Schaal Z, Zhang T, Löbmann K, Leng D. A Comparative Study between A Protein Based Amorphous Formulation and Other Dissolution Rate Enhancing Approaches: A Case Study with Rifaximin. Pharmaceutics. 2023; 15(1):126. https://doi.org/10.3390/pharmaceutics15010126
Chicago/Turabian StyleZhuo, Xuezhi, Maud Margrethe Brekstad Kjellin, Zarah Schaal, Tengyu Zhang, Korbinian Löbmann, and Donglei Leng. 2023. "A Comparative Study between A Protein Based Amorphous Formulation and Other Dissolution Rate Enhancing Approaches: A Case Study with Rifaximin" Pharmaceutics 15, no. 1: 126. https://doi.org/10.3390/pharmaceutics15010126







