Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration
Abstract
:1. Introduction
2. Hydrogels: Distinctive Properties for Craniomaxillofacial Bone Regeneration
2.1. Unique Physicochemical Properties Enabling Communication with Cells
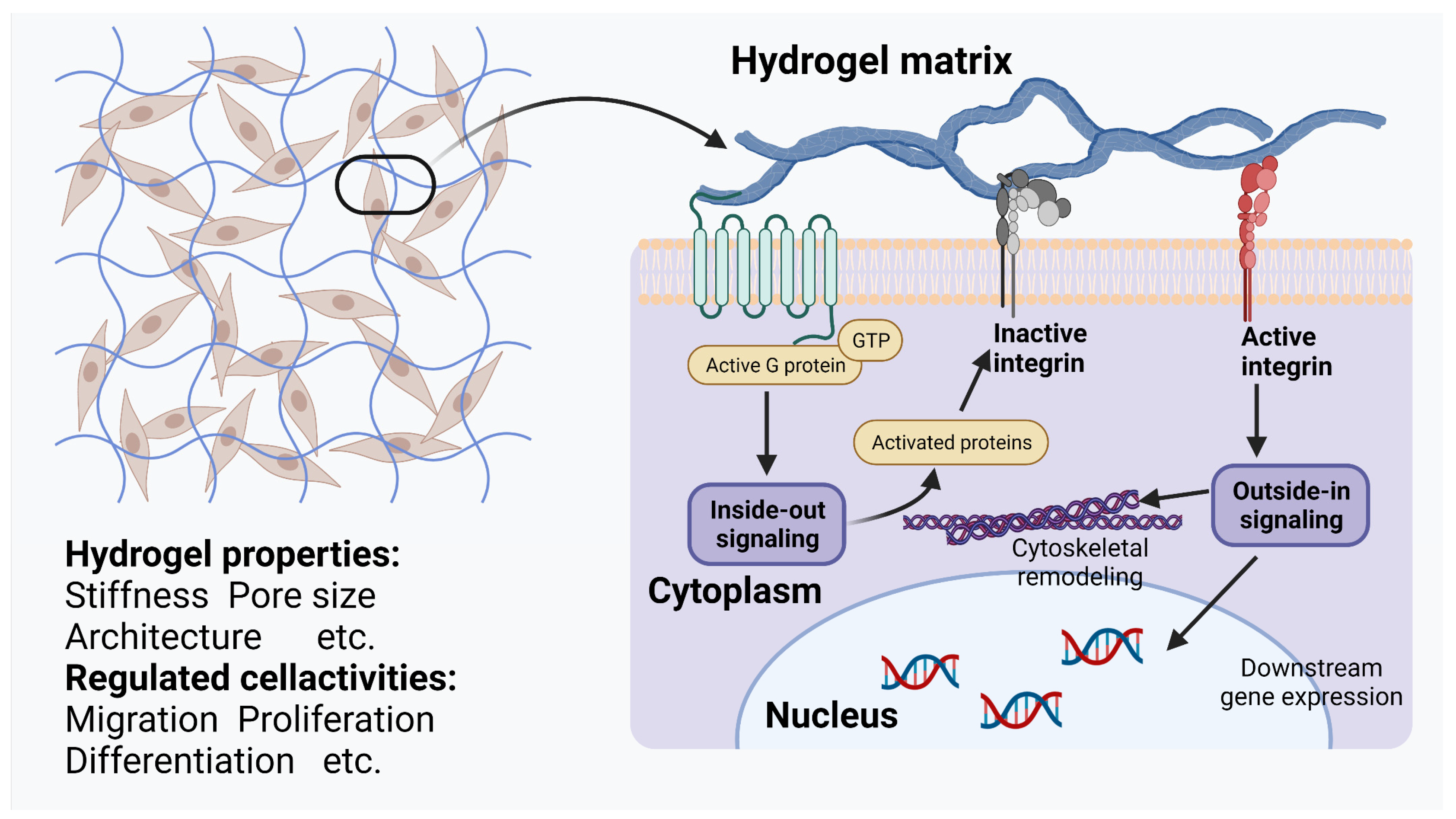
2.2. Wide Variety of Drug Delivery Methods
2.2.1. Physical Loading
2.2.2. Covalent Conjugation and Electrostatic Interaction
2.3. Stimuli-Responsive Hydrogels That Can Be Applied to Craniomaxillofacial Bone Reconstruction
2.3.1. Enzyme-Responsive Hydrogels
2.3.2. Temperature-Responsive Hydrogels
2.3.3. Light-Responsive Hydrogels
2.3.4. pH-Responsive Hydrogels
3. Smart Hydrogels: Application in Craniomaxillofacial Bone Engineering
3.1. Appropriate Structure for Carrying Cargo
3.2. Smarter “Packing” of a Wide Range of Cargoes
3.3. Responding More Intelligently to “Deliver” Cargoes
4. Discussion
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Gsaxner, C.; Pepe, A.; Morais, A.; Alves, V.; von Campe, G.; Wallner, J.; Egger, J. Synthetic Skull Bone Defects for Automatic Patient-Specific Craniofacial Implant Design. Sci. Data 2021, 8, 36. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Zivkovic, P.; Petrovic, D.; Stefanovic, V. Craniofacial Bone Tissue Engineering. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. Hydrogels for Soft Machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Dehli, F.; Rebers, L.; Stubenrauch, C.; Southan, A. Highly Ordered Gelatin Methacryloyl Hydrogel Foams with Tunable Pore Size. Biomacromolecules 2019, 20, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An Engineered Cell-Laden Adhesive Hydrogel Promotes Craniofacial Bone Tissue Regeneration in Rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An Introduction to Bone Tissue Engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent Progress in Extrusion 3D Bioprinting of Hydrogel Biomaterials for Tissue Regeneration: A Comprehensive Review with Focus on Advanced Fabrication Techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, e2000095. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Z.; Zhao, X.; Luo, Y.; Ou, Y.; Kang, P.; Tian, M. A Bone Regeneration Strategy via Dual Delivery of Demineralized Bone Matrix Powder and Hypoxia-Pretreated Bone Marrow Stromal Cells Using an Injectable Self-Healing Hydrogel. J. Mater. Chem. B 2021, 9, 479–493. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Gao, R.; Liu, X.; Feng, Z.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Biomimetic Glycopeptide Hydrogel Coated PCL/NHA Scaffold for Enhanced Cranial Bone Regeneration via Macrophage M2 Polarization-Induced Osteo-Immunomodulation. Biomaterials 2022, 285, 121538. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Sarrion, P.; Hasani-Sadrabadi, M.M.; Aghaloo, T.; Wu, B.M.; Moshaverinia, A. Regulation of the Fate of Dental-Derived Mesenchymal Stem Cells Using Engineered Alginate-GelMA Hydrogels. J. Biomed. Mater. Res. A 2017, 105, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.A.; Carreira, A.C.O.; Sipert, C.R.; Uehara, C.M.; Moreira, M.S.N.; Freire, L.; Pelissari, C.; Kossugue, P.M.; de Araújo, D.R.; Sogayar, M.C.; et al. Photobiomodulation of Mesenchymal Stem Cells Encapsulated in an Injectable RhBMP4-Loaded Hydrogel Directs Hard Tissue Bioengineering. J. Cell. Physiol. 2018, 233, 4907–4918. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug. Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Hung, W.-C.; Chen, S.-H.; Paul, C.D.; Stroka, K.M.; Lo, Y.-C.; Yang, J.T.; Konstantopoulos, K. Distinct Signaling Mechanisms Regulate Migration in Unconfined versus Confined Spaces. J. Cell Biol. 2013, 202, 807–824. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Kim, M.J.; Lam, T.Y.; McGrail, D.J.; Dawson, M.R. Architectural and Mechanical Cues Direct Mesenchymal Stem Cell Interactions with Crosslinked Gelatin Scaffolds. Tissue Eng. Part A 2014, 20, 3252–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of Cell Binding to Collagen and Gelatin: A Study of the Effect of 2D and 3D Architecture and Surface Chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Cha, J.; Jang, M.; Kim, P. Hyaluronic Acid-Based Extracellular Matrix Triggers Spontaneous M2-like Polarity of Monocyte/Macrophage. Biomater. Sci. 2019, 7, 2264–2271. [Google Scholar] [CrossRef]
- Roncada, T.; Bonithon, R.; Blunn, G.; Roldo, M. Soft Substrates Direct Stem Cell Differentiation into the Chondrogenic Lineage without the Use of Growth Factors. J. Tissue Eng. 2022, 13, 20417314221122120. [Google Scholar] [CrossRef]
- Ting, M.S.; Travas-Sejdic, J.; Malmström, J. Modulation of Hydrogel Stiffness by External Stimuli: Soft Materials for Mechanotransduction Studies. J. Mater. Chem. B 2021, 9, 7578–7596. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Whelan, I.T.; Sathy, B.N.; Carroll, S.F.; Kelly, D.J. Soft Hydrogel Environments That Facilitate Cell Spreading and Aggregation Preferentially Support Chondrogenesis of Adult Stem Cells. Macromol. Biosci. 2022, 22, 2100365. [Google Scholar] [CrossRef]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the Role of Nanoparticle Shape in Enhancing Hydrogel Adhesive and Mechanical Properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.; Babhadiashar, N.; Barrett-Catton, E.; Asuri, P. Role of Nanoparticle–Polymer Interactions on the Development of Double-Network Hydrogel Nanocomposites with High Mechanical Strength. Polymers 2020, 12, 470. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Pacelli, S.; Paul, A. Self-Healing DNA-Based Injectable Hydrogels with Reversible Covalent Linkages for Controlled Drug Delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Kuang, T.; Chen, Z.; Liao, G. Double Network Hydrogel for Tissue Engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.-R.V.; Tseng, K.-F.; Lai, H.-Y.; Lin, C.-H.; Lee, O.K. Matrix Stiffness Regulation of Integrin-Mediated Mechanotransduction during Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Bone Miner. Res. 2011, 26, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular Matrix Stiffness Controls Osteogenic Differentiation of Mesenchymal Stem Cells Mediated by Integrin A5. Stem Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A Multiscale Model for Solute Diffusion in Hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.H.; Turnbull, D. Molecular Transport in Liquids and Glasses. J. Chem. Phys. 1959, 31, 1164–1169. [Google Scholar] [CrossRef] [Green Version]
- Lust, S.T.; Hoogland, D.; Norman, M.D.A.; Kerins, C.; Omar, J.; Jowett, G.M.; Yu, T.T.L.; Yan, Z.; Xu, J.Z.; Marciano, D.; et al. Selectively Cross-Linked Tetra-PEG Hydrogels Provide Control over Mechanical Strength with Minimal Impact on Diffusivity. ACS Biomater. Sci. Eng. 2021, 7, 4293–4304. [Google Scholar] [CrossRef]
- Cukier, R.I. Diffusion of Brownian Spheres in Semidilute Polymer Solutions. Macromolecules 1984, 17, 252–255. [Google Scholar] [CrossRef]
- Mackie, J.S.; Meares, P.; Rideal, E.K. The Diffusion of Electrolytes in a Cation-Exchange Resin Membrane I. Theoretical. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1955, 232, 498–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, X.; Zhang, L.; Wang, H.; Zhang, T.; Bai, R.; Sun, Q.; Wang, X.; Yu, T.; Wu, D.; et al. Facile Fabrication of a Biocompatible Composite Gel with Sustained Release of Aspirin for Bone Regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef]
- Tang, G.; Zhu, L.; Wang, W.; Zuo, D.; Shi, C.; Yu, X.; Chen, R. Alendronate-Functionalized Double Network Hydrogel Scaffolds for Effective Osteogenesis. Front. Chem. 2022, 10, 977419. [Google Scholar] [CrossRef] [PubMed]
- Schoonraad, S.A.; Trombold, M.L.; Bryant, S.J. The Effects of Stably Tethered BMP-2 on MC3T3-E1 Preosteoblasts Encapsulated in a PEG Hydrogel. Biomacromolecules 2021, 22, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth Factor Delivery: Defining the next Generation Platforms for Tissue Engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Dorval Courchesne, N.-M.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive Growth Factor Delivery from a Polyelectrolyte Coating Promotes Synergistic Bone Tissue Repair and Reconstruction. Proc Natl. Acad. Sci. USA 2014, 111, 12847–12852. [Google Scholar] [CrossRef] [Green Version]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An Alginate-Based Hybrid System for Growth Factor Delivery in the Functional Repair of Large Bone Defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Shen, Y.; Zhang, X.; Ding, L.; Meng, Z.; Wang, X.; Han, M.; Guo, Y.; Wang, X. Poly(Methacrylate Citric Acid) as a Dual Functional Carrier for Tumor Therapy. Pharmaceutics 2022, 14, 1765. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of Liposomes for Enhanced Local Release of Drugs by Hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-Responsive Polymer Hydrogels for Therapeutic Delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid. Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2001341. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Najafi, M.; Asadi, H.; van den Dikkenberg, J.; van Steenbergen, M.J.; Fens, M.H.A.M.; Hennink, W.E.; Vermonden, T. Conversion of an Injectable MMP-Degradable Hydrogel into Core-Cross-Linked Micelles. Biomacromolecules 2020, 21, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Ekerdt, B.L.; Fuentes, C.M.; Lei, Y.; Adil, M.M.; Ramasubramanian, A.; Segalman, R.A.; Schaffer, D.V. Thermoreversible Hyaluronic Acid-PNIPAAm Hydrogel Systems for 3D Stem Cell Culture. Adv. Healthc. Mater. 2018, 7, e1800225. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, X.-Z.; Chu, C.-C. Effect of the Molecular Weight of Polyethylene Glycol (PEG) on the Properties of Chitosan-PEG-Poly(N-Isopropylacrylamide) Hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 2865–2872. [Google Scholar] [CrossRef]
- Johnson, H.J.; Chakraborty, S.; Muckom, R.J.; Balsara, N.P.; Schaffer, D.V. A Scalable and Tunable Thermoreversible Polymer for 3D Human Pluripotent Stem Cell Biomanufacturing. iScience 2022, 25, 104971. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Lim, W.; Kim, S.Y.; Jo, D.; Jung, J.S.; Jo, G.; Um, S.; Lee, D.-W.; Yang, D.H. Injectable Visible Light-Cured Glycol Chitosan Hydrogels with Controlled Release of Anticancer Drugs for Local Cancer Therapy in Vivo: A Feasible Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” Materials-Based near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Habault, D.; Branda, N.R.; Zhao, Y. Near Infrared Light Triggered Release of Biomacromolecules from Hydrogels Loaded with Upconversion Nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Huang, W.; Wang, H.; Wang, L.; Ding, D.; Zhou, H.; Long, J.; Wang, T.; Yang, Z. Rational Design of a Photo-Responsive UVR8-Derived Protein and a Self-Assembling Peptide-Protein Conjugate for Responsive Hydrogel Formation. Nanoscale 2015, 7, 16666–16670. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Luo, J.; Hsing, I.-M.; Sun, F. B12-Dependent Photoresponsive Protein Hydrogels for Controlled Stem Cell/Protein Release. Proc. Natl. Acad. Sci. USA 2017, 114, 5912–5917. [Google Scholar] [CrossRef] [Green Version]
- Duan, T.; Bian, Q.; Li, H. Light-Responsive Dynamic Protein Hydrogels Based on LOVTRAP. Langmuir 2021, 37, 10214–10222. [Google Scholar] [CrossRef]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef]
- Stayton, P.S.; El-Sayed, M.E.H.; Murthy, N.; Bulmus, V.; Lackey, C.; Cheung, C.; Hoffman, A.S. “Smart” Delivery Systems for Biomolecular Therapeutics. Orthod. Craniofac. Res. 2005, 8, 219–225. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. PH-Responsive Drug-Delivery Systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Zhang, N.; Hu, L.; Jing, W.; Pan, J. Interleukin-4-loaded Hydrogel Scaffold Regulates Macrophages Polarization to Promote Bone Mesenchymal Stem Cells Osteogenic Differentiation via TGF-β1/Smad Pathway for Repair of Bone Defect. Cell Prolif. 2020, 53, e12907. [Google Scholar] [CrossRef]
- Fraser, D.; Nguyen, T.; Kotelsky, A.; Lee, W.; Buckley, M.; Benoit, D.S.W. Hydrogel Swelling-Mediated Strain Induces Cell Alignment at Dentin Interfaces. ACS Biomater. Sci. Eng. 2022, 8, 3568–3575. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, K.; Jiang, H.; Deng, R.; Chu, L.; Cao, Y.; Zheng, Y.; Lu, W.; Deng, Z.; Liang, B. A Three-in-One Strategy: Injectable Biomimetic Porous Hydrogels for Accelerating Bone Regeneration via Shape-Adaptable Scaffolds, Controllable Magnesium Ion Release, and Enhanced Osteogenic Differentiation. Biomacromolecules 2021, 22, 4552–4568. [Google Scholar] [CrossRef]
- Park, Y.; Lin, S.; Bai, Y.; Moeinzadeh, S.; Kim, S.; Huang, J.; Lee, U.; Huang, N.F.; Yang, Y.P. Dual Delivery of BMP2 and IGF1 Through Injectable Hydrogel Promotes Cranial Bone Defect Healing. Tissue Eng. Part A 2022, 28, 760–769. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G.; et al. Mussel-Inspired Bisphosphonated Injectable Nanocomposite Hydrogels with Adhesive, Self-Healing, and Osteogenic Properties for Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.; Jiang, K.; Yang, X.; Watanabe, N.; Mayo, K.H.; et al. Injectable Hydrogel-Loaded Nano-Hydroxyapatite That Improves Bone Regeneration and Alveolar Ridge Promotion. Mater. Sci. Eng. C 2020, 116, 111158. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, W.; Lin, J.; Fang, C.; Deng, J.; Zhang, P.; Zhou, M.; Liu, P.; Weng, J.; Yu, F.; et al. Magnesium Oxide Nanoparticle Coordinated Phosphate-Functionalized Chitosan Injectable Hydrogel for Osteogenesis and Angiogenesis in Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 7592–7608. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan Hydrogel Incorporated with Dental Pulp Stem Cell-Derived Exosomes Alleviates Periodontitis in Mice via a Macrophage-Dependent Mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-Hydroxyapatite Use in Dentistry: A Systematic Review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Rajula, M.P.B.; Narayanan, V.; Venkatasubbu, G.D.; Mani, R.C.; Sujana, A. Nano-Hydroxyapatite: A Driving Force for Bone Tissue Engineering. J. Pharm. Bioallied. Sci. 2021, 13, S11–S14. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale Hydroxyapatite Particles for Bone Tissue Engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Kang, M.; Huang, C.-C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone Regeneration Is Mediated by Macrophage Extracellular Vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular Vesicles in Bone and Periodontal Regeneration: Current and Potential Therapeutic Applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The Tissue Origin Effect of Extracellular Vesicles on Cartilage and Bone Regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Liu, H.; Cao, Z.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Ma, D.; Liu, M. Polysaccharides Based Injectable Hydrogel Compositing Bio-Glass for Cranial Bone Repair. Carbohydr. Polym. 2017, 175, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Tian, J.; Gu, P.; Cao, H.; Fan, X.; Zhang, W. In Situ Bone Regeneration Enabled by a Biodegradable Hybrid Double-Network Hydrogel. Biomater. Sci. 2019, 7, 3266–3276. [Google Scholar] [CrossRef]
- Ai, Y.; She, W.; Wu, S.; Shao, Q.; Jiang, Z.; Chen, P.; Mei, L.; Zou, C.; Peng, Y.; He, Y. AM1241-Loaded Poly(Ethylene Glycol)-Dithiothreitol Hydrogel Repairs Cranial Bone Defects by Promoting Vascular Endothelial Growth Factor and COL-1 Expression. Front. Cell Dev. Biol. 2022, 10, 888598. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Wu, J.; Zheng, B.; Chen, S.; Ma, M.; Shi, Y.; He, H.; Wang, X. An On-Demand and on-Site Shape-Designable Mineralized Hydrogel with Calcium Supply and Inflammatory Warning Properties for Cranial Repair Applications. J. Mater. Chem. B 2022, 10, 3541–3549. [Google Scholar] [CrossRef]
- Gan, M.; Zhou, Q.; Ge, J.; Zhao, J.; Wang, Y.; Yan, Q.; Wu, C.; Yu, H.; Xiao, Q.; Wang, W.; et al. Precise In-Situ Release of MicroRNA from an Injectable Hydrogel Induces Bone Regeneration. Acta Biomater. 2021, 135, 289–303. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Milward, M.R.; Hadis, M.A.; Kuehne, S.A.; Cooper, P.R. Photobiomodulation of Mineralisation in Mesenchymal Stem Cells. Photochem. Photobiol. Sci. 2021, 20, 699–714. [Google Scholar] [CrossRef]
- Jarai, B.M.; Stillman, Z.; Fromen, C.A. Hydrogel Nanoparticle Degradation Influences the Activation and Survival of Primary Macrophages. J. Mater. Chem. B 2021, 9, 7246–7257. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, M.A.; Ding, M.-H.; Gutierrez, A.S.; Chew, S.A. The Application of MicroRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering. Biotechnol. J. 2019, 14, e1900084. [Google Scholar] [CrossRef]
- Leng, Q.; Chen, L.; Lv, Y. RNA-Based Scaffolds for Bone Regeneration: Application and Mechanisms of MRNA, MiRNA and SiRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Veiseh, O.; Appel, E.A.; Xue, K.; Webber, M.J.; Tang, B.C.; Yang, X.-W.; Weir, G.C.; Langer, R.; et al. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid–Glucose Complexation. Langmuir 2016, 32, 8743–8747. [Google Scholar] [CrossRef]
- Gohil, S.V.; Padmanabhan, A.; Kan, H.-M.; Khanal, M.; Nair, L.S. Degradation-Dependent Protein Release from Enzyme Sensitive Injectable Glycol Chitosan Hydrogel. Tissue Eng. Part A 2021, 27, 867–880. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable Hydrogel-Based Drug Delivery Systems for Local Cancer Therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed]



| Strategies | Carriers | Cargoes | Reference |
|---|---|---|---|
| Dual drug delivery | Double-crosslinking architecture of borax-mixed alginate dialdehyde (ADA) and gelatin | Demineralized bone matrix (DBM) powder (mimicking the ECM environment of bone trauma) and hypoxia-pretreated bone marrow stromal cells | [15] |
| >Aginate/collagen-based hydrogel | gMP-BMP2, pMP-IGF1 | [70] | |
| Nanoparticles | Poly (lactic-co-glycolic acid) (PLGA)-dextran (PLGA-Dex) | Nano-hydroxyapatite (nHA) | [71] |
| Chitosan/hyaluronic acid-aldehyde hydrogel | Injectable soft self-repairing hydrogel–hydroxyapatite scaffold | [72] | |
| PLGA | MgO/MgCO3 | [69] | |
| Phosphocreatine-functionalized chitosan (CSMP) | MgO | [73] | |
| Extracellular vesicles (EVs) | Chitosan-based hydrogel | Dental pulp stem cell (DPSC)-derived exosomes | [74] |
| Gelatin | Bone marrow mesenchymal stem cells (BMMSCs) | [75] | |
| Biomimic coating | Glycopeptide hydrogel | Self-assembly of β-sheet RADA16-grafted glucomannan | [16] |
| Stimulus-Responsive System | Methods | Functional Block | Reference |
|---|---|---|---|
| Ion-strength | Ca2+ can respond to free phosphate ions at the local bone defect | Ca–gellan gum (GG) hydrogel | [67] |
| pH | Acylhydrazone bond cross-linking and DA click covalent cross-linking | Bio-glass (BG) | [83] |
| Changes in osteoconductivity and osteoinductivity | Silica-based NPs | [84] | |
| Temperature | PEG-dithiothreitol (DTT) | [85] | |
| Pluronic F-127 (PEO®100PPO65PEO100) | [18] | ||
| Calcium lactate (CaL) | [86] | ||
| Light | UV-cleavable ester bond | Cholesterol-modified noncoding microRNA Chol-miR-26a | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Yu, T.; Wang, X.; Liu, D. Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration. Pharmaceutics 2023, 15, 150. https://doi.org/10.3390/pharmaceutics15010150
Yu Y, Yu T, Wang X, Liu D. Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration. Pharmaceutics. 2023; 15(1):150. https://doi.org/10.3390/pharmaceutics15010150
Chicago/Turabian StyleYu, Yi, Tingting Yu, Xing Wang, and Dawei Liu. 2023. "Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration" Pharmaceutics 15, no. 1: 150. https://doi.org/10.3390/pharmaceutics15010150
APA StyleYu, Y., Yu, T., Wang, X., & Liu, D. (2023). Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration. Pharmaceutics, 15(1), 150. https://doi.org/10.3390/pharmaceutics15010150







