Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spherical PLGA Nanoparticles
2.3. Preparation of Differently Shaped Nanoparticles
2.4. In Vitro Characterization of Blank and HSA-Loaded PLGA Nanoparticles
2.4.1. Determination of Particle Size, Polydispersity Index, and Zeta Potential
2.4.2. Morphology of Differently Shaped PLGA Nanoparticles
2.4.3. Encapsulation Efficiency (EE)
2.4.4. Drug Release Study
2.5. Cell Culture Studies
2.5.1. Effect of Nanoparticle Shape on Safety
2.5.2. Cellular Uptake Study
2.6. In Vivo Biodistribution of Nanoparticles
2.7. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Characterization of Blank and HSA-Loaded PLGA Nanoparticles
3.1.1. Determination of Particle Size, Polydispersity Index, and Zeta Potential
3.1.2. Morphology of Differently Shaped PLGA Nanoparticles
3.1.3. Encapsulation Efficiency
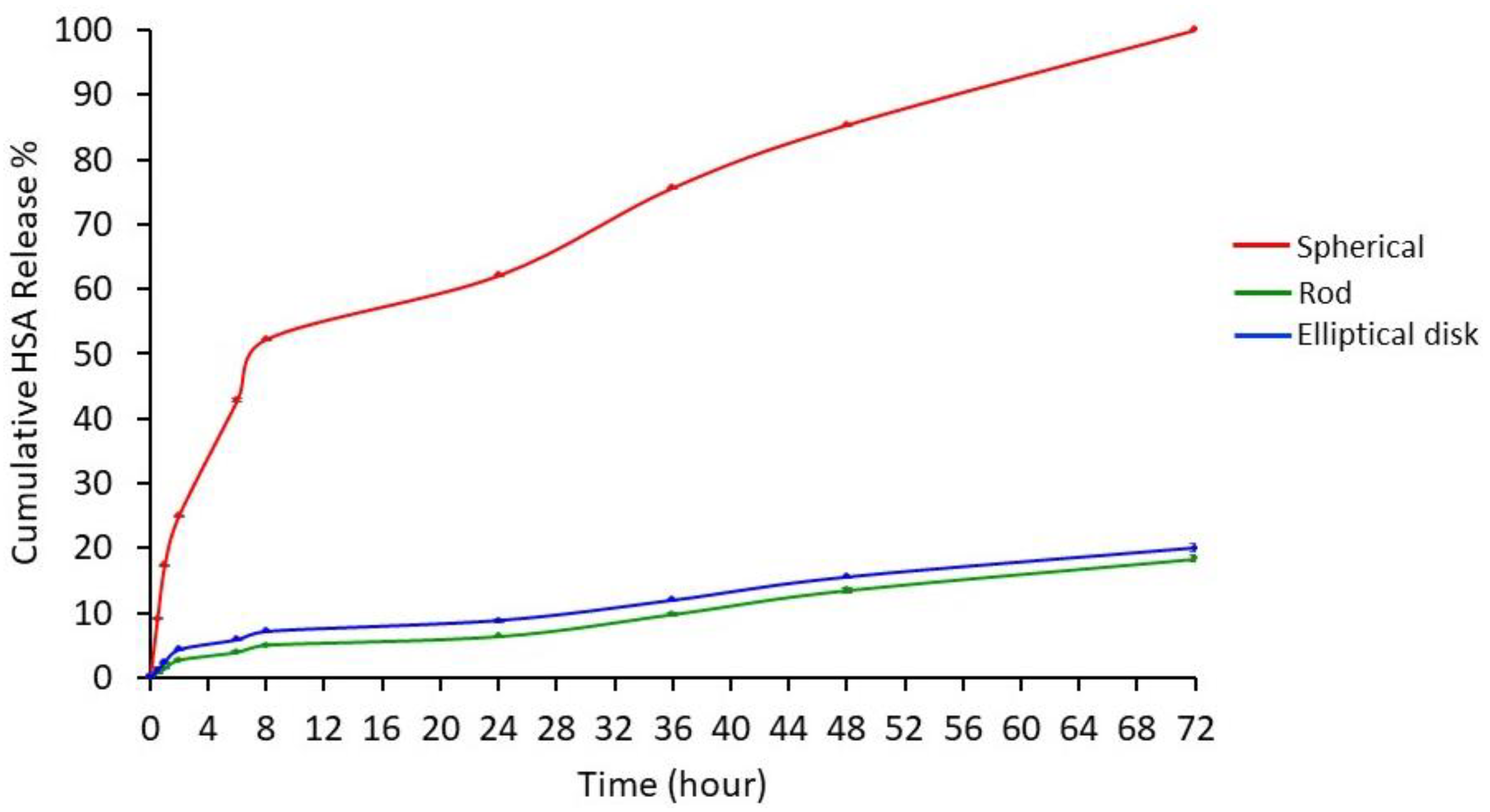
3.1.4. Drug Release Study
3.2. Cell Culture Studies
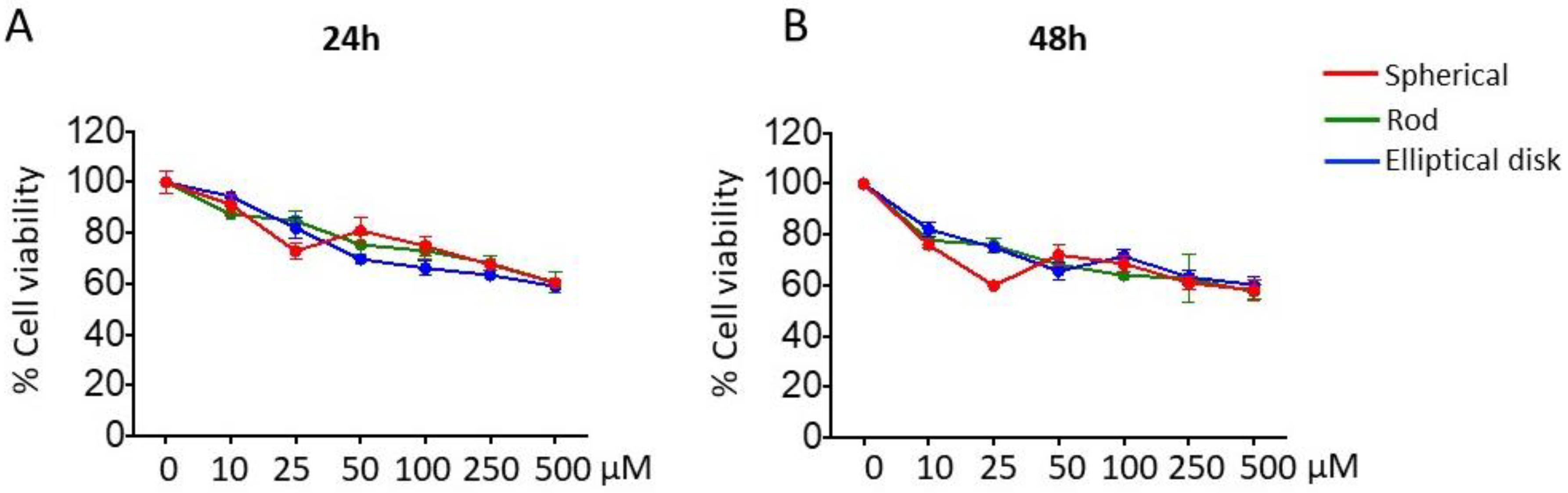
3.2.1. Effect of Nanoparticle Shape on Safety
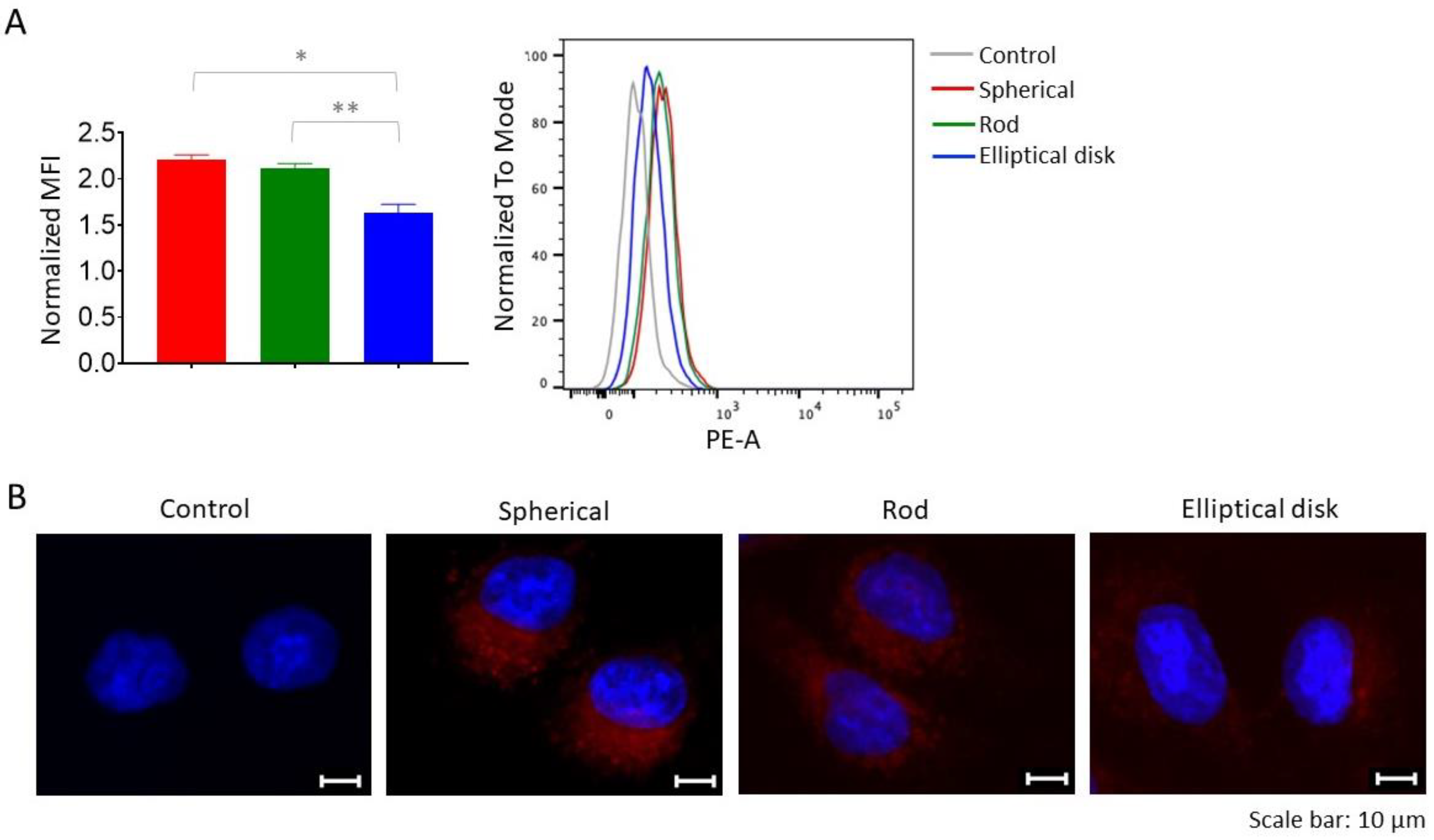
3.2.2. Based on Different Shapes, Uptake of the Nanoparticles may Change
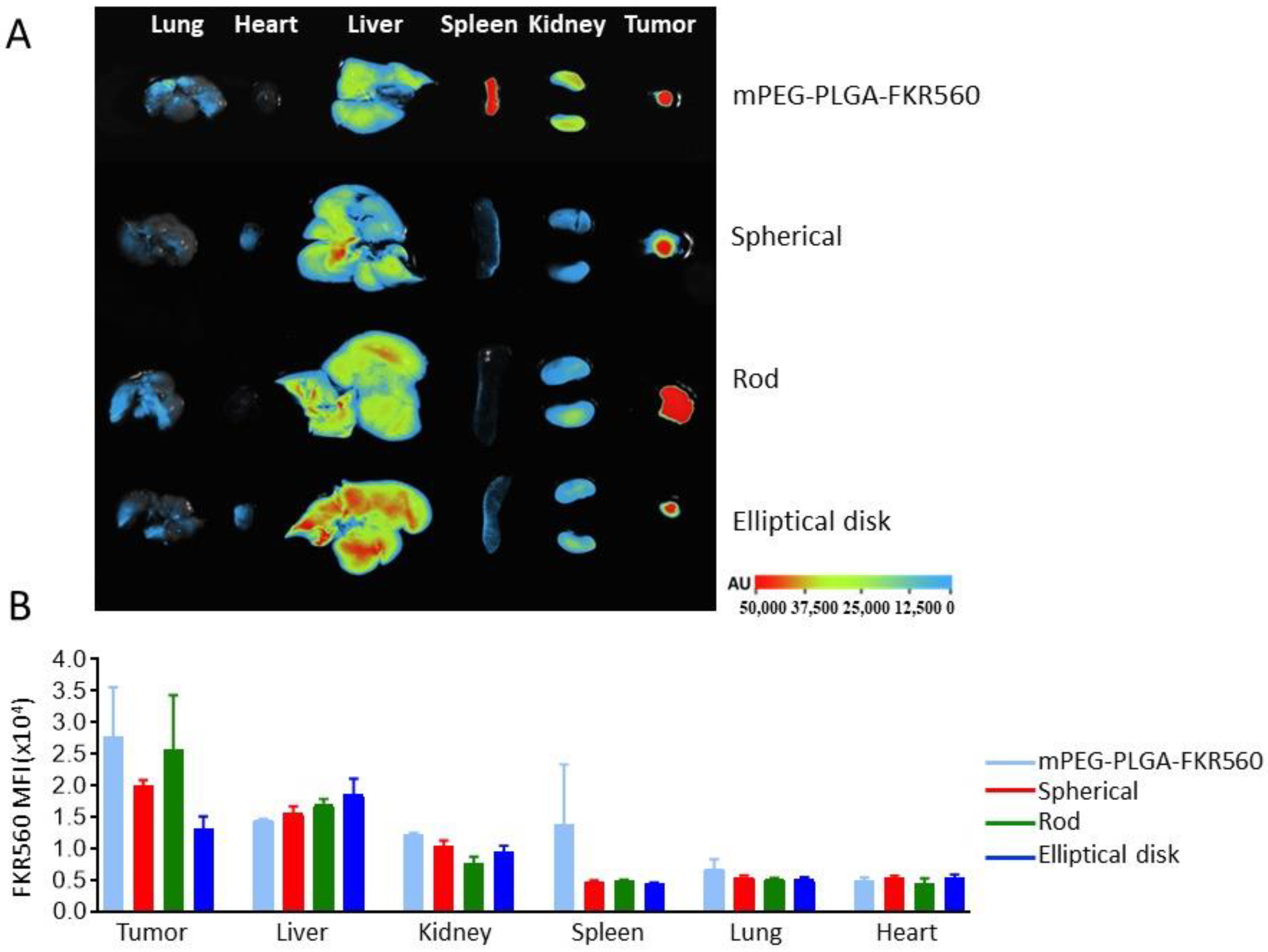
3.3. Biodistribution and Targeting Efficiency of Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- About Nanotechnology. Available online: https://www.nano.gov/about-nanotechnology (accessed on 26 October 2022).
- Li, J.; Kataoka, K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: The next generation. J. Am. Chem. Soc. 2020, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Candal, A.; Brown, T.; Krishnan, V.; Lopez-Loureiro, I.; Ávila-Gómez, P.; Pusuluri, A.; Pérez-Díaz, A.; Correa-Paz, C.; Hervella, P.; Castillo, J. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J. Control. Release 2019, 309, 94–105. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Santander-Ortega, M.; Jódar-Reyes, A.; Csaba, N.; Bastos-González, D.; Ortega-Vinuesa, J. Colloidal stability of pluronic F68-coated PLGA nanoparticles: A variety of stabilisation mechanisms. J. Colloid Interface Sci. 2006, 302, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Pei, Y.-Y.; Zhang, X.-Y.; Gu, Z.-H.; Zhou, Z.-H.; Yuan, W.-F.; Zhou, J.-J.; Zhu, J.-H.; Gao, X.-J. PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control. Release 2001, 71, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic polyglycerol-derived nano-architectures as delivery platforms of gemcitabine for pancreatic cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Rajani, C.; Borisa, P.; Karanwad, T.; Borade, Y.; Patel, V.; Rajpoot, K.; Tekade, R.K. Cancer-targeted chemotherapy: Emerging role of the folate anchored dendrimer as drug delivery nanocarrier. In Pharmaceutical Applications of Dendrimers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–198. [Google Scholar]
- Jindal, A.B. The effect of particle shape on cellular interaction and drug delivery applications of micro-and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Non-spherical micro-and nanoparticles: Fabrication, characterization and drug delivery applications. Expert Opin. Drug Deliv. 2015, 12, 481–492. [Google Scholar] [CrossRef]
- Kapate, N.; Clegg, J.R.; Mitragotri, S. Non-spherical micro-and nanoparticles for drug delivery: Progress over 15 years. Adv. Drug Deliv. Rev. 2021, 177, 113807–113823. [Google Scholar] [CrossRef]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The impact of nanoparticle shape on cellular internalisation and transport: What do the different analysis methods tell us? Mater. Horiz. 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Liu, Y.; Wang, L.; Xu, L.; Bai, R.; Ji, Y.; Wu, X.; Zhao, Y.; Li, Y.; Chen, C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 2010, 31, 7606–7619. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Rolland, J.P.; Maynor, B.W.; Euliss, L.E.; Exner, A.E.; Denison, G.M.; DeSimone, J.M. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005, 127, 10096–10100. [Google Scholar] [CrossRef]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of monodisperse particles by using microfluidics: Control over size, shape, and composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making polymeric micro-and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904. [Google Scholar] [CrossRef]
- Meyer, R.A.; Meyer, R.S.; Green, J.J. An automated multidimensional thin film stretching device for the generation of anisotropic polymeric micro-and nanoparticles. J. Biomed. Mater. Res. Part A 2015, 103, 2747–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-S.; Park, J.-S. Design and evaluation of the anticancer activity of paclitaxel-loaded anisotropic-poly (lactic-co-glycolic acid) nanoparticles with PEGylated chitosan surface modifications. Int. J. Biol. Macromol. 2020, 162, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lung, P.S.; Zhao, S.; Chu, Z.; Chrzanowski, W.; Li, Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci. Rep. 2017, 7, 7315. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Choi, J.-S.; Oshi, M.A.; Lee, J.; Hasan, N.; Kim, J.; Yoo, J.-W. Development of PLGA micro-and nanorods with high capacity of surface ligand conjugation for enhanced targeted delivery. Asian J. Pharm. Sci. 2019, 14, 86–94. [Google Scholar] [CrossRef]
- Ordikhani, F.; Uehara, M.; Kasinath, V.; Dai, L.; Eskandari, S.K.; Bahmani, B.; Yonar, M.; Azzi, J.R.; Haik, Y.; Sage, P.T. Targeting antigen-presenting cells by anti–PD-1 nanoparticles augments antitumor immunity. JCI Insight 2018, 3, 122700. [Google Scholar] [CrossRef] [Green Version]
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Gupta, A.S. Influence of particle size and shape on their margination and wall-adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 10, 15350–15364. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, C. Tuning the size of poly (lactic-co-glycolic acid)(PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef]
- Patel, J.; Amrutiya, J.; Bhatt, P.; Javia, A.; Jain, M.; Misra, A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J. Microencapsul. 2018, 35, 204–217. [Google Scholar] [CrossRef]
- Au, L.; Zhang, Q.; Cobley, C.M.; Gidding, M.; Schwartz, A.G.; Chen, J.; Xia, Y. Quantifying the cellular uptake of antibody-conjugated Au nanocages by two-photon microscopy and inductively coupled plasma mass spectrometry. AcS Nano 2010, 4, 35–42. [Google Scholar] [CrossRef]
- D’Souza, S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef] [Green Version]
- Diez-Ahedo, R.; Mendibil, X.; Márquez-Posadas, M.C.; Quintana, I.; González, F.; Rodríguez, F.J.; Zilic, L.; Sherborne, C.; Glen, A.; Taylor, C.S. UV-casting on methacrylated PCL for the production of a peripheral nerve implant containing an array of porous aligned microchannels. Polymers 2020, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, E.; Husemann, U.; Greller, G.; Barbaroux, M.; Fenge, C. Verification of a new biocompatible single-use film formulation with optimized additive content for multiple bioprocess applications. Biotechnol. Prog. 2014, 30, 1171–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.; Carraway, J.; Geertsma, R. In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices. In Biocompatibility and Performance of Medical Devices; Elsevier: Cambridge, MA, USA, 2020; pp. 123–166. [Google Scholar]
- Öztürk, K.; Esendağlı, G.; Gürbüz, M.U.; Tülü, M.; Çalış, S. Effective targeting of gemcitabine to pancreatic cancer through PEG-cored Flt-1 antibody-conjugated dendrimers. Int. J. Pharm. 2017, 517, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Varan, C.; Öztürk, S.C.; Benito, J.M.; Esendağlı, G.; Bilensoy, E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials 2021, 11, 515. [Google Scholar] [CrossRef]
- Ho, C.; Keller, A.; Odell, J.; Ottewill, R. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid Polym. Sci. 1993, 271, 469–479. [Google Scholar] [CrossRef]
- Amini, A.; Kamali, M.; Amini, B.; Najafi, A. Enhanced antibacterial activity of imipenem immobilized on surface of spherical and rod gold nanoparticles. J. Phys. D Appl. Phys. 2018, 52, 065401. [Google Scholar] [CrossRef]
- Kaga, S.; Truong, N.P.; Esser, L.; Senyschyn, D.; Sanyal, A.; Sanyal, R.; Quinn, J.F.; Davis, T.P.; Kaminskas, L.M.; Whittaker, M.R. Influence of size and shape on the biodistribution of nanoparticles prepared by polymerization-induced self-assembly. Biomacromolecules 2017, 18, 3963–3970. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Leng, D.; Thanki, K.; Fattal, E.; Foged, C.; Yang, M. Engineering of budesonide-loaded lipid-polymer hybrid nanoparticles using a quality-by-design approach. Int. J. Pharm. 2018, 548, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Baby, T.; Liu, Y.; Middelberg, A.P.; Zhao, C.-X. Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles. Chem. Eng. Sci. 2017, 169, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 324–333. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: New York, NY, USA, 2011; pp. 63–70. [Google Scholar]
- Haryadi, B.M.; Hafner, D.; Amin, I.; Schubel, R.; Jordan, R.; Winter, G.; Engert, J. Nonspherical nanoparticle shape stability is affected by complex manufacturing aspects: Its implications for drug delivery and targeting. Adv. Healthc. Mater. 2019, 8, 1900352. [Google Scholar] [CrossRef] [PubMed]
- Feczkó, T.; Tóth, J.; Dósa, G.; Gyenis, J. Optimization of protein encapsulation in PLGA nanoparticles. Chem. Eng. Process. Process Intensif. 2011, 50, 757–765. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [Green Version]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: Are we doing it right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Choo, P.; Liu, T.; Odom, T.W. Nanoparticle shape determines dynamics of targeting nanoconstructs on cell membranes. J. Am. Chem. Soc. 2021, 143, 4550–4555. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; Van den Bogaart, G. Modulation of immune responses by particle size and shape. Front. Immunol. 2021, 11, 3854. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.; Izat, N.; Kir, F.; Gulsun, T.; Sahin, S. Evaluation of Pharmacokinetics and Biodistribution of Targeted Nanoparticles. In Drug Delivery with Targeted Nanoparticles: In Vitro and In Vivo Evaluation Methods; Jenny Stanford Publishing: Singapore, 2021; p. 345. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]

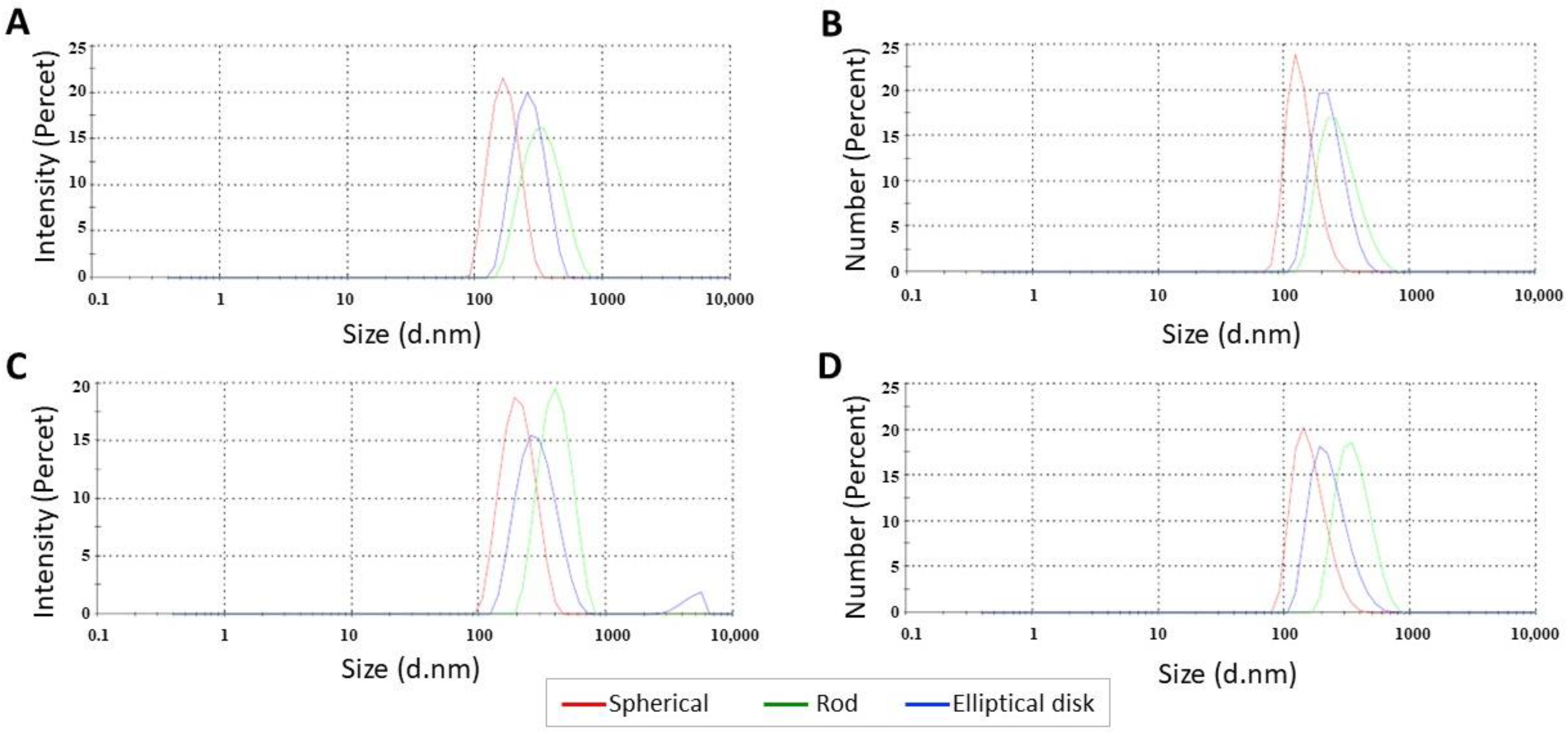

| Formulation | Particle Size(nm) | Aspect Ratio | Polydispersity Index (PDI) | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|---|
| SN | 163.2 ± 0.7 | 1.0 | 0.032 ± 0.70 | −17.20 ± 0.15 | - |
| RN | 312.9 ± 5.4 | 4.0 ± 0.5 | 0.079 ± 0.03 | −4.08 ± 0.16 | - |
| EDN | 256.8 ± 4.7 | 7.5 ± 0.5 | 0.067 ± 0.01 | −9.52 ± 0.34 | - |
| SN-HSA | 193.3 ± 0.9 | - | 0.055 ± 0.03 | −14.50 ± 0.51 | 91 |
| RN-HSA | 375.3 ± 3.5 | - | 0.112 ± 0.03 | −0.62 ± 0.03 | 86.3 |
| EDN-HSA | 294.3 ± 1.6 | - | 0.215 ± 0.01 | −12.90 ± 0.70 | 86.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, M.; Öztürk, K.; Öztürk, S.C.; Tavukçuoğlu, E.; Esendağlı, G.; Calis, S. Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation. Pharmaceutics 2023, 15, 175. https://doi.org/10.3390/pharmaceutics15010175
Kaplan M, Öztürk K, Öztürk SC, Tavukçuoğlu E, Esendağlı G, Calis S. Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation. Pharmaceutics. 2023; 15(1):175. https://doi.org/10.3390/pharmaceutics15010175
Chicago/Turabian StyleKaplan, Meryem, Kıvılcım Öztürk, Süleyman Can Öztürk, Ece Tavukçuoğlu, Güneş Esendağlı, and Sema Calis. 2023. "Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation" Pharmaceutics 15, no. 1: 175. https://doi.org/10.3390/pharmaceutics15010175
APA StyleKaplan, M., Öztürk, K., Öztürk, S. C., Tavukçuoğlu, E., Esendağlı, G., & Calis, S. (2023). Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation. Pharmaceutics, 15(1), 175. https://doi.org/10.3390/pharmaceutics15010175





