Creating a Microenvironment to Give Wings to Dental Pulp Regeneration—Bioactive Scaffolds
Abstract
:1. Introduction
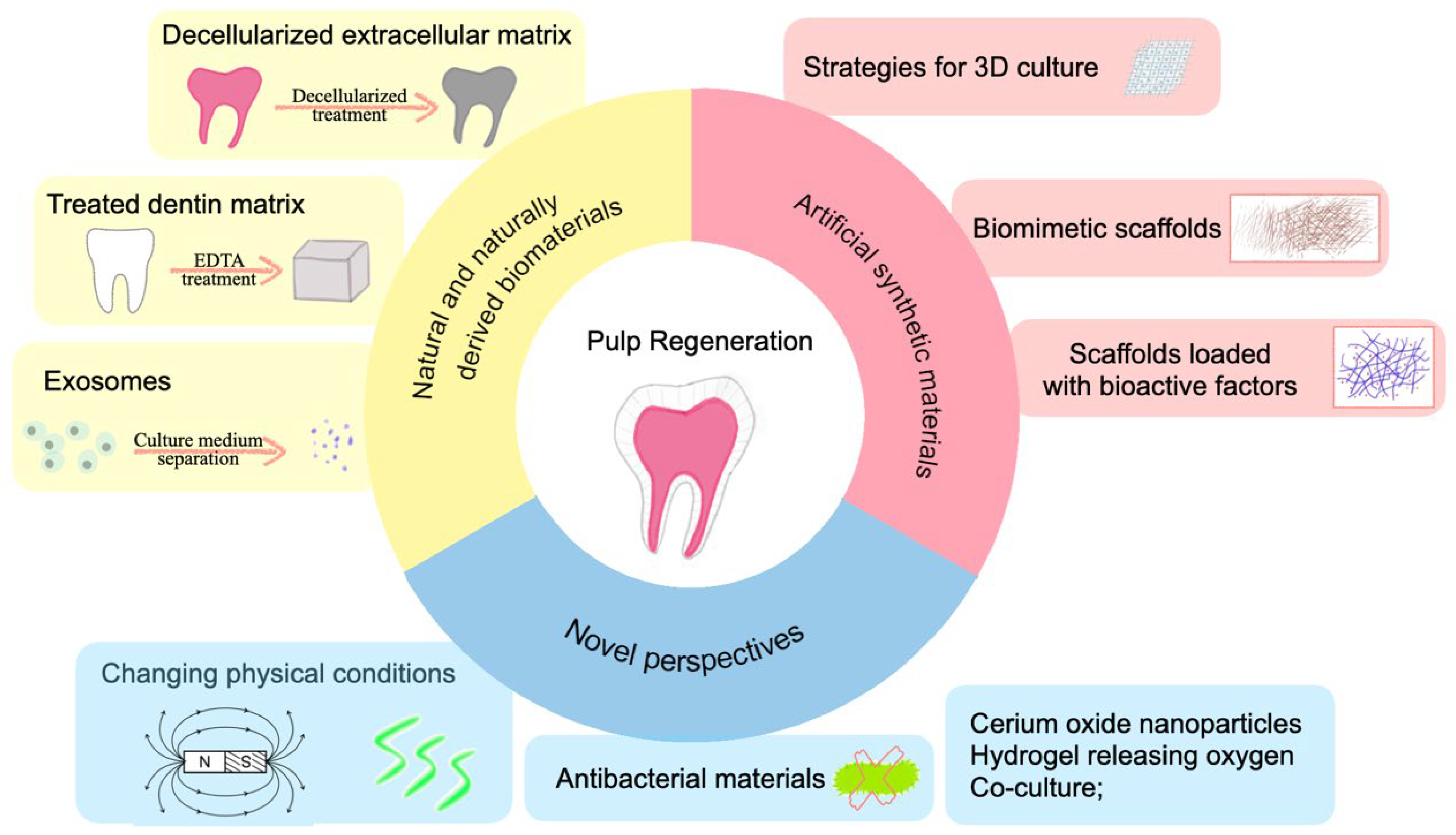
2. Strategies
2.1. Natural and Naturally Derived Biomaterials
2.1.1. Decellularized Extracellular Matrix
2.1.2. Treated Dentin Matrix
2.1.3. Exosomes
2.1.4. Other Perspectives
2.2. Artificial Synthetic Material
2.2.1. Agents for Creating a 3D Environment
Self-Assembled Peptide Hydrogel
Microspheres
3D Printing
2.2.2. Biomimetic Scaffolds
2.2.3. Scaffolds Loaded with Bioactive Factors
Bioactive Factors
Scaffolds Loaded with Bioactive Factors
3. Novel Perspectives of Pulp Regeneration
3.1. Changing Physical Conditions
3.2. Antibacterial Material
3.3. Other Novel Viewpoints
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulabivala, K.; Ng, Y.L. 1—Tooth organogenesis, morphology and physiology. In Endodontics (Fourth Edition); Gulabivala, K., Ng, Y.-L., Eds.; Mosby/Elsevier: Edinburgh, UK, 2014; pp. 2–32. [Google Scholar]
- Si, Y.; Tai, B.; Hu, D.; Lin, H.; Wang, B.; Wang, C.; Zheng, S.; Liu, X.; Rong, W.; Wang, W.; et al. Oral health status of Chinese residents and suggestions for prevention and treatment strategies. Glob. Health J. 2019, 3, 50–54. [Google Scholar] [CrossRef]
- Qin, X.; Zi, H.; Zeng, X. Changes in the global burden of untreated dental caries from 1990 to 2019: A systematic analysis for the Global Burden of Disease study. Heliyon 2022, 8, e10714. [Google Scholar] [CrossRef] [PubMed]
- Krastl, G.; Allgayer, N.; Lenherr, P.; Filippi, A.; Taneja, P.; Weiger, R. Tooth discoloration induced by endodontic materials: A literature review. Dent. Traumatol. 2013, 29, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, Z.; Hou, B. Diverse bacterial profile in extraradicular biofilms and periradicular lesions associated with persistent apical periodontitis. Int. Endod. J. 2021, 54, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Venkateshbabu, N.; John, A.; Deenadhayalan, G.; Kandaswamy, D. A comparative assessment of fracture resistance of endodontically treated and re-treated teeth: An in vitro study. J. Conserv. Dent. 2014, 17, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Winters, J.; Cameron, A.C.; Widmer, R.P. 7—Pulp therapy for primary and immature permanent teeth. In Handbook of Pediatric Dentistry (Fourth Edition); Cameron, A.C., Widmer, R.P., Eds.; Mosby: Canberra, Australia, 2013; pp. 103–122. [Google Scholar]
- He, L.; Kim, S.G.; Gong, Q.; Zhong, J.; Wang, S.; Zhou, X.; Ye, L.; Ling, J.; Mao, J.J. Regenerative Endodontics for Adult Patients. J. Endod. 2017, 43, S57–S64. [Google Scholar] [CrossRef]
- Nanci, A. (Ed.) Chapter 5—Development of the Tooth and Its Supporting Tissues. In Ten Cate’s Oral Histology (Ninth Edition); Elsevier: St. Louis, MO, USA, 2016; pp. 68–83. [Google Scholar]
- Balic, A.; Thesleff, I. Chapter Seven—Tissue Interactions Regulating Tooth Development and Renewal. In Current Topics in Developmental Biology; Chai, Y., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 115, pp. 157–186. [Google Scholar]
- Ilan Rotstein, D.; John, I.; Ingle, D. 2—Structure and Function of the Pulp–Dentin Complex. In Ingle’s Endodontics; PMPH USA, Limited: Hamilton, ON, Canada, 2019; pp. 59–84. [Google Scholar]
- Provenza, D.V. The blood vascular supply of the dental pulp with emphasis on capillary circulation. Circ. Res. 1958, 6, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Iijima, T.; Zhang, J.Q. Three-dimensional wall structure and the innervation of dental pulp blood vessels. Microsc. Res. Tech. 2002, 56, 32–41. [Google Scholar] [CrossRef]
- Luukko, K.; Kettunen, P. Coordination of tooth morphogenesis and neuronal development through tissue interactions: Lessons from mouse models. Exp. Cell Res. 2014, 325, 72–77. [Google Scholar] [CrossRef]
- Yu, T.; Volponi, A.A.; Babb, R.; An, Z.; Sharpe, P.T. Chapter Eight—Stem Cells in Tooth Development, Growth, Repair, and Regeneration. In Current Topics in Developmental Biology; Chai, Y., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 115, pp. 187–212. [Google Scholar]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra269. [Google Scholar] [CrossRef]
- He, P.; Zheng, L.; Zhou, X. IGFs in Dentin Formation and Regeneration: Progress and Remaining Challenges. Stem Cells Int. 2022, 2022, 3737346. [Google Scholar] [CrossRef]
- Sagomonyants, K.; Kalajzic, I.; Maye, P.; Mina, M. Enhanced Dentinogenesis of Pulp Progenitors by Early Exposure to FGF2. J. Dent. Res. 2015, 94, 1582–1590. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Liu, M.; Simchon, S.; Dörscher-Kim, J.E. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc. Finn. Dent. Soc. 1992, 88 (Suppl. 1), 387–392. [Google Scholar]
- Bletsa, A.; Berggreen, E.; Fristad, I.; Tenstad, O.; Wiig, H. Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. J. Physiol. 2006, 573, 225–236. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharm. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, C.; Giraud, T.; Jeanneau, C.; About, I. Pulp Vascularization during Tooth Development, Regeneration, and Therapy. J. Dent. Res. 2017, 96, 137–144. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of Angiogenesis in Endodontics: Contributions of Stem Cells and Proangiogenic and Antiangiogenic Factors to Dental Pulp Regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Sosa, J.F.; Cardier, J.E.; Caviedes-Bucheli, J. The hypoxia-dependent angiogenic process in dental pulp. J. Oral Biosci. 2022, 64, 381–391. [Google Scholar] [CrossRef]
- Luukko, K.; Kettunen, P.; Fristad, I.; Berggreen, E. Chapter 12—Structure and Functions of the Dentin-Pulp Complex. In Cohen’s Pathways of the Pulp (Tenth Edition); Hargreaves, K.M., Cohen, S., Eds.; Mosby: St. Louis, MO, USA, 2011; pp. 452–503. [Google Scholar]
- Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers 2019, 11, 1437. [Google Scholar] [CrossRef] [Green Version]
- Lumelsky, N. Creating a Pro-Regenerative Tissue Microenvironment: Local Control is the Key. Front. Bioeng. Biotechnol. 2021, 9, 712685. [Google Scholar] [CrossRef]
- Kaushik, S.N.; Kim, B.; Walma, A.M.; Choi, S.C.; Wu, H.; Mao, J.J.; Jun, H.W.; Cheon, K. Biomimetic microenvironments for regenerative endodontics. Biomater. Res. 2016, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental Pulp Tissue Engineering with Stem Cells from Exfoliated Deciduous Teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Araújo, I.J.; Münchow, E.A.; Tootla, S.; Bottino, M.C. Chapter 13—Dental pulp tissue regeneration. In Tissue Engineering; Sharma, C.P., Chandy, T., Thomas, V., Thankam, F.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 313–346. [Google Scholar]
- Smith, J.G.; Smith, A.J.; Shelton, R.M.; Cooper, P.R. Dental Pulp Cell Behavior in Biomimetic Environments. J. Dent. Res. 2015, 94, 1552–1559. [Google Scholar] [CrossRef]
- Krebs, N.J.; Neville, C.; Vacanti, J.P. 12—Cellular Transplants for Liver Diseases. In Cellular Transplantation; Halberstadt, C., Emerich, D., Eds.; Academic Press: Burlington, MA, USA, 2007; pp. 215–240. [Google Scholar]
- Hoffman, B.D.; Grashoff, C.; Schwartz, M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011, 475, 316–323. [Google Scholar] [CrossRef]
- Collins, K.L.; Gates, E.M.; Gilchrist, C.L.; Hoffman, B.D. Chapter 1—Bio-Instructive Cues in Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine. In Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Brown, J.L., Kumbar, S.G., Banik, B.L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–35. [Google Scholar]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp Stem Cell-Mediated Functional Pulp Regeneration. J. Dent. Res. 2019, 98, 27–35. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Poel, W.E. Preparation of Acellular Homogenates from Muscle Samples. Science 1948, 108, 390–391. [Google Scholar] [CrossRef]
- Smoak, M.M.; Hogan, K.J.; Grande-Allen, K.J.; Mikos, A.G. Bioinspired electrospun dECM scaffolds guide cell growth and control the formation of myotubes. Sci. Adv. 2021, 7, eabg4123. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Chen, M.; Liu, Y.; Cui, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Childhood Cartilage ECM Enhances the Chondrogenesis of Endogenous Cells and Subchondral Bone Repair of the Unidirectional Collagen-dECM Scaffolds in Combination with Microfracture. ACS Appl. Mater. Interfaces 2021, 13, 57043–57057. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L.; et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, e2103526. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.-L.; Zhao, Y.-Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Juhasz, I.; Kiss, B.; Lukacs, L.; Erdei, I.; Peter, Z.; Remenyik, E. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol. Res. Pract. 2010, 2010, 210150. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Nazzal, H.; Raif, E.M.; Wilshaw, S.P.; Esteves, F.; Duggal, M. Ex-vivo recellularisation and stem cell differentiation of a decellularised rat dental pulp matrix. Sci. Rep. 2020, 10, 21553. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Duggal, M.S.; Nazzal, H.; Esteves, F.; Raïf, E. A biocompatible decellularized pulp scaffold for regenerative endodontics. Int. Endod. J. 2018, 51, 663–673. [Google Scholar] [CrossRef]
- Alqahtani, Q.; Zaky, S.H.; Patil, A.; Beniash, E.; Ray, H.; Sfeir, C. Decellularized Swine Dental Pulp Tissue for Regenerative Root Canal Therapy. J. Dent. Res. 2018, 97, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Pezeshki-Modaress, M.; Kiaipour, Z.; Shafiee, M.; Ellini, M.R.; Mazidi, A.; Rajabi, S.; Zamanlui Benisi, S.; Ostad, S.N.; Galler, K.; et al. Pulp ECM-derived macroporous scaffolds for stimulation of dental-pulp regeneration process. Dent. Mater. 2020, 36, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Rajabi, S.; Pezeshki-Modaress, M.; Ellini, M.R.; Panahinia, M.; Alijani, S.; Mazidi, A.; Kamali, A.; Azarpazhooh, A.; Kishen, A. Optimizing Methods for Bovine Dental Pulp Decellularization. J. Endod. 2021, 47, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Alghutaimel, H.; Yang, X.; Drummond, B.; Nazzal, H.; Duggal, M.; Raïf, E. Investigating the vascularization capacity of a decellularized dental pulp matrix seeded with human dental pulp stem cells: In vitro and preliminary in vivo evaluations. Int. Endod. J. 2021, 54, 1300–1316. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Gao, Z.; Xu, J.; Zhu, Z.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Decellularized Swine Dental Pulp as a Bioscaffold for Pulp Regeneration. Biomed Res. Int. 2017, 2017, 9342714. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeon, M.; Cheon, K.; Kim, S.H.; Jung, H.S.; Shin, Y.; Kang, C.M.; Kim, S.O.; Choi, H.J.; Lee, H.S.; et al. In Vivo Evaluation of Decellularized Human Tooth Scaffold for Dental Tissue Regeneration. Appl. Sci. 2021, 11, 8472. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized Human Dental Pulp as a Scaffold for Regenerative Endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef]
- Tan, Q.; Cao, Y.; Zheng, X.; Peng, M.; Huang, E.; Wang, J. BMP4-regulated human dental pulp stromal cells promote pulp-like tissue regeneration in a decellularized dental pulp matrix scaffold. Odontology 2021, 109, 895–903. [Google Scholar] [CrossRef]
- Fu, J.; Chen, J.; Li, W.; Yang, X.; Yang, J.; Quan, H.; Huang, H.; Chen, G. Laminin-Modified Dental Pulp Extracellular Matrix for Dental Pulp Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 595096. [Google Scholar] [CrossRef]
- Zhang, W.; Vazquez, B.; Oreadi, D.; Yelick, P.C. Decellularized Tooth Bud Scaffolds for Tooth Regeneration. J. Dent. Res. 2017, 96, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef]
- Traphagen, S.B.; Fourligas, N.; Xylas, J.F.; Sengupta, S.; Kaplan, D.L.; Georgakoudi, I.; Yelick, P.C. Characterization of natural, decellularized and reseeded porcine tooth bud matrices. Biomaterials 2012, 33, 5287–5296. [Google Scholar] [CrossRef] [Green Version]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, H.; Lee, G.H.; Hoang, T.H.; Kim, H.R.; Kim, G.H. Fabrication of bone-derived decellularized extracellular matrix/ceramic-based biocomposites and their osteo/odontogenic differentiation ability for dentin regeneration. Bioeng. Transl. Med. 2022, 7, e10317. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Ashoori, A.; Rajabi, S.; Pezeshki-Modaress, M.; Ayati, A.; Mousavi, M.R.; Ellini, M.R.; Kamali, A.; Azarpazhooh, A.; Kishen, A. Human amniotic membrane extracellular matrix scaffold for dental pulp regeneration in vitro and in vivo. Int. Endod. J. 2022, 55, 374–390. [Google Scholar] [CrossRef]
- Nowwarote, N.; Petit, S.; Ferre, F.C.; Dingli, F.; Laigle, V.; Loew, D.; Osathanon, T.; Fournier, B.P.J. Extracellular Matrix Derived From Dental Pulp Stem Cells Promotes Mineralization. Front. Bioeng. Biotechnol. 2021, 9, 740712. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Sun, J.; Luo, X.; Yang, H.; Xie, L.; Yang, B.; Guo, W.; Tian, W. Cell-derived micro-environment helps dental pulp stem cells promote dental pulp regeneration. Cell Prolif. 2017, 50, e12361. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Narayanan, R.; Warshawsky, N.; Ravindran, S. Dual ECM Biomimetic Scaffolds for Dental Pulp Regenerative Applications. Front. Physiol. 2018, 9, 495. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Gendviliene, I.; Barasa, P.; Rinkunaite, I.; Kaupinis, A.; Seinin, D.; Rutkunas, V.; Bukelskiene, V. Dental pulp stem cell-derived extracellular matrix: Autologous tool boosting bone regeneration. Cytotherapy 2022, 24, 597–607. [Google Scholar] [CrossRef]
- Ravindran, S.; Zhang, Y.; Huang, C.C.; George, A. Odontogenic induction of dental stem cells by extracellular matrix-inspired three-dimensional scaffold. Tissue Eng. Part A 2014, 20, 92–102. [Google Scholar] [CrossRef]
- Aksel, H.; Sarkar, D.; Lin, M.H.; Buck, A.; Huang, G.T.J. Cell-derived Extracellular Matrix Proteins in Colloidal Microgel as a Self-Assembly Hydrogel for Regenerative Endodontics. J. Endod. 2022, 48, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhu, S.; Xu, J.; Yuan, C.; Gong, T.; Zhang, C. Effects of decellularized matrices derived from periodontal ligament stem cells and SHED on the adhesion, proliferation and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue Cell 2016, 48, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng. Regen. Med. 2022, 19, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; He, Y.; Zhang, X.; Lu, W.; Wang, C.; Yu, H.; Liu, Y.; Li, Y.; Zhou, Y.; Zhou, J.; et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 2009, 30, 6708–6723. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Lu, Q.; Chen, D.; Zhou, M.; Kuang, Y.; Ying, S.; Song, J. Proteomics and N-glycoproteomics analysis of an extracellular matrix-based scaffold-human treated dentin matrix. J. Tissue Eng. Regen. Med. 2019, 13, 1164–1177. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, T.A.; Wu, S.Y.; Lin, C.P.; Chang, H.H. Regeneration of Tooth with Allogenous, Autoclaved Treated Dentin Matrix with Dental Pulpal Stem Cells: An In Vivo Study. J. Endod. 2020, 46, 1256–1264. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yuan, S.; Yang, Y.; Gong, Y.; Wang, Y.; Guo, R.; Zhang, X.; Liu, Y.; Mi, H.; et al. Treated dentin matrix induces odontogenic differentiation of dental pulp stem cells via regulation of Wnt/β-catenin signaling. Bioact. Mater. 2022, 7, 85–97. [Google Scholar] [CrossRef]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med 2016, 10, 261–270. [Google Scholar] [CrossRef]
- Chen, J.; Cui, C.; Qiao, X.; Yang, B.; Yu, M.; Guo, W.; Tian, W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3428–3436. [Google Scholar] [CrossRef]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M.; Kawana, K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2021, 25, 2101–2112. [Google Scholar] [CrossRef]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: A 2-year randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 4621–4634. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Ren, N.; Bai, S.; Zhao, Y. Human Freeze-dried Dentin Matrix as a Biologically Active Scaffold for Tooth Tissue Engineering. J. Endod. 2019, 45, 1321–1331. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Wu, M.; Zhao, W.; He, X.; Sui, B.; Dong, Z.; Wang, L.; Shi, S.; Huang, X.; et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials 2021, 279, 121223. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, H.; Ye, Y.; Hu, Z.; Sun, W.; Cui, L.; Zhao, X. Translational and Clinical Applications of Dental Stem Cell-Derived Exosomes. Front. Genet. 2021, 12, 750990. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Yang, X.; Chen, J.; Yang, B.; Tian, W. The Application of Pulp Tissue Derived-Exosomes in Pulp Regeneration: A Novel Cell-Homing Approach. Int. J. Nanomed. 2022, 17, 465–476. [Google Scholar] [CrossRef]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect. Tissue Res. 2021, 62, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Ji, L.; Jiang, H.; Liu, Y.; Liu, X.; Bi, J.; Zhao, W.; Ding, Z.; Chen, X. Exosomes Derived from Stem Cells from the Apical Papilla Promote Dentine-Pulp Complex Regeneration by Inducing Specific Dentinogenesis. Stem Cells Int. 2020, 2020, 5816723. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, X.; Li, Z.; Huang, X.; Guo, H.; Guo, X.; Yang, X.; Li, B.; Xuan, K.; Jin, Y. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 2021, 54, e13074. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qin, L.; Liu, C.; Mi, J.; Zhang, Q.; Wang, S.; Zhuang, D.; Xu, Q.; Chen, W.; Guo, J.; et al. Exosomes Derived From Hypoxia-Conditioned Stem Cells of Human Deciduous Exfoliated Teeth Enhance Angiogenesis via the Transfer of let-7f-5p and miR-210-3p. Front. Cell Dev. Biol. 2022, 10, 879877. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lyu, Y.; Yang, Y.; Zhang, S.; Chen, G.; Pan, J.; Tian, W. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 2022, 140, 610–624. [Google Scholar] [CrossRef]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Liu, S.; Liu, X.; Huan, Y.; Ye, Q.; Yang, X.; Guo, H.; Liu, A.; Huang, X.; et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol. Ther. 2022, 30, 3193–3208. [Google Scholar] [CrossRef]
- Zhang, S.; Thiebes, A.L.; Kreimendahl, F.; Ruetten, S.; Buhl, E.M.; Wolf, M.; Jockenhoevel, S.; Apel, C. Extracellular Vesicles-Loaded Fibrin Gel Supports Rapid Neovascularization for Dental Pulp Regeneration. Int. J. Mol. Sci. 2020, 21, 4226. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- Xu, X.; Liang, C.; Gao, X.; Huang, H.; Xing, X.; Tang, Q.; Yang, J.; Wu, Y.; Li, M.; Li, H.; et al. Adipose Tissue–derived Microvascular Fragments as Vascularization Units for Dental Pulp Regeneration. J. Endod. 2021, 47, 1092–1100. [Google Scholar] [CrossRef]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, aaf3227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yang, T.; Zhang, R.; Liang, X.; Wang, G.; Tian, Y.; Xie, L.; Tian, W. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021, 136, 441–455. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.; Jin, R.; Yuan, G.; Kanter, V.; Miron, R.J.; Zhang, Y. Effect of Liquid Platelet-rich Fibrin and Platelet-rich Plasma on the Regenerative Potential of Dental Pulp Cells Cultured under Inflammatory Conditions: A Comparative Analysis. J. Endod. 2019, 45, 1000–1008. [Google Scholar] [CrossRef]
- Rizk, H.M.; Salah Al-Deen, M.S.M.; Emam, A.A. Comparative evaluation of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) scaffolds in regenerative endodontic treatment of immature necrotic permanent maxillary central incisors: A double blinded randomized controlled trial. Saudi Dent. J. 2020, 32, 224–231. [Google Scholar] [CrossRef]
- Nageh, M.; Ahmed, G.M.; El-Baz, A.A. Assessment of Regaining Pulp Sensibility in Mature Necrotic Teeth Using a Modified Revascularization Technique with Platelet-rich Fibrin: A Clinical Study. J. Endod. 2018, 44, 1526–1533. [Google Scholar] [CrossRef]
- Kim, J.-H.; Woo, S.-M.; Choi, N.-K.; Kim, W.-J.; Kim, S.-M.; Jung, J.-Y. Effect of Platelet-rich Fibrin on Odontoblastic Differentiation in Human Dental Pulp Cells Exposed to Lipopolysaccharide. J. Endod. 2017, 43, 433–438. [Google Scholar] [CrossRef]
- ElSheshtawy, A.S.; Nazzal, H.; El Shahawy, O.I.; El Baz, A.A.; Ismail, S.M.; Kang, J.; Ezzat, K.M. The effect of platelet-rich plasma as a scaffold in regeneration/revitalization endodontics of immature permanent teeth assessed using 2-dimensional radiographs and cone beam computed tomography: A randomized controlled trial. Int. Endod. J. 2020, 53, 905–921. [Google Scholar] [CrossRef]
- Rahul, M.; Lokade, A.; Tewari, N.; Mathur, V.; Agarwal, D.; Goel, S.; Keshari, P.; Sharma, S.; Bansal, K. Effect of intracanal scaffolds on the success outcomes of Regenerative Endodontic Therapy—A systematic review and meta-analysis. J. Endod. 2022. [Google Scholar] [CrossRef]
- Son, Y.B.; Bharti, D.; Kim, S.B.; Jo, C.H.; Bok, E.Y.; Lee, S.L.; Kang, Y.H.; Rho, G.J. Comparison of Pluripotency, Differentiation, and Mitochondrial Metabolism Capacity in Three-Dimensional Spheroid Formation of Dental Pulp-Derived Mesenchymal Stem Cells. Biomed Res. Int. 2021, 2021, 5540877. [Google Scholar] [CrossRef] [PubMed]
- Afami, M.E.; El Karim, I.; About, I.; Krasnodembskaya, A.D.; Laverty, G.; Lundy, F.T. Multicomponent Peptide Hydrogels as an Innovative Platform for Cell-Based Tissue Engineering in the Dental Pulp. Pharmaceutics 2021, 13, 1575. [Google Scholar] [CrossRef]
- Fu, K.; Wu, H.; Su, Z. Self-assembling peptide-based hydrogels: Fabrication, properties, and applications. Biotechnol. Adv. 2021, 49, 107752. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, Z.; Sun, J.; Li, K.; Li, Y.; Ren, C.; Meng, Q.; Yang, J. Self-Assembling Peptide-Based Hydrogels in Angiogenesis. Int. J. Nanomed. 2020, 15, 10257–10269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nör, J.E. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Koohi-Moghadam, M.; Chen, Q.; Zhang, L.; Chopra, H.; Zhang, J.; Dissanayaka, W.L. HIF-1α Stabilization Boosts Pulp Regeneration by Modulating Cell Metabolism. J. Dent. Res. 2022, 101, 1214–1226. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Lin, X.; Zou, L.; Li, Y.; Ge, X.; Fu, W.; Zhang, Z.; Xiao, K.; Lv, H. Functionalized self-assembled peptide RAD/Dentonin hydrogel scaffold promotes dental pulp regeneration. Biomed. Mater. 2021, 17, 015009. [Google Scholar] [CrossRef]
- Xia, K.; Chen, Z.; Chen, J.; Xu, H.; Xu, Y.; Yang, T.; Zhang, Q. RGD- and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int. J. Nanomed. 2020, 15, 6631–6647. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Sarkar, B.; Kim, K.K.; Kadincesme, N.; Paul, R.; Kumar, A.; Kobayashi, Y.; Roy, A.; Choudhury, M.; Yang, J.; et al. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021, 126, 109–118. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Gao, W.; Patel, S.D.; Siddiqui, Z.; Weiner, S.; Shimizu, E.; Sarkar, B.; Kumar, V.A. Self-Assembly of a Dentinogenic Peptide Hydrogel. ACS Omega 2018, 3, 5980–5987. [Google Scholar] [CrossRef]
- Mu, X.; Shi, L.; Pan, S.; He, L.; Niu, Y.; Wang, X. A Customized Self-Assembling Peptide Hydrogel-Wrapped Stem Cell Factor Targeting Pulp Regeneration Rich in Vascular-Like Structures. ACS Omega 2020, 5, 16568–16574. [Google Scholar] [CrossRef]
- Leong, W.; Wang, D.A. Cell-laden Polymeric Microspheres for Biomedical Applications. Trends Biotechnol. 2015, 33, 653–666. [Google Scholar] [CrossRef]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Kuang, R.; Zhang, Z.; Jin, X.; Hu, J.; Gupte, M.J.; Ni, L.; Ma, P.X. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv. Healthc. Mater. 2015, 4, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Kuang, R.; Zhang, Z.; Jin, X.; Hu, J.; Shi, S.; Ni, L.; Ma, P.X. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016, 33, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Zhang, Q.; Xie, L.; Zhang, R.; Qian, R.; Tian, Y.; Chen, G.; Tian, W. hDPSC-laden GelMA microspheres fabricated using electrostatic microdroplet method for endodontic regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111850. [Google Scholar] [CrossRef]
- Liang, X.; Xie, L.; Zhang, Q.; Wang, G.; Zhang, S.; Jiang, M.; Zhang, R.; Yang, T.; Hu, X.; Yang, Z.; et al. Gelatin methacryloyl-alginate core-shell microcapsules as efficient delivery platforms for prevascularized microtissues in endodontic regeneration. Acta Biomater. 2022, 144, 242–257. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Xie, X.; Sun, H.; Liu, X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016, 35, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yuan, Z.; Wang, Y.; Wan, Z.; Wang, X.; Yu, S.; Han, J.; Huang, J.; Xiong, C.; Ge, L.; et al. Vascularized pulp regeneration via injecting simvastatin functionalized GelMA cryogel microspheres loaded with stem cells from human exfoliated deciduous teeth. Mater. Today Bio 2022, 13, 100209. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jeong, W.; Kim, M.K.; Nam, S.H.; Park, E.K.; Kang, H.W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Hsu, T.T.; Liu, Y.W.; Kao, C.T.; Huang, T.H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [Green Version]
- Qu, T.; Liu, X. Nano-Structured Gelatin/Bioactive Glass Hybrid Scaffolds for the Enhancement of Odontogenic Differentiation of Human Dental Pulp Stem Cells. J. Mater. Chem. B 2013, 1, 4764–4772. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Qu, T.; Chang, B.; Jing, Y.; Feng, J.Q.; Liu, X. 3D Maskless Micropatterning for Regeneration of Highly Organized Tubular Tissues. Adv. Healthc. Mater. 2018, 7, 1700738. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Y.; Ni, S.; Zhang, X.; Sun, H.; Song, W.; Li, X. Mussel-Inspired Biocoating for Improving the Adhesion of Dental Pulp Stem Cells in Dental Pulp Regeneration. Macromol. Rapid Commun. 2020, 41, e2000102. [Google Scholar] [CrossRef]
- Tu, M.G.; Ho, C.C.; Hsu, T.T.; Huang, T.H.; Lin, M.J.; Shie, M.Y. Mineral Trioxide Aggregate with Mussel-inspired Surface Nanolayers for Stimulating Odontogenic Differentiation of Dental Pulp Cells. J. Endod. 2018, 44, 963–970. [Google Scholar] [CrossRef]
- He, H.; Yu, J.; Liu, Y.; Lu, S.; Liu, H.; Shi, J.; Jin, Y. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol. Int. 2008, 32, 827–834. [Google Scholar] [CrossRef]
- Mathieu, S.; Jeanneau, C.; Sheibat-Othman, N.; Kalaji, N.; Fessi, H.; About, I. Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J. Endod. 2013, 39, 228–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zou, T.; Qi, Y.; Yi, B.; Dissanayaka, W.L.; Zhang, C. DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling. Stem Cell Res. Ther. 2021, 12, 281. [Google Scholar] [CrossRef]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Zhang, G.; Qi, W.; Zhang, P.; Li, P.; Zeng, Y.; Zhao, W.; Tan, Y. Extracellular Ca2+ Promotes Odontoblastic Differentiation of Dental Pulp Stem Cells via BMP2-Mediated Smad1/5/8 and Erk1/2 Pathways. J. Cell Physiol. 2015, 230, 2164–2173. [Google Scholar] [CrossRef]
- Kong, Y.; Hu, X.; Zhong, Y.; Xu, K.; Wu, B.; Zheng, J. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res. Ther. 2019, 10, 378. [Google Scholar] [CrossRef]
- Vandomme, J.; Touil, Y.; Ostyn, P.; Olejnik, C.; Flamenco, P.; El Machhour, R.; Segard, P.; Masselot, B.; Bailliez, Y.; Formstecher, P.; et al. Insulin-like growth factor 1 receptor and p38 mitogen-activated protein kinase signals inversely regulate signal transducer and activator of transcription 3 activity to control human dental pulp stem cell quiescence, propagation, and differentiation. Stem Cells Dev. 2014, 23, 839–851. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Xiao, J.; Zhou, Y.; Zhou, X.; Liu, Y.; Peng, Y.; Yu, Y.; Li, H.; Zhou, X.; Yuan, Q.; et al. Epiregulin enhances odontoblastic differentiation of dental pulp stem cells via activating MAPK signalling pathway. Cell Prolif. 2019, 52, e12680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, F.; Zhang, X.; Wang, S.; Jin, Y.; Zhang, W.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Human Dental Pulp Stem Cells Mediated Dentin-Pulp Complex Regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Banzai, Y. Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int. Endod. J. 2008, 41, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, N.; Na, J.; Li, C.; Yue, G.; Fan, Y.; Zheng, L. Wnt/β-catenin plays a dual function in calcium hydroxide induced proliferation, migration, osteogenic differentiation and mineralization in vitro human dental pulp stem cells. Int. Endod. J. 2022, 56, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hill, R.G.; Rawlinson, S.C. Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair? Acta Biomater. 2016, 38, 201–211. [Google Scholar] [CrossRef]
- Kulthanaamondhita, P.; Kornsuthisopon, C.; Photichailert, S.; Manokawinchoke, J.; Limraksasin, P.; Osathanon, T. Specific microRNAs Regulate Dental Pulp Stem Cell Behavior. J. Endod. 2022, 48, 688–698. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, N.; Li, L.; Ge, L.; Jia, H.; Fan, Z. miR-140-3p enhanced the osteo/odontogenic differentiation of DPSCs via inhibiting KMT5B under hypoxia condition. Int. J. Oral Sci. 2021, 13, 41. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, N.; Li, F.; Han, D.; Liu, Y.; Liu, H.; Sun, S.; Wang, Y.; Feng, H. miR-675 promotes odontogenic differentiation of human dental pulp cells by epigenetic regulation of DLX3. Exp. Cell Res. 2018, 367, 104–111. [Google Scholar] [CrossRef]
- Botero, T.M.; Son, J.S.; Vodopyanov, D.; Hasegawa, M.; Shelburne, C.E.; Nör, J.E. MAPK signaling is required for LPS-induced VEGF in pulp stem cells. J. Dent. Res. 2010, 89, 264–269. [Google Scholar] [CrossRef]
- Janebodin, K.; Zeng, Y.; Buranaphatthana, W.; Ieronimakis, N.; Reyes, M. VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. J. Dent. Res. 2013, 92, 524–531. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lian, M.; Cao, P.; Bao, G.; Xu, G.; Sun, Y.; Wang, L.; Chen, J.; Wang, Y.; Feng, G.; et al. Effects of Nerve Growth Factor and Basic Fibroblast Growth Factor Promote Human Dental Pulp Stem Cells to Neural Differentiation. Neurochem. Res. 2017, 42, 1015–1025. [Google Scholar] [CrossRef]
- Han, Q.; Wang, Q.; Wu, J.; Li, M.; Fang, Y.; Zhu, H.; Wang, X. Nell-1 promotes the neural-like differentiation of dental pulp cells. Biochem. Biophys. Res. Commun. 2019, 513, 515–521. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, Y.F.; Wan, C.Y.; Sun, Z.Y.; Nie, S.; Jian, S.J.; Zhang, L.; Song, G.T.; Chen, Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Ma, L.; Jin, L.; Zhang, W.; Xiao, M.; Yu, Q. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci. Rep. 2017, 7, 40161. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Dangaria, S.; Gopinathan, G.; Yan, X.; Lu, X.; Kolokythas, A.; Niu, Y.; Luan, X. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling—Potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev. Rep. 2013, 9, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Iohara, K.; Hayashi, Y.; Okuwa, Y.; Kurita, K.; Nakashima, M. Enhanced regeneration potential of mobilized dental pulp stem cells from immature teeth. Oral Dis. 2017, 23, 620–628. [Google Scholar] [CrossRef]
- Murakami, M.; Horibe, H.; Iohara, K.; Hayashi, Y.; Osako, Y.; Takei, Y.; Nakata, K.; Motoyama, N.; Kurita, K.; Nakashima, M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 2013, 34, 9036–9047. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, J.; Guan, M.; Zhou, T.; Duan, X.; Xiang, Z. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int. J. Nanomed. 2020, 15, 6097–6111. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liang, Q.; Xu, X.; Liu, X.; Gao, X.; Li, M.; Yang, J.; Xing, X.; Huang, H.; Tang, Q.; et al. Bone morphogenetic protein 7 mediates stem cells migration and angiogenesis: Therapeutic potential for endogenous pulp regeneration. Int. J. Oral Sci. 2022, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, M.; Biguetti, C.; Cavalla, F.; Nieves, F.; Bessey, C.; Bohluli, P.; Garlet, G.P.; Letra, A.; Fakhouri, W.D.; Silva, R.M. Characterization of a Vascular Endothelial Growth Factor-loaded Bioresorbable Delivery System for Pulp Regeneration. J. Endod. 2017, 43, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Anovazzi, G.; Bordini, E.A.F.; Zuta, U.O.; Silva Leite, M.L.A.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Biological Analysis of Simvastatin-releasing Chitosan Scaffold as a Cell-free System for Pulp-dentin Regeneration. J. Endod. 2018, 44, 971–976.e971. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.G.; Bordini, E.A.F.; Bronze-Uhle, E.S.; Cassiano, F.B.; Silva, I.S.P.; Gallinari, M.O.; Matheus, H.R.; Almeida, J.M.; Cintra, L.T.A.; Hebling, J.; et al. Chitosan-Calcium-Simvastatin Scaffold as an Inductive Cell-Free Platform. J. Dent. Res. 2021, 100, 1118–1126. [Google Scholar] [CrossRef]
- Soares, D.G.; Zhang, Z.; Mohamed, F.; Eyster, T.W.; de Souza Costa, C.A.; Ma, P.X. Simvastatin and nanofibrous poly(l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater. 2018, 68, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.G.; Bordini, E.A.F.; Cassiano, F.B.; Bronze-Uhle, E.S.; Pacheco, L.E.; Zabeo, G.; Hebling, J.; Lisboa-Filho, P.N.; Bottino, M.C.; de Souza Costa, C.A. Characterization of novel calcium hydroxide-mediated highly porous chitosan-calcium scaffolds for potential application in dentin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2546–2559. [Google Scholar] [CrossRef]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.H.; Kim, H.W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin-Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Zhao, S.; Wu, H.; Xu, H.H. Porous chitosan bilayer membrane containing TGF-β1 loaded microspheres for pulp capping and reparative dentin formation in a dog model. Dent. Mater. 2014, 30, 172–181. [Google Scholar] [CrossRef]
- Wang, S.; Niu, Y.; Jia, P.; Liao, Z.; Guo, W.; Chaves, R.C.; Tran-Ba, K.H.; He, L.; Bai, H.; Sia, S.; et al. Alkaline activation of endogenous latent TGFβ1 by an injectable hydrogel directs cell homing for in situ complex tissue regeneration. Bioact. Mater. 2022, 15, 316–329. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Qu, T.; Jing, J.; Ren, Y.; Ma, C.; Feng, J.Q.; Yu, Q.; Liu, X. Complete pulpodentin complex regeneration by modulating the stiffness of biomimetic matrix. Acta Biomater. 2015, 16, 60–70. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef]
- Lew, W.Z.; Feng, S.W.; Lin, C.T.; Huang, H.M. Use of 0.4-Tesla static magnetic field to promote reparative dentine formation of dental pulp stem cells through activation of p38 MAPK signalling pathway. Int. Endod. J. 2019, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Kamei, N.; Adachi, N.; Ochi, M. Magnetic cell delivery for the regeneration of musculoskeletal and neural tissues. Regen. Ther. 2018, 9, 116–119. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, C.-H.; Yang, G.; Sun, N.; Jiang, F.; Zhou, M.; Wu, X.; Luo, J.; Huang, C.; Zhang, W.; et al. A Rapidly Magnetically Assembled Stem Cell Microtissue with “Hamburger” Architecture and Enhanced Vascularization Capacity. Bioact. Mater. 2021, 6, 3756–3765. [Google Scholar] [CrossRef]
- Madanagopal, T.T.; Tai, Y.K.; Lim, S.H.; Fong, C.H.; Cao, T.; Rosa, V.; Franco-Obregón, A. Pulsed electromagnetic fields synergize with graphene to enhance dental pulp stem cell-derived neurogenesis by selectively targeting TRPC1 channels. Eur. Cell Mater. 2021, 41, 216–232. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically actuated mechanical stimuli on Fe3O4/mineralized collagen coatings to enhance osteogenic differentiation of the MC3T3-E1 cells. Acta Biomater. 2018, 71, 49–60. [Google Scholar] [CrossRef]
- Moreira, M.S.; Sarra, G.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.V.; Pedroni, A.C.F.; Lascala, C.A.; Catalani, L.H.; Marques, M.M. Physical and Biological Properties of a Chitosan Hydrogel Scaffold Associated to Photobiomodulation Therapy for Dental Pulp Regeneration: An In Vitro and In Vivo Study. Biomed Res. Int. 2021, 2021, 6684667. [Google Scholar] [CrossRef]
- Theocharidou, A.; Bakopoulou, A.; Kontonasaki, E.; Papachristou, E.; Hadjichristou, C.; Bousnaki, M.; Theodorou, G.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K.; et al. Odontogenic differentiation and biomineralization potential of dental pulp stem cells inside Mg-based bioceramic scaffolds under low-level laser treatment. Lasers Med. Sci. 2017, 32, 201–210. [Google Scholar] [CrossRef]
- Niyazi, M.; Zibaii, M.I.; Chavoshinezhad, S.; Hamidabadi, H.G.; Dargahi, L.; Bojnordi, M.N.; Alizadeh, R.; Heravi, M.; Karimi, H.; Hosseini, M.; et al. Neurogenic differentiation of human dental pulp stem cells by optogenetics stimulation. J. Chem. Neuroanat. 2020, 109, 101821. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, X.; Xu, C.; Zhang, Z.; Qi, G.; Jin, Y. Thermoplasmonic Regulation of the Mitochondrial Metabolic State for Promoting Directed Differentiation of Dental Pulp Stem Cells. Anal. Chem. 2022, 94, 9564–9571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, L.; Pan, S.; Zhang, L.; Zhang, W.; Yi, H.; Niu, Y. Three-dimensional simulated microgravity culture improves the proliferation and odontogenic differentiation of dental pulp stem cell in PLGA scaffolds implanted in mice. Mol. Med. Rep. 2017, 15, 873–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Pan, S.; Li, Y.; Zhang, L.; Zhang, W.; Yi, H.; Song, C.; Niu, Y. Increased proliferation and adhesion properties of human dental pulp stem cells in PLGA scaffolds via simulated microgravity. Int. Endod. J. 2016, 49, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Zhang, W.; Huang, Z.; Huang, J.; Wang, J.; Li, W.; Gu, S. Graphene Oxide-Copper Nanocomposites Suppress Cariogenic Streptococcus mutans Biofilm Formation. Int. J. Nanomed. 2021, 16, 7727–7739. [Google Scholar] [CrossRef]
- Li, W.; Mao, M.; Hu, N.; Wang, J.; Huang, J.; Zhang, W.; Gu, S. A graphene oxide-copper nanocomposite for the regeneration of the dentin-pulp complex: An odontogenic and neurovascularization-inducing material. Chem. Eng. J. 2021, 417, 129299. [Google Scholar] [CrossRef]
- Li, Z.; Xie, K.; Yang, S.; Yu, T.; Xiao, Y.; Zhou, Y. Multifunctional Ca-Zn-Si-based micro-nano spheres with anti-infective, anti-inflammatory, and dentin regenerative properties for pulp capping application. J. Mater. Chem. B 2021, 9, 8289–8299. [Google Scholar] [CrossRef]
- Mahapatra, C.; Singh, R.K.; Lee, J.-H.; Jung, J.; Hyun, J.K.; Kim, H.-W. Nano-shape varied cerium oxide nanomaterials rescue human dental stem cells from oxidative insult through intracellular or extracellular actions. Acta Biomater. 2017, 50, 142–153. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H.Y. Coculture of Dental Pulp Stem Cells with Endothelial Cells Enhances Osteo-/Odontogenic and Angiogenic Potential In Vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Jiang, S.; Zhang, Y.; Liu, J.; Yi, B.; Qi, Y.; Dissanayaka, W.L.; Zhang, C. In Situ Oxygen Generation Enhances the SCAP Survival in Hydrogel Constructs. J. Dent. Res. 2021, 100, 1127–1135. [Google Scholar] [CrossRef]
| Main Function | Bioactive Factors | Related Articles | Notes |
|---|---|---|---|
| Odontoblastic/Odontogenic differentiation | TGF-β1 | [143,144,145] | Angiogenesis |
| BMP2, BMP7 | [146,147,148,149] | Extracellular Ca2+, Mg2+ can enhance their effects | |
| FGF | [18] | Angiogenesis | |
| IGF | [17,150] | Cell proliferation and migration | |
| EREG | [151] | / | |
| PDGF-BB | [152] | Migration capability | |
| Ca2+ | [153,154] | ||
| Mg2+ | [149] | / | |
| Sr2+ | [155] | / | |
| miRNA | [156] | For example: miR-140-3p [157], miR-675 [158], etc. | |
| Angiogenesis | VEGF | [159,160] | / |
| HIF-1α | [161] | / | |
| miRNA | [93] | For example: miR-26a (SHED) | |
| Neurogenesis | BDNF | [162] | / |
| bFGF + NGF | [163] | / | |
| Nell-1 | [164] | / | |
| Chemotactic function | SDF-1α | [95,165,166] | Homing factors in dental pulp regeneration |
| SCF | [166,167] | ||
| G-CSF | [168,169] | Ameliorate regeneration potential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, N.; Li, W.; Jiang, W.; Wen, J.; Gu, S. Creating a Microenvironment to Give Wings to Dental Pulp Regeneration—Bioactive Scaffolds. Pharmaceutics 2023, 15, 158. https://doi.org/10.3390/pharmaceutics15010158
Hu N, Li W, Jiang W, Wen J, Gu S. Creating a Microenvironment to Give Wings to Dental Pulp Regeneration—Bioactive Scaffolds. Pharmaceutics. 2023; 15(1):158. https://doi.org/10.3390/pharmaceutics15010158
Chicago/Turabian StyleHu, Nan, Weiping Li, Wentao Jiang, Jin Wen, and Shensheng Gu. 2023. "Creating a Microenvironment to Give Wings to Dental Pulp Regeneration—Bioactive Scaffolds" Pharmaceutics 15, no. 1: 158. https://doi.org/10.3390/pharmaceutics15010158
APA StyleHu, N., Li, W., Jiang, W., Wen, J., & Gu, S. (2023). Creating a Microenvironment to Give Wings to Dental Pulp Regeneration—Bioactive Scaffolds. Pharmaceutics, 15(1), 158. https://doi.org/10.3390/pharmaceutics15010158









