Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment
Abstract
:1. Introduction
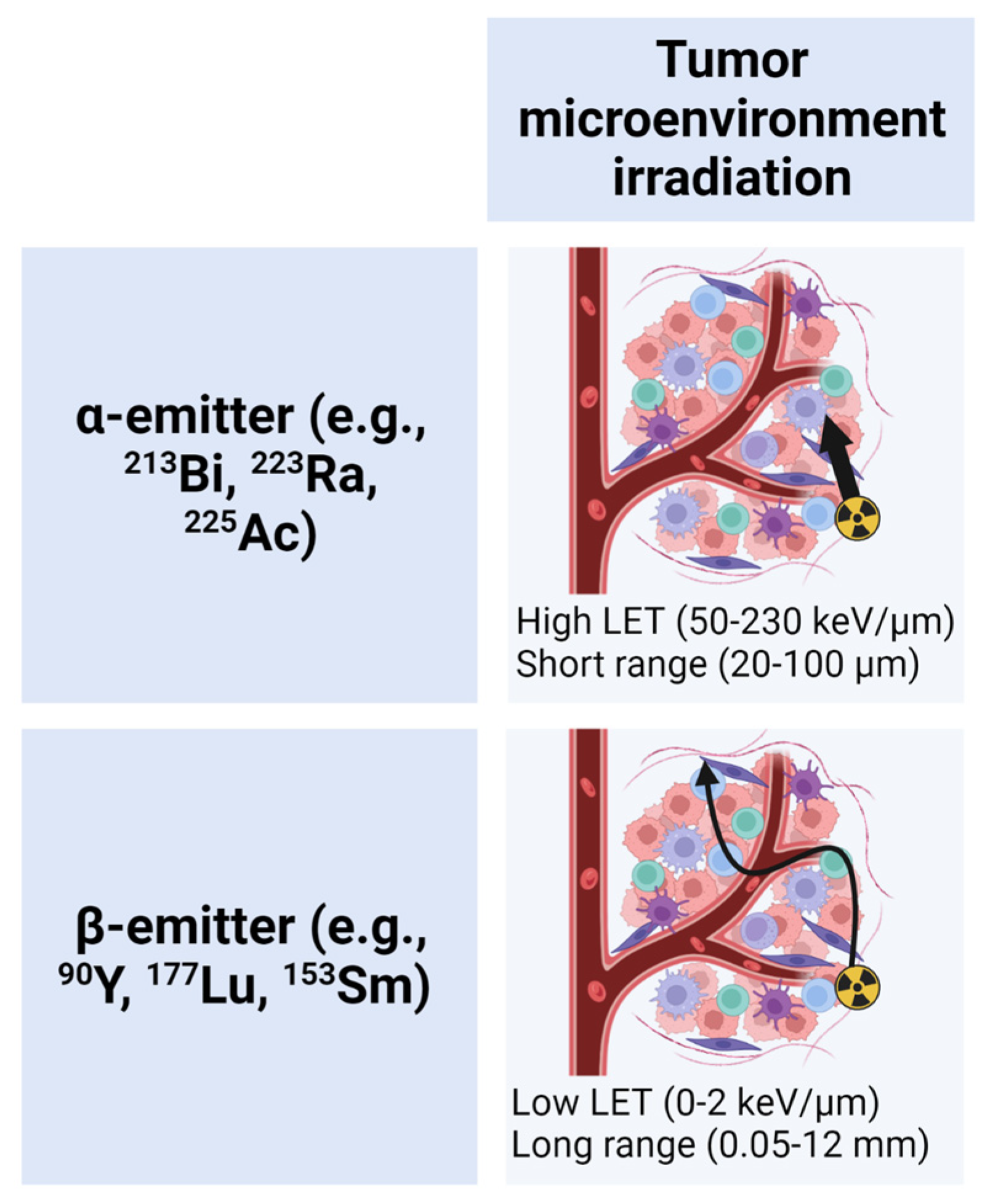
1.1. TRT Radiobiology
1.2. Immunomodulation by TRT
2. Combination TRT and Immunotherapies
2.1. Preclinical Studies
2.2. Clinical Studies
3. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, J.B.; Hussey, R.G.; Nakahara, W.; Sturm, E. Studies on X-ray effects: Vi. Effect of the cellular reaction induced by X-rays on cancer grafts. J. Exp. Med. 1921, 33, 299–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-h2ax in recognition and signaling of dna double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef] [PubMed]
- Rückert, M.; Deloch, L.; Fietkau, R.; Frey, B.; Hecht, M.; Gaipl, U.S. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther. Onkol. 2018, 194, 509–519. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and damps in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Yamazaki, T.; Vanpouille-Box, C.; Demaria, S.; Galluzzi, L. Immunogenic cell death driven by radiation-impact on the tumor microenvironment. Cancer Treat. Res. 2020, 180, 281–296. [Google Scholar]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Helen Barcellos-Hoff, M.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [Green Version]
- Carlson, P.; Morris, Z. Translational development and testing of theranostics in combination with immunotherapies. In Nuclear Medicine and Immunology; Springer: Cham, Switzerland, 2022; pp. 267–280. [Google Scholar]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Pilones, K.A.; Hensler, M.; Daviaud, C.; Kraynak, J.; Fucikova, J.; Galluzzi, L.; Demaria, S.; Formenti, S.C. Converging focal radiation and immunotherapy in a preclinical model of triple negative breast cancer: Contribution of vista blockade. Oncoimmunology 2020, 9, 1830524. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; Van Der Noort, V.; De Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; De Langen, A.J.; et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer. JAMA Oncol. 2019, 5, 1276. [Google Scholar] [CrossRef]
- Raventos, A. An abscopal effect of x-ray upon mouse spleen weight. Radiat. Res. 1954, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Law, A.W.; Mole, R.H. Direct and abscopal effects of x-radiation on the thymus of the weanling rat. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1961, 3, 233–248. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.S.; Guy, E.I.; Francis, D.M.; Gressett, M.M.; Werner, L.R.; Carmichael, L.L.; Yang, R.K.; Armstrong, E.A.; Huang, S.; Navid, F.; et al. In situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Res. 2016, 76, 3929–3941. [Google Scholar] [CrossRef] [Green Version]
- Morris, Z.S.; Guy, E.I.; Werner, L.R.; Carlson, P.M.; Heinze, C.M.; Kler, J.S.; Busche, S.M.; Jaquish, A.A.; Sriramaneni, R.N.; Carmichael, L.L.; et al. Tumor-specific inhibition of in situ vaccination by distant untreated tumor sites. Cancer Immunol. Res. 2018, 6, 825–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated t cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N.; et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed b-cell non-hodgkin’s lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463. [Google Scholar] [CrossRef]
- Bernier, M.-O.; Leenhardt, L.; Hoang, C.; Aurengo, A.; Mary, J.-Y.; Menegaux, F.; Enkaoua, E.; Turpin, G.; Chiras, J.; Saillant, G.; et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2001, 86, 1568–1573. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)lu]-psma-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (lupsma trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Fitzgerald, P.A.; Goldsby, R.E.; Huberty, J.P.; Price, D.C.; Hawkins, R.A.; Veatch, J.J.; Cruz, F.D.; Jahan, T.M.; Linker, C.A.; Damon, L.; et al. Malignant pheochromocytomas and paragangliomas: A phase ii study of therapy with high-dose 131i-metaiodobenzylguanidine (131i-mibg). Ann. N. Y. Acad. Sci. 2006, 1073, 465–490. [Google Scholar] [CrossRef]
- Coldwell, D.; Sangro, B.; Salem, R.; Wasan, H.; Kennedy, A. Radioembolization in the treatment of unresectable liver tumors: Experience across a range of primary cancers. Am. J. Clin. Oncol. 2012, 35, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czernin, J. Molecular imaging and therapy with a purpose: A renaissance of nuclear medicine. J. Nucl. Med. 2017, 58, 21a–22a. [Google Scholar]
- Dolgin, E. Radioactive drugs emerge from the shadows to storm the market. Nat. Biotechnol. 2018, 36, 1125–1127. [Google Scholar] [CrossRef]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–psma-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and nontargeted alpha-particle therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef]
- Timm, S.; Lorat, Y.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Clustered dna damage concentrated in particle trajectories causes persistent large-scale rearrangements in chromatin architecture. Radiother. Oncol. 2018, 129, 600–610. [Google Scholar] [CrossRef]
- Brady, D.; O’Sullivan, J.; Prise, K. What is the role of the bystander response in radionuclide therapies? Front. Oncol. 2013, 3, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G. Mird pamphlet no. 22 (abridged): Radiobiology and dosimetry of α-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, S.; Devita, V.; Rosenberg, S. Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Pouget, J.P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015, 2, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, A.A.; Pedley, R.B.; Green, A.J.; Boxer, G.M.; Boden, R.; Bhatia, J.; Morris, R.; Begent, R.H. Antibody and radionuclide characteristics and the enhancement of the effectiveness of radioimmunotherapy by selective dose delivery to radiosensitive areas of tumour. Int. J. Radiat. Biol. 2002, 78, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Haeck, J.; Bol, K.; Bison, S.; van Tiel, S.; Koelewijn, S.; de Jong, M.; Veenland, J.; Bernsen, M. Optimized time-resolved imaging of contrast kinetics (tricks) in dynamic contrast-enhanced mri after peptide receptor radionuclide therapy in small animal tumor models. Contrast Media Mol. Imaging 2015, 10, 413–420. [Google Scholar] [CrossRef]
- Bussink, J.; Kaanders, J.H.; van der Kogel, A.J. Tumor hypoxia at the micro-regional level: Clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother. Oncol. 2003, 67, 3–15. [Google Scholar] [CrossRef]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Wahl, R.L.; Sunderland, J. Radiopharmaceutical dosimetry for cancer therapy: From theory to practice. J. Nucl. Med. 2021, 62, 1S. [Google Scholar] [CrossRef]
- Capala, J.; Graves, S.A.; Scott, A.; Sgouros, G.; James, S.S.; Zanzonico, P.; Zimmerman, B.E. Dosimetry for radiopharmaceutical therapy: Current practices and commercial resources. J. Nucl. Med. 2021, 62, 3S. [Google Scholar] [CrossRef]
- Sgouros, G.; Dewaraja, Y.K.; Escorcia, F.; Graves, S.A.; Hope, T.A.; Iravani, A.; Pandit-Taskar, N.; Saboury, B.; James, S.S.; Zanzonico, P.B. Tumor response to radiopharmaceutical therapies: The knowns and the unknowns. J. Nucl. Med. 2021, 62, 12S. [Google Scholar]
- Wahl, R.L.; Sgouros, G.; Iravani, A.; Jacene, H.; Pryma, D.; Saboury, B.; Capala, J.; Graves, S.A. Normal-tissue tolerance to radiopharmaceutical therapies, the knowns and the unknowns. J. Nucl. Med. 2021, 62, 23S. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Peterson, A.; Van, B.; Fedrigo, R.; Carlson, J.; Sunderland, J.; Frey, E.; Dewaraja, Y.K. An international study of factors affecting variability of dosimetry calculations, part 1: Design and early results of the snmmi dosimetry challenge. J. Nucl. Med. 2021, 62, 36S. [Google Scholar] [CrossRef]
- Graves, S.A.; Bageac, A.; Crowley, J.R.; Merlino, D.A.M. Reimbursement approaches for radiopharmaceutical dosimetry: Current status and future opportunities. J. Nucl. Med. 2021, 62, 48S. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Iravani, A.; Lee, D.; Jacene, H.; Pryma, D.; Hope, T.; Saboury, B.; Capala, J.; Wahl, R.L. Dosimetry in clinical radiopharmaceutical therapy of cancer: Practicality versus perfection in current practice. J. Nucl. Med. 2021, 62, 60S. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Eberlein, U.; Gear, J.; Konijnenberg, M.; Kunikowska, J. Dosimetry for radiopharmaceutical therapy: The european perspective. J. Nucl. Med. 2021, 62, 73S. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, J.; Benboubker, V.; Akil, H.; Hennino, A.; Auzeloux, P.; Besse, S.; Pereira, B.; Delorme, S.; Mansard, S.; D’Incan, M.; et al. Immune checkpoint inhibitors reverse tolerogenic mechanisms induced by melanoma targeted radionuclide therapy. Cancer Immunol. Immunother. 2020, 69, 2075–2088. [Google Scholar] [CrossRef]
- Malamas, A.S.; Gameiro, S.R.; Knudson, K.M.; Hodge, J.W. Sublethal exposure to alpha radiation (223ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic t lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget 2016, 7, 86937–86947. [Google Scholar] [CrossRef] [Green Version]
- Gorin, J.B.; Ménager, J.; Gouard, S.; Maurel, C.; Guilloux, Y.; Faivre-Chauvet, A.; Morgenstern, A.; Bruchertseifer, F.; Chérel, M.; Davodeau, F.; et al. Antitumor immunity induced after α irradiation. Neoplasia 2014, 16, 319–328. [Google Scholar] [CrossRef]
- Chakraborty, M.; Wansley, E.K.; Carrasquillo, J.A.; Yu, S.; Paik, C.H.; Camphausen, K.; Becker, M.D.; Goeckeler, W.F.; Schlom, J.; Hodge, J.W. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance t cell-mediated killing. Clin. Cancer Res. 2008, 14, 4241–4249. [Google Scholar] [CrossRef] [Green Version]
- Jagodinsky, J.C.; Jin, W.J.; Bates, A.M.; Hernandez, R.; Grudzinski, J.J.; Marsh, I.R.; Chakravarty, I.; Arthur, I.S.; Zangl, L.M.; Brown, R.J.; et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics 2021, 11, 6120–6137. [Google Scholar] [CrossRef]
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabb3631. [Google Scholar] [CrossRef]
- Creemers, J.H.A.; van der Doelen, M.J.; van Wilpe, S.; Hermsen, R.; Duiveman-de Boer, T.; Somford, D.M.; Janssen, M.J.R.; Sedelaar, J.P.M.; Mehra, N.; Textor, J.; et al. Immunophenotyping reveals longitudinal changes in circulating immune cells during radium-223 therapy in patients with metastatic castration-resistant prostate cancer. Front. Oncol. 2021, 11, 667658. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Walker, K.L.; Grudzinski, J.J.; Aluicio-Sarduy, E.; Patel, R.; Zahm, C.D.; Pinchuk, A.N.; Massey, C.F.; Bitton, A.N.; Brown, R.J.; et al. 90y-nm600 targeted radionuclide therapy induces immunologic memory in syngeneic models of t-cell non-hodgkin’s lymphoma. Commun. Biol. 2019, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Fu, K.; Lin, Q.; Wen, X.; Jacobson, O.; Sun, L.; Wu, H.; Zhang, X.; Guo, Z.; et al. Integrin alphavbeta3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 2019, 9, 7948–7960. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zeng, X.; Shi, C.; Liu, J.; Zhang, Y.; Shi, M.; Li, J.; Chen, H.; Zhuang, R.; Chen, X.; et al. Optimum combination of radiopharmaceuticals-based targeting-triggering-therapy effect and pd-l1 blockade immunotherapy. Adv. Ther. 2022, 2200193. [Google Scholar] [CrossRef]
- Zukotynski, K.; Jadvar, H.; Capala, J.; Fahey, F. Targeted radionuclide therapy: Practical applications and future prospects: Supplementary issue: Biomarkers and their essential role in the development of personalised therapies (a). Biomark. Cancer 2016, 8, BIC-S31804. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, M.; Gelbard, A.; Carrasquillo, J.A.; Yu, S.; Mamede, M.; Paik, C.H.; Camphausen, K.; Schlom, J.; Hodge, J.W. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol. Immunother. 2008, 57, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; Karacay, H.; Johnson, C.R.; Litwin, S.; Rossi, E.A.; McBride, W.J.; Chang, C.H.; Goldenberg, D.M. Pretargeted versus directly targeted radioimmunotherapy combined with anti-cd20 antibody consolidation therapy of non-hodgkin lymphoma. J. Nucl. Med. 2009, 50, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Beaino, W.; Fecek, R.J.; Fabian, K.P.L.; Laymon, C.M.; Kurland, B.F.; Storkus, W.J.; Anderson, C.J. Combined vla-4-targeted radionuclide therapy and immunotherapy in a mouse model of melanoma. J. Nucl. Med. 2018, 59, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Jiao, R.; Allen, K.J.H.; Malo, M.E.; Rickles, D.; Dadachova, E. Evaluating the combination of radioimmunotherapy and immunotherapy in a melanoma mouse model. Int. J. Mol. Sci. 2020, 21, 773. [Google Scholar] [CrossRef] [Green Version]
- Malo, M.E.; Allen, K.J.H.; Jiao, R.; Frank, C.; Rickles, D.; Dadachova, E. Mechanistic insights into synergy between melanin-targeting radioimmunotherapy and immunotherapy in experimental melanoma. Int. J. Mol. Sci. 2020, 21, 8721. [Google Scholar] [CrossRef] [PubMed]
- Dabagian, H.; Taghvaee, T.; Martorano, P.; Martinez, D.; Samanta, M.; Watkins, C.M.; Chai, R.; Mansfield, A.; Graham, T.J.; Maris, J.M.; et al. Parp targeted alpha-particle therapy enhances response to pd-1 immune-checkpoint blockade in a syngeneic mouse model of glioblastoma. ACS Pharmacol. Transl. Sci. 2021, 4, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Current, K.; Mona, C.E.; Nyiranshuti, L.; Hikmat, F.; Radu, C.G.; Lückerath, K. Immune-checkpoint blockade enhances (225)ac-psma617 efficacy in a mouse model of prostate cancer. J. Nucl. Med. 2021, 62, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, D.; Lee, D.; Cheng, Y.; Baumhover, N.J.; Marks, B.M.; Sagastume, E.A.; Ballas, Z.K.; Johnson, F.L.; Morris, Z.S.; et al. Targeted alpha-particle radiotherapy and immune checkpoint inhibitors induces cooperative inhibition on tumor growth of malignant melanoma. Cancers 2021, 13, 3676. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.; Nigam, S.; Tatum, D.S.; Raphael, I.; Xu, J.; Kumar, R.; Plakseychuk, E.; Latoche, J.D.; Vincze, S.; Li, B.; et al. Novel theranostic agent for pet imaging and targeted radiopharmaceutical therapy of tumour-infiltrating immune cells in glioma. eBioMedicine 2021, 71, 103571. [Google Scholar] [CrossRef]
- Guzik, P.; Siwowska, K.; Fang, H.Y.; Cohrs, S.; Bernhardt, P.; Schibli, R.; Müller, C. Promising potential of [(177)lu]lu-dota-folate to enhance tumor response to immunotherapy-a preclinical study using a syngeneic breast cancer model. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, P.; Cruciani, V.; Berg-Larsen, A.; Schlicker, A.; Mobergslien, A.; Bartnitzky, L.; Berndt, S.; Zitzmann-Kolbe, S.; Kamfenkel, C.; Stargard, S.; et al. Immunostimulatory effects of targeted thorium-227 conjugates as single agent and in combination with anti-pd-l1 therapy. J. Immunother. Cancer 2021, 9, e002387. [Google Scholar] [CrossRef]
- Ferreira, C.d.A.; Heidari, P.; Ataeinia, B.; Sinevici, N.; Granito, A.; Kumar, H.M.; Wehrenberg-Klee, E.; Mahmood, U. Immune checkpoint inhibitor-mediated cancer theranostics with radiolabeled anti-granzyme b peptide. Pharmaceutics 2022, 14, 1460. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, X.; Cheng, X.; Zeng, X.; Liu, J.; Zhang, Y.; Li, Y.; Chen, H.; Huang, J.; Guo, Z.; et al. Pd-l1-targeted radionuclide therapy combined with αpd-l1 antibody immunotherapy synergistically improves the antitumor effect. Mol. Pharm. 2022, 19, 3612–3622. [Google Scholar] [CrossRef]
- Potluri, H.K.; Ferreira, C.A.; Grudzinski, J.; Massey, C.; Aluicio-Sarduy, E.; Engle, J.W.; Kwon, O.; Marsh, I.R.; Bednarz, B.P.; Hernandez, R.; et al. Antitumor efficacy of (90)y-nm600 targeted radionuclide therapy and pd-1 blockade is limited by regulatory t cells in murine prostate tumors. J. Immunother. Cancer 2022, 10, e005060. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to ctla-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Heery, C.R.; Madan, R.A.; Stein, M.N.; Stadler, W.M.; Di Paola, R.S.; Rauckhorst, M.; Steinberg, S.M.; Marté, J.L.; Chen, C.C.; Grenga, I.; et al. Samarium-153-edtmp (quadramet®) with or without vaccine in metastatic castration-resistant prostate cancer: A randomized phase 2 trial. Oncotarget 2016, 7, 69014–69023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B. Sipuleucel-t immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase iii trial of immunologic therapy with sipuleucel-t (apc8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006, 24, 3089–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, C.H.; Fu, W.; Wang, H.; Park, J.C.; DeWeese, T.L.; Tran, P.T.; Song, D.Y.; King, S.; Afful, M.; Hurrelbrink, J.; et al. Randomized phase ii trial of sipuleucel-t with or without radium-223 in men with bone-metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2021, 27, 1623–1630. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. ²¹³bi-dotatoc receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225ac-psma-617 for psma-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.D.; Kwak, L.; Cheung, A.; Tripathi, A.; Pace, A.F.; Van Allen, E.M.; Kilbridge, K.L.; Wei, X.X.; McGregor, B.A.; Pomerantz, M.; et al. Randomized phase ii study evaluating the addition of pembrolizumab to radium-223 in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2021, 39, 98. [Google Scholar] [CrossRef]
- Fong, L.; Morris, M.J.; Sartor, O.; Higano, C.S.; Pagliaro, L.; Alva, A.; Appleman, L.J.; Tan, W.; Vaishampayan, U.; Porcu, R.; et al. A phase ib study of atezolizumab with radium-223 dichloride in men with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2021, 27, 4746–4756. [Google Scholar] [CrossRef]
- Sandhu, S.; Joshua, A.M.; Emmett, L.; Spain, L.A.; Horvath, L.; Crumbaker, M.; Anton, A.; Wallace, R.; Pasam, A.; Bressel, M.; et al. Prince: Phase i trial of 177lu-psma-617 in combination with pembrolizumab in patients with metastatic castration-resistant prostate cancer (mcrpc). J. Clin. Oncol. 2022, 40, 5017. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Subramaniam, D.S.; Torres, T.; Loda, M.; Esposito, G.; Giaccone, G. Phase i study of the (177)lu-dota(0)-tyr(3)-octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J. Immunother. Cancer 2020, 8, e000980. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Sindrilaru, A.; Crisan, D.; Thaiss, W.; Steinacker, J.; Beer, M.; Wiegel, T.; Scharffetter-Kochanek, K.; Beer, A.J.; Prasad, V. Short-interval, low-dose peptide receptor radionuclide therapy in combination with pd-1 checkpoint immunotherapy induces remission in immunocompromised patients with metastatic merkel cell carcinoma. Pharmaceutics 2022, 14, 1466. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandus, J.; Fendler, W.P.; Lueckerath, K.; Berliner, C.; Kurzidem, S.; Hadaschik, E.; Klode, J.; Zimmer, L.; Livingstone, E.; Schadendorf, D.; et al. Response to combined peptide receptor radionuclide therapy and checkpoint immunotherapy with ipilimumab plus nivolumab in metastatic merkel cell carcinoma. J. Nucl. Med. 2022, 63, 396–398. [Google Scholar] [CrossRef] [PubMed]

| Publication | Publication Year | TRT | Target | ICI | Disease Model |
|---|---|---|---|---|---|
| Choi et al. [61] | 2018 | 177Lu-LLP2A | VLA-4 | anti-PD-1/anti-PD-L1 + anti-CTLA-4 | B16F10 melanoma |
| Chen et al. [56] | 2019 | 177Lu-EB-RGD | Integrin αVβ3 | anti-PD-L1 | MC38 colon adenocarcinoma |
| Rouanet et al. [48] | 2020 | 131I-ICF01012 | Melanin | anti-CTLA-4, anti-PD-L1, anti-PD-1 | B16F10 melanoma |
| Jiao et al. [62] | 2020 | 213Bi-h8C3 | Melanin | anti-PD-1 | Cloudman S91 melanoma |
| Malo et al. [63] | 2020 | 177Lu- or 225Ac-h8C3 | Melanin | anti-PD-1 | Cloudman S91 melanoma |
| Dabagian et al. [64] | 2021 | 211At-MM4 | PARP-1 | anti-PD-1 | GL261 glioblastoma |
| Czernin et al. [65] | 2021 | 225Ac-PSMA617 | Prostate Specific Membrane Antigen (PSMA) | anti-PD-1 | RM1-PGLS prostate cancer |
| Jagodinsky et al. [52] | 2021 | 90Y-NM600 | Lipid rafts | anti-CTLA-4 + anti-PD-L1 | MOC2 head and neck squamous cell carcinoma |
| Patel et al. [53] | 2021 | 90Y-NM600 | Lipid rafts | anti-CTLA-4 +/− anti-PD-L1 | B78 melanoma, B16 melanoma, 4T1 breast cancer, NXS2 neuroblastoma |
| Li et al. [66] | 2021 | 212Pb-VMT01 | Melanocortin 1 receptor | anti-CTLA-4 + anti-PD-1 | B16F10 melanoma, YUMM1.7 melanoma |
| Foster et al. [67] | 2021 | 177Lu-Lumi804-αCD11b | CD11b+ cells | anti-CTLA-4 + anti-PD-1 | GL261 glioma |
| Guzik et al. [68] | 2021 | [177Lu]Lu-DOTA-folate | Folate receptor | anti-CTLA-4 | NF9006 breast cancer |
| Lejeune et al. [69] | 2021 | 227Th-anetumab corixetan | Mesothelin | anti-PD-L1 | MC38-hMSLN (human mesothelin) colon adenocarcinoma |
| Ferreira et al. [70] | 2022 | 90Y-GZP | Granzyme B | anti-CTLA-4 + anti-PD-1 | MC38 colon adenocarcinoma, CT26 colon carcinoma |
| Wen et al. [71] | 2022 | 131I-anti-PD-L1 | Programmed death receptor ligand 1 (PD-L1) | anti-PD-L1 | MC38 colon adenocarcinoma, CT26 colon carcinoma |
| Potluri et al. [72] | 2022 | 90Y-NM600 | Lipid rafts | anti-CTLA-4 +/− anti-PD-1 | TRAMP-C1 prostate cancer, MycCaP prostate cancer |
| Wen et al. [57] | 2022 | 177Lu-DOTA-EB-cRGDfK | Integrin αVβ3 | anti-PD-L1 | MC38 colon adenocarcinoma, CT26 colon carcinoma |
| Clinical Trial Identifier | Study Start Date | TRT | Target | Immunotherapy | Disease | Status |
|---|---|---|---|---|---|---|
| NCT00438880 | Oct 2004 | 90Y ibritumomab tiuxetan | CD20 | Agatolimod sodium | Non-Hodgkin lymphoma | Phase I/II; Completed |
| NCT00450619 | Feb 2007 | 153Sm-EDTMP | Bone metastases | Prostate-specific antigen (PSA)/TRICOM vaccine | Metastatic castration resistant prostate cancer (mCRPC) | Phase II; Completed |
| NCT02463799 | Feb 2016 | 223Ra | Bone metastases | Sipuleucel-T | mCRPC | Phase II; Completed |
| NCT02814669 | Sep 2016 | 223Ra | Bone metastases | Atezolizumab | mCRPC | Phase Ib; Completed |
| NCT03093428 | Jun 2017 | 223Ra | Bone metastases | Pembrolizumab | mCRPC | Phase II; Active, not recruiting |
| NCT03215095 | Jul 2017 | 131I | Recombinant human thyroid stimulating hormone (rhTSH) stimulated thyroid | Durvalumab | Thyroid cancer | Phase I; Active, not recruiting |
| NCT03325816 | Nov 2017 | 177Lu-DOTA0-Tyr3-Octreotate | Somatostatin Receptor (SSTR) | Nivolumab | Advanced lung neuroendocrine tumors (NETs) | Phase I; Completed |
| NCT02914405 (MiNivAN) | May 2018 | 131I-meta-iodobenzylguanidine (MIBG) | Norepinephrine Transporter | Nivolumab | Relapsed/ refractory pediatric neuroblastoma | Phase I; Recruiting |
| NCT03457948 | Aug 2018 | 177Lu-DOTA0-Tyr3-Octreotate | SSTR | Pembrolizumab | Neuroendocrine neoplasm | Phase II; Recruiting |
| NCT03805594 | May 2019 | 177Lu-PSMA-617 | Prostate-Specific Membrane Antigen (PSMA) | Pembrolizumab | mCRPC | Phase Ib; Active, not recruiting |
| NCT03658447 (PRINCE) | Jul 2019 | 177Lu-PSMA-617 | PSMA | Pembrolizumab | mCRPC | Phase Ib/II; Active, not recruiting |
| NCT04071236 | Dec 2019 | 223Ra | Bone metastases | Avelumab | mCRPC | Phase I/II; Recruiting |
| NCT04261855 (GoTHAM) | Mar 2020 | 177Lu-DOTATATE | SSTR | Avelumab | Metastatic Merkel Cell Carcinoma | Phase Ib/II; Recruiting |
| NCT03996473 | Mar 2020 | 223Ra | Bone metastases | Pembrolizumab | Non-small cell lung cancer (NSCLC) | Phase I; Active, not recruiting |
| NCT04109729 | Aug 2020 | 223Ra | Bone metastases | Nivolumab | mCRPC | Phase Ib/II; Recruiting |
| NCT04946370 | Aug 2021 | 225Ac-J591 | PSMA | Pembrolizumab | mCRPC | Phase I/II; Recruiting |
| NCT05583708 | Est. Feb 2023 | 177Lu-DOTATATE | SSTR | Pembrolizumab | Merkel Cell Carcinoma | Phase II; Not yet recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerr, C.P.; Grudzinski, J.J.; Nguyen, T.P.; Hernandez, R.; Weichert, J.P.; Morris, Z.S. Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics 2023, 15, 128. https://doi.org/10.3390/pharmaceutics15010128
Kerr CP, Grudzinski JJ, Nguyen TP, Hernandez R, Weichert JP, Morris ZS. Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics. 2023; 15(1):128. https://doi.org/10.3390/pharmaceutics15010128
Chicago/Turabian StyleKerr, Caroline P., Joseph J. Grudzinski, Thanh Phuong Nguyen, Reinier Hernandez, Jamey P. Weichert, and Zachary S. Morris. 2023. "Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment" Pharmaceutics 15, no. 1: 128. https://doi.org/10.3390/pharmaceutics15010128
APA StyleKerr, C. P., Grudzinski, J. J., Nguyen, T. P., Hernandez, R., Weichert, J. P., & Morris, Z. S. (2023). Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics, 15(1), 128. https://doi.org/10.3390/pharmaceutics15010128






