Recent Developments in 3D Bio-Printing and Its Biomedical Applications
Abstract
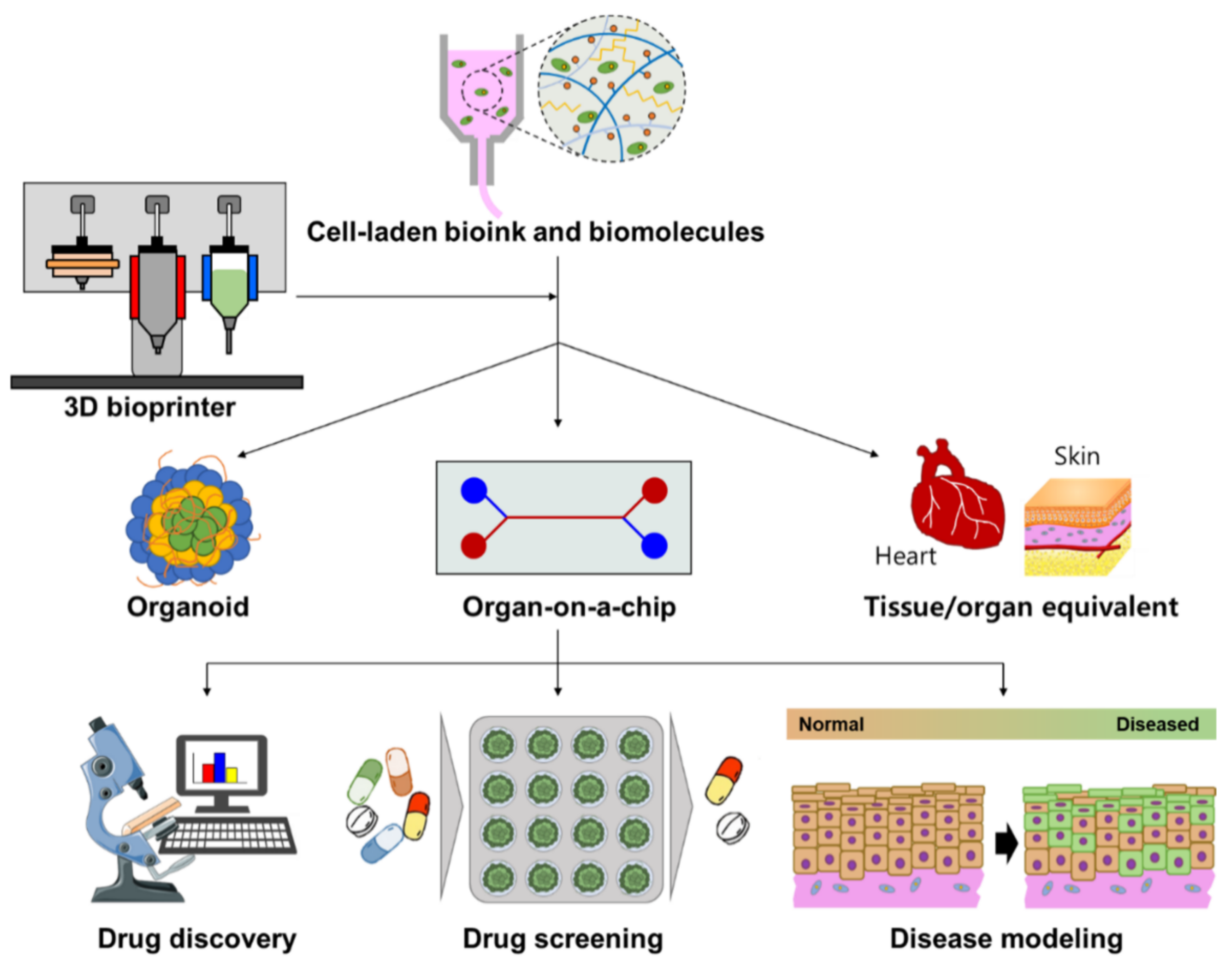
:1. Introduction
2. Materials for 3D Bio-Printing
2.1. Synthetic Polymers
- Selecting the materials for the scaffold and the bone tissue;
- Selecting the cell structure to be used;
- Bio-printing the cells into the scaffoldings;
- Determining the viability of the cells;
- Conducting experiments on animals.
2.2. Natural Polymers
- Strong biocompatibility;
- Low mechanical strength;
- Quick biodegradability.
2.2.1. Gelatin
- Poor mechanical potency;
- Organizational unsteadiness at physiological temperatures (such as 37 °C).
2.2.2. Alginate
2.2.3. Collagen
- In contrast to the conventional tissue-engineering of porous scaffolds, most 3D-printed scaffolds characteristically scale up via networks that are useful for transporting nutrients, oxygen, and metabolites;
- The gradient structural morphology and material composition are advantageous for realizing diverse functions in 3D-printed scaffolds;
- For hard or soft TE, living cells can be directly inserted into biocompatible material.
2.2.4. Hyaluronic Acid
2.2.5. Chitosan
2.2.6. Decellularized Extracellular Matrix
2.2.7. Other Materials
2.3. Material Characteristics of Bio-Inks
2.3.1. Printability
2.3.2. Biocompatibility
2.3.3. Gelation
2.3.4. Mechanical Properties
2.3.5. Viscosity
2.3.6. Biodegradability and Surface Characteristics
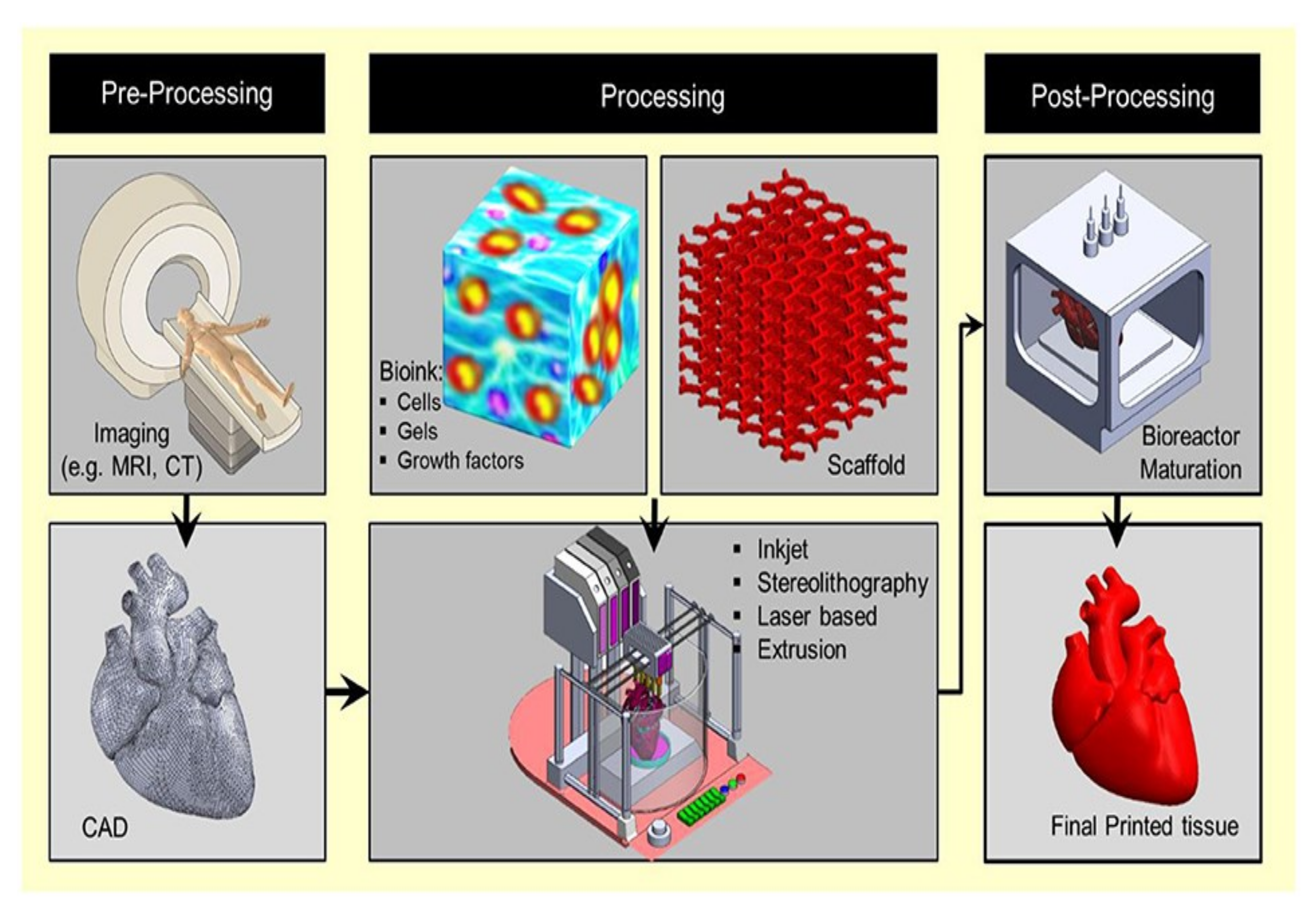
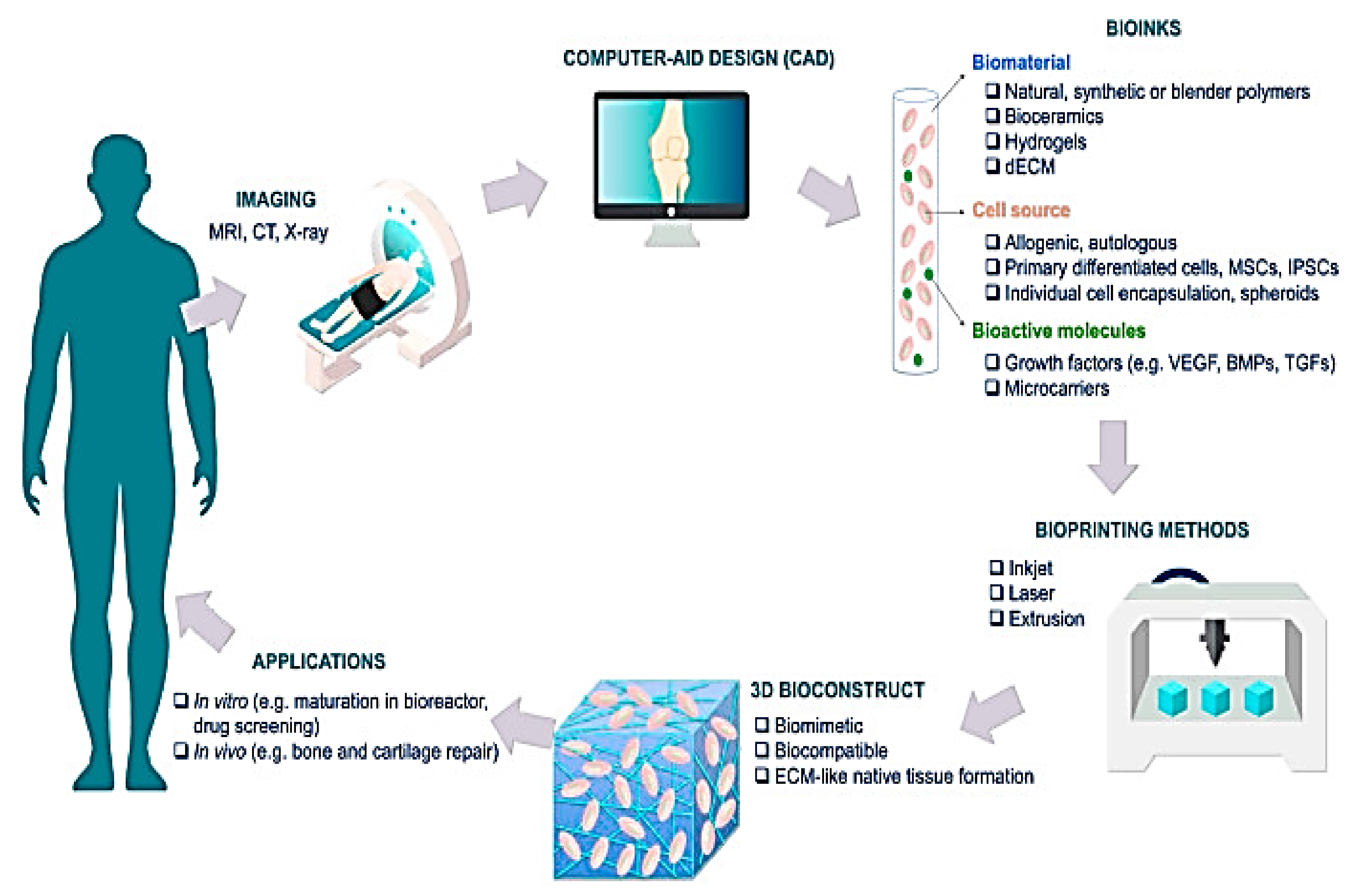
3. Fundamentals of 3D Bio-Printing
4. Classification of 3D Bio-Printing
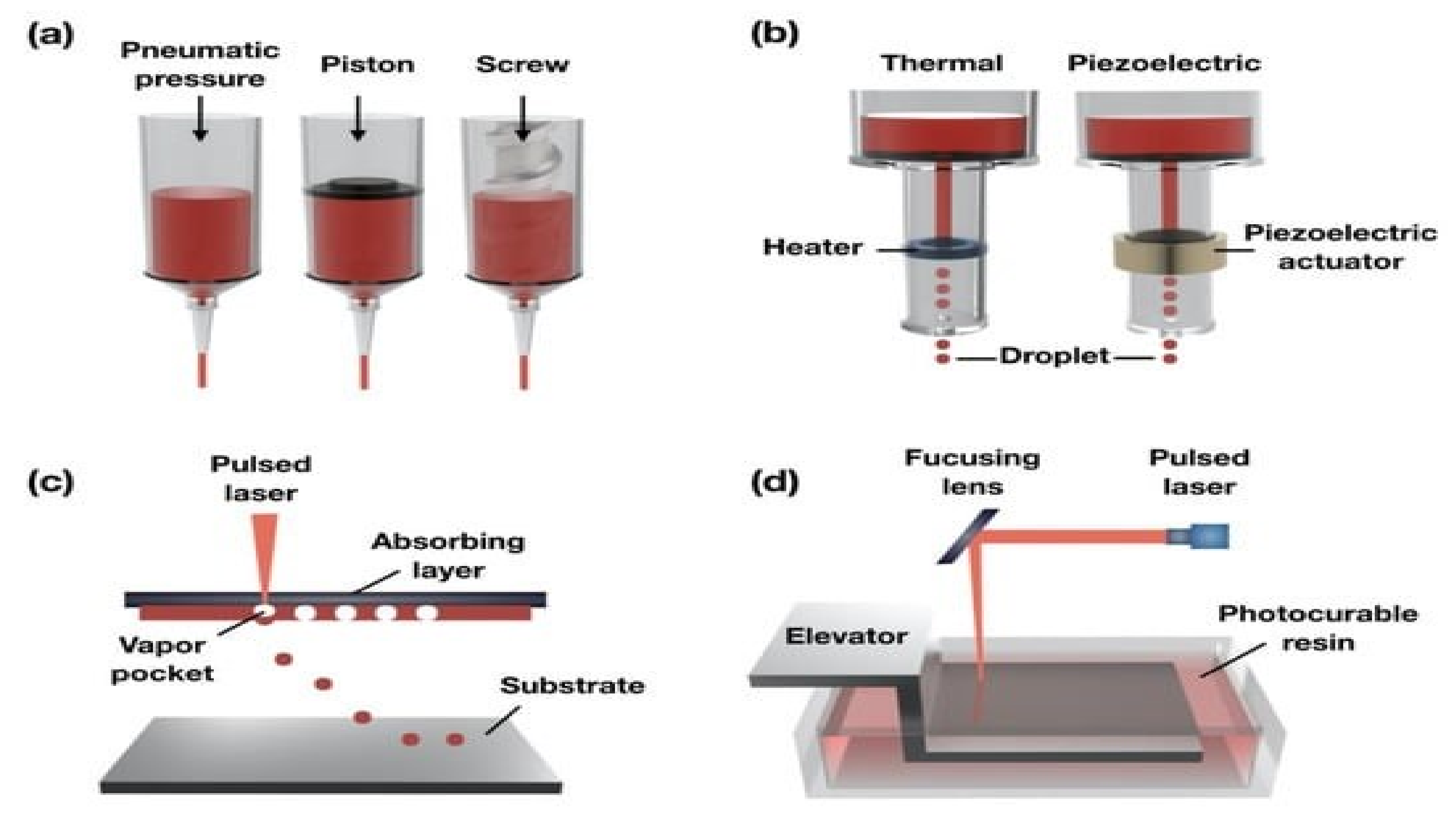
4.1. Droplet-Based Bio-Printing
4.2. Laser-Assisted Bio-Printing
4.3. Extrusion Bio-Printing
4.4. Stereolithography
4.5. Other Printing Techniques
- Incubating cells with nanoparticles in an external magnetic field to create gel through electrostatic interactions;
- Combining a label-free cell with a paramagnetic buffer.
5. Biomedical Applications of 3D Bio-Printing
5.1. Tissue and Organ Regeneration
5.1.1. Bone
5.1.2. Cartilage
5.1.3. Skin
5.1.4. Cardiac and other Tissues
5.2. Drug Delivery and Screening
6. Pros and Cons of 3D Bio-Printing
7. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| AM | Additive Manufacturing |
| CAD | Computer-Aided Design |
| dECM | Decellularized Extracellular Matrix |
| DLP | Digital Light Processing |
| ECM | Extracellular Matrix |
| GelMA | Gelatin Methacryloyl |
| HCMPC | Human Fetal Cardio Myocyte Progenitor Cells |
| HSFs | Human Skin Fibroblasts |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IBBP | Inkjet-Based Bioprinting |
| ITOP | Integrated Tissue–Organ Printer |
| LAB | Laser-Assisted Bioprinting |
| LBL | Layer-By-Layer |
| MSC | Mesenchymal Stem Cells |
| NSCs | Neural Stem Cells |
| PASMCs | Porcine Aortic Smooth-Muscle Cells |
| PBT | Polybutylene Terephthalate |
| PDLLA | Poly-D, L-Lactic Acid |
| PGA | Poly-Glycolic Acid |
| PRs | Printing Resources |
| PU | Polyurethane |
| RM | Regenrative Medicine |
| RP | Rapid Prototyping |
| SFM | Solid Free-form Manufacturing |
| SL | Stereolithography |
| TE | Tissue Engineering |
| TM | Tissue Model |
| VEGF | Vascular Endothelial Growth Factor |
References
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wohlers, T.T. Wohlers Report 2014: 3D Printing and Additive Manufacturing, State of the Industry, Annual Worldwide Progress Report; Wohlers Associates Incorporated: Fort Collins, CO, USA, 2014. [Google Scholar]
- Singh, I.; George, S.M.; Tiwari, A.; Ramkumar, J.; Balani, K. Influence of laser surface texturing on the wettability and antibacterial properties of metallic, ceramic, and polymeric surfaces. J. Mater. Res. 2021, 36, 3985–3999. [Google Scholar] [CrossRef]
- Yao, S.S.; Jin, F.L.; Rhee, K.Y.; Hui, D.; Park, S.J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. Part B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Boparai, K.S.; Singh, R.; Singh, H. Experimental investigations for development of Nylon6-Al-Al2O3 alternative FDM filament. Rapid Prototyp. J. 2016, 22, 217–224. [Google Scholar] [CrossRef]
- Khatri, B.; Lappe, K.; Noetzel, D.; Pursche, K.; Hanemann, T. A 3D-printable polymer-metal soft-magnetic functional composite—Development and characterization. Materials 2018, 11, 189. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.J.; Dixon, S.; Hale, L.R.; Darbyshire, A.; Martin, D.; de Mel, A. Biomimetic heterogenous elastic tissue development. NPJ Regen. Med. 2017, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D bioprinting methods and techniques: Applications on artificial blood vessel fabrication. Acta Cardiol. Sin. 2019, 35, 284. [Google Scholar]
- Cohen, S.; Baño, M.C.; Cima, L.G.; Allcock, H.R.; Vacanti, J.P.; Vacanti, C.A.; Langer, R. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clin. Mater. 1993, 13, 3–10. [Google Scholar] [CrossRef]
- Munaz, A.; Vadivelu, R.K.; John, J.S.; Barton, M.; Kamble, H.; Nguyen, N.T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Janani, G.; Priya, S.; Dey, S.; Mandal, B.B. Mimicking native liver lobule microarchitecture in vitro with parenchymal and non-parenchymal cells using 3D bioprinting for drug toxicity and drug screening applications. ACS Appl. Mater. Interfaces 2022, 14, 10167–10186. [Google Scholar] [CrossRef]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef] [Green Version]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [Green Version]
- Hespel, A.M.; Wilhite, R.; Hudson, J. Invited review-applications for 3d printers in veterinary medicine. Vet. Radiol. Ultrasound 2014, 55, 347–358. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Rocco, N.; Papallo, I.; Nava, M.B.; Catanuto, G.; Accurso, A.; Onofrio, I.; Oliviero, O.; Improta, G.; Speranza, D.; Domingos, M.; et al. Additive manufacturing and technical strategies for improving outcomes in breast reconstructive surgery. Acta IMEKO 2020, 9, 74–79. [Google Scholar] [CrossRef]
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.; Kaplan, D.L. Evolution of bioinks and additive manufacturing technologies for 3D bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. 3D bioprinting at the frontier of regenerative medicine, pharmaceutical, and food industries. Front. Med. Technol. 2021, 2, 607648. [Google Scholar] [CrossRef]
- Tammaro, D.; Detry AL, H.; Landonfi, L.; Napolitano, F.; Villone, M.M.; Maffettone, P.L.; Squillace, A. Bio-lightweight structures by 3D foam printing. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Naples, Italy, 6–9 September 2021; IEEE: New York, NY, USA, 2021; pp. 47–51. [Google Scholar]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef] [Green Version]
- Assad, H.; Kumar, A. Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. J. Mol. Liq. 2021, 344, 117755. [Google Scholar] [CrossRef]
- Assad, H.; Ganjoo, R.; Sharma, S. A theoretical insight to understand the structures and dynamics of thiazole derivatives. J. Phys. Conf. Ser. 2022, 2267, 012063. [Google Scholar] [CrossRef]
- Assad, H.; Fatma, I.; Kumar, A. An overview of the application of graphene-based materials in anticorrosive coatings. Mater. Lett. 2022, 330, 133287. [Google Scholar] [CrossRef]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Bae, E.-B.; et al. Effects of 3D-printed polycaprolactone/β-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atala, A.; Yoo, J.J. Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, B.J.; Balikov, D.A.; Lippmann, E.S.; Bellan, L.M. Spatiotemporal control of morphogen delivery to pattern stem cell differentiation in three-dimensional hydrogels. Curr. Protoc. Stem Cell Biol. 2019, 51, e97. [Google Scholar] [CrossRef]
- Griffin, M.D.; Pereira, S.R.; DeBari, M.K.; Abbott, R.D. Mechanisms of action, chemical characteristics, and model systems of obesogens. BMC Biomed. Eng. 2020, 2, 6. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Serra, T.; Mateos-Timoneda, M.A.; Planell, J.A.; Navarro, M. 3D printed PLA-based scaffolds: A versatile tool in regenerative medicine. Organogenesis 2013, 9, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Gao, C.; Wang, Y.; Wang, Y.; Mao, C.; Wang, Q.; Economidou, S.N.; Douroumis, D.; Wen, F.; Tan, L.P.; et al. Investigation of bone reconstruction using an attenuated immunogenicity xenogenic composite scaffold fabricated by 3D printing. Bio Des. Manuf. 2020, 3, 396–409. [Google Scholar] [CrossRef]
- Murphy, C.; Kolan, K.; Li, W.; Semon, J.; Day, D.; Leu, M. 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int. J. Bioprinting 2017, 3, 005. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNT s for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [Green Version]
- Kutikov, A.B.; Gurijala, A.; Song, J. Rapid prototyping amphiphilic polymer/hydroxyapatite composite scaffolds with hydration-induced self-fixation behavior. Tissue Eng. Part C Methods 2015, 21, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef]
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014. [Google Scholar] [CrossRef]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The effects of Biodentine/polycaprolactone three-dimensional-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, e291–e300. [Google Scholar] [CrossRef] [Green Version]
- Pati, F.; Song, T.H.; Rijal, G.; Jang, J.; Kim, S.W.; Cho, D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 2015, 37, 230–241. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in organ 3D bioprinting. Int. J. Bioprinting 2018, 4, 182. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Springer: New York, NY, USA, 2010; pp. 269–284. [Google Scholar]
- Hou, R.; Nie, L.; Du, G.; Xiong, X.; Fu, J. Natural polysaccharides promote chondrocyte adhesion and proliferation on magnetic nanoparticle/PVA composite hydrogels. Colloids Surf. B Biointerfaces 2015, 132, 146–154. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135, 091011–0910119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane–cell/hydrogel construct. J. Bioact. Compat. Polym. 2013, 28, 303–319. [Google Scholar] [CrossRef]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and characterization of types A and B gelatin methacryloyl for bioink applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef] [Green Version]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yi, H.G.; Kim, S.W.; Cho, D.W. 3D cell printed tissue analogues: A new platform for theranostics. Theranostics 2017, 7, 3118. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chang, T.; Kulyk, W.; Chen, X.; Eames, B.F. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng. Part C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Luo, G.; Gelinsky, M.; Huang, P.; Ruan, C. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2017, 189, 295–298. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Salemizadehparizi, F.; Vanaei, H.R. An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Nagel, T.; Kelly, D.J. The composition of engineered cartilage at the time of implantation determines the likelihood of regenerating tissue with a normal collagen architecture. Tissue Eng. Part A 2013, 19, 824–833. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Seol, Y.-J.; Forsythe, S.D.; Bishop, C.; Shupe, T.; Soker, S.; Atala, A. Bioprinting cellularized constructs using a tissue-specific hydrogel bioink. J. Vis. Exp. 2016, 110, e53606. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Yang, X.; Ke, L.; Hu, Y. The properties, biotechnologies, and applications of antifreeze proteins. Int. J. Biol. Macromol. 2020, 153, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; Armiento, A.R.; Grijpma, D.; Alini, M.; Eglin, D.; D’este, M. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 2018, 10, 044104. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Abbadessa, A.; Mouser, V.H.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.; Hennink, W.E.; Malda, J.; Vermonden, T. A synthetic thermosensitive hydrogel for cartilage bioprinting and its biofunctionalization with polysaccharides. Biomacromolecules 2016, 17, 2137–2147. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A polyurethane-gelatin hybrid construct for manufacturing implantable bioartificial livers. J. Bioact. Compat. Polym. 2008, 23, 409–422. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv. Healthc. Mater. 2020, 9, 2000737. [Google Scholar] [CrossRef]
- Cui, T.; Wang, X.; Tan, Y.; Zhang, R. Rapid prototyping a double-layer polyurethane—Collagen conduit and its Schwann cell compatibility. J. Bioact. Compat. Polym. 2009, 24, 5–17. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.C. 3D printing of microstructured and stretchable chitosan hydrogel for guided cell growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mat. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. Engineering human neural tissue by 3D bioprinting. In Biomaterials for Tissue Engineering; Humana Press: New York, NY, USA, 2018; pp. 129–138. [Google Scholar]
- Cheng, Y.L.; Chen, F. Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C 2017, 81, 66–73. [Google Scholar] [CrossRef]
- Kingsley, D.M.; Dias, A.D.; Corr, D.T. Microcapsules and 3D customizable shelled microenvironments from laser direct-written microbeads. Biotechnol. Bioeng. 2016, 113, 2264–2274. [Google Scholar] [CrossRef]
- Lee, C.M.; Yang, S.W.; Jung, S.C.; Kim, B.H. Oxygen plasma treatment on 3D-printed chitosan/gelatin/hydroxyapatite scaffolds for bone tissue engineering. J. Nanosci. Nanotechnol. 2017, 17, 2747–2750. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-derived decellularized extracellular matrix: A game changer for bioink manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef]
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Sellaro, T.L.; Ranade, A.; Faulk, D.M.; McCabe, G.P.; Dorko, K.; Badylak, S.F.; Strom, S.C. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 2010, 16, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef]
- Wong, Y.S.; Tay, C.Y.; Wen, F.; Venkatraman, S.S.; Tan, L.P. Engineered polymeric biomaterials for tissue engineering. Curr. Tissue Eng. 2012, 1, 41–53. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Srinivasan, V.; Lather, V.; Pandita, D.; Vasanthan, K.S. Insights of 3D bioprinting and focusing the paradigm shift towards 4D printing for biomedical applications. J. Mater. Res. 2022, 1–30. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, S.; Cheng, H.; Yoo, J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, C.L.; Collin, E.; Redondo-Gomez, C.; Castrejón-Pita, J.R.; Mata, A.; Ng, K.W.; Castrejón-Pita, A.A. A self-assembly based supramolecular bioink with hierarchical control as a new bioprinting tool. In Proceedings of the Biofabrication for Hierarchical in Vitro Tissue Models, Hernstein, Austria, 5–9 June 2017. [Google Scholar]
- Loo, Y.; Hauser, C.A. Bioprinting synthetic self-assembling peptide hydrogels for biomedical applications. Biomed. Mater. 2015, 11, 014103. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P. Re: How Can We Find the Number of Papers per Year Dedicated to a Certain Topic? 2022. Available online: https://www.researchgate.net/post/How-can-we-find-the-number-of-papers-per-year-dedicated-to-a-certain-topic/61e42848f7181f153356f673/citation/download (accessed on 8 January 2023).
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Deschamps, A.A.; van Apeldoorn, A.A.; Hayen, H.; de Bruijn, J.D.; Karst, U.; Grijpma, D.W.; Feijen, J. In vivo and in vitro degradation of poly (ether ester) block copolymers based on poly (ethylene glycol) and poly (butylene terephthalate). Biomaterials 2004, 25, 247–258. [Google Scholar] [CrossRef]
- Oka, M.; Ushio, K.; Kumar, P.; Ikeuchi, K.; Hyon, S.H.; Nakamura, T.; Fujita, H. Development of artificial articular cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2000, 214, 59–68. [Google Scholar] [CrossRef]
- Nie, L.; Wang, C.; Hou, R.; Li, X.; Sun, M.; Suo, J.; Wang, Z.; Cai, R.; Yin, B.; Fang, L.; et al. Preparation and characterization of dithiol-modified graphene oxide nanosheets reinforced alginate nanocomposite as bone scaffold. SN Appl. Sci. 2019, 1, 545. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wang, T.; Wang, C.; Wang, Z.; Yang, Y.; Li, P.; Cai, R.; Sun, M.; Yuan, H.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124081. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Göhl, J.; Markstedt, K.; Mark, A.; Håkansson, K.; Gatenholm, P.; Edelvik, F. Simulations of 3D bioprinting: Predicting bioprintability of nanofibrillar inks. Biofabrication 2018, 10, 034105. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, Y.B.; Yeon, Y.K.; Lee, Y.J.; Park, H.S.; Sultan, T.; Lee, J.M.; Lee, J.S.; Lee, O.J.; Hong, H.; et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 2020, 260, 120281. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 014102. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Li, D.; Long, S.; Zhang, G.; Wu, Z. Dual ionically cross-linked double-network hydrogels with high strength, toughness, swelling resistance, and improved 3D printing processability. ACS Appl. Mater. Interfaces 2018, 10, 31198–31207. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.-J.; Atala, A.; Yoo, J.-J.; Lee, S.-J. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Hoornaert, A.; Vidal, L.; Besnier, R.; Morlock, J.F.; Louarn, G.; Layrolle, P. Biocompatibility and osseointegration of nanostructured titanium dental implants in minipigs. Clin. Oral Implant. Res. 2020, 31, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Lerman, A. Bioprinting a cardiac valve. Biotechnol. Adv. 2015, 33, 1503–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.G.; Leong, K.; Fisher, J.P. (Eds.) 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Zhang, X.Y.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratheesh, G.; Vaquette, C.; Xiao, Y. Patient-specific bone particles bioprinting for bone tissue engineering. Adv. Healthc. Mater. 2020, 9, 2001323. [Google Scholar] [CrossRef]
- Dubbin, K.; Hori, Y.; Lewis, K.K.; Heilshorn, S.C. Dual-stage crosslinking of a gel-phase bioink improves cell viability and homogeneity for 3D bioprinting. Adv. Healthc. Mater. 2016, 5, 2488–2492. [Google Scholar] [CrossRef]
- Gillispie, G.; Han, A.; Uzun-Per, M.; Fisher, J.; Mikos, A.G.; Niazi, M.K.K.; Yoo, J.J.; Lee, S.J.; Atala, A. The influence of printing parameters and cell density on bioink printing outcomes. Tissue Eng. Part A 2020, 26, 1349–1358. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- OOuyang, L.; Armstrong, J.P.K.; Lin, Y.; Wojciechowski, J.P.; Lee-Reeves, C.; Hachim, D.; Zhou, K.; Burdick, J.A.; Stevens, M.M. Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci. Adv. 2020, 6, eabc5529. [Google Scholar] [CrossRef]
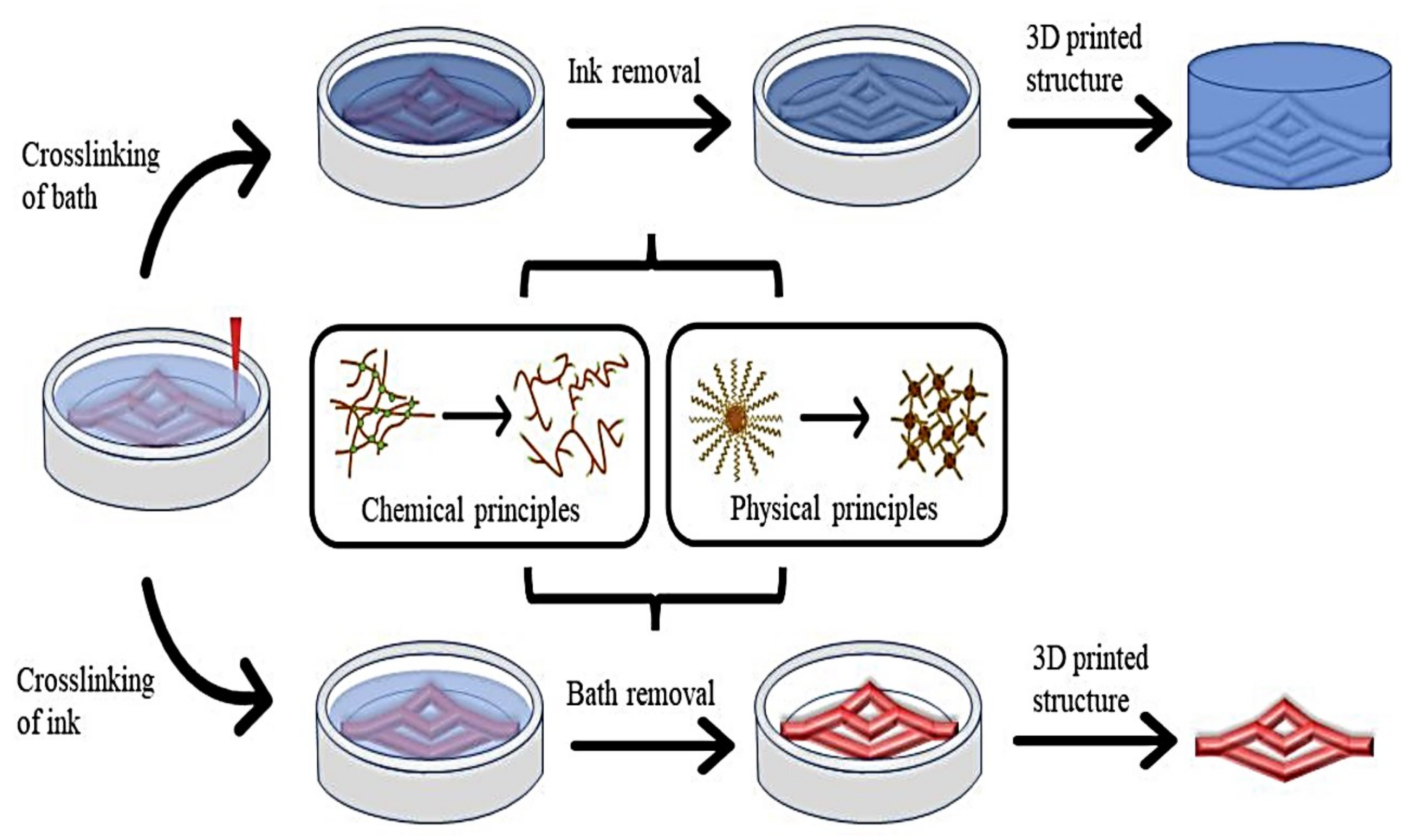
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Varchanis, S.; Haward, S.J.; Hopkins, C.C.; Syrakos, A.; Shen, A.Q.; Dimakopoulos, Y.; Tsamopoulos, J. Transition between solid and liquid state of yield-stress fluids under purely extensional deformations. Proc. Natl. Acad. Sci. USA 2020, 117, 12611–12617. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Chen, X.B. Modeling the flow behavior and flow rate of medium viscosity alginate for scaffold fabrication with a three-dimensional bioplotter. J. Manuf. Sci. Eng. 2017, 139, 081002. [Google Scholar] [CrossRef]
- Sweeney, M.; Campbell, L.L.; Hanson, J.; Pantoya, M.L.; Christopher, G.F. Characterizing the feasibility of processing wet granular materials to improve rheology for 3D printing. J. Mater. Sci. 2017, 52, 13040–13053. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, D.; Zheng, Y.; Zhao, L.; Xu, T.; Guo, Z.; Hussain, M.I.; Zeng, J.; Lou, L.; Sun, Y.; et al. A novel waterborne polyurethane with biodegradability and high flexibility for 3D printing. Biofabrication 2020, 12, 035015. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah AA, A.; Iftikhar, F.J.; Bano, B.; Khana, N.; Afridi, M.A.; Han, S.S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Gao, G.; Cui, X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol. Lett. 2016, 38, 203–211. [Google Scholar] [CrossRef]
- Liu, S.; Wang, T.; Li, S.; Wang, X. Application status of sacrificial biomaterials in 3D bioprinting. Polymers 2022, 14, 2182. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Nealey, P.F.; Murphy, C.J. Responses of human keratocytes to micro-and nanostructured substrates. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 369–376. [Google Scholar] [CrossRef]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Xiongfa, J.; Hao, Z.; Liming, Z.; Jun, X. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen. Med. 2018, 13, 73–87. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [Green Version]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D bioprinting processes: A perspective on classification and terminology. Int. J. Bioprinting 2018, 4, 151. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D bioprinting strategies for the regeneration of functional tubular tissues and organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Gu, Z.; Xie, M.; Fu, J.; Lin, H. Why choose 3D bioprinting? Part II: Methods and bioprinters. Bio Des. Manuf. 2020, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Lee, Y.; Li, Z.; Hu, J.; Park, S.S.; Kim, K. Optimized 3D bioprinting technology based on machine learning: A review of recent trends and advances. Micromachines 2022, 13, 363. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Chahal, D.; Ahmadi, A.; Cheung, K.C. Improving piezoelectric cell printing accuracy and reliability through neutral buoyancy of suspensions. Biotechnol. Bioeng. 2012, 109, 2932–2940. [Google Scholar] [CrossRef]
- Wijshoff, H. The dynamics of the piezo inkjet printhead operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M. Reconstruction of biological three-dimensional tissues: Bioprinting and biofabrication using inkjet technology. In Cell and Organ Printing; Springer: Dordrecht, The Netherlands, 2010; pp. 23–33. [Google Scholar]
- Demirci, U.; Montesano, G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip 2007, 7, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Greenhough, S.; King, J.A.; Gardner, J.; Courtney, A.; Shu, W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013, 5, 015013. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.F.; Ho, V.B.; Gaston, J.D. 3D Bioprinting and its application to military medicine. Mil. Med. 2020, 185, e1510–e1519. [Google Scholar] [CrossRef]
- Duocastella, M.; Colina, M.; Fernández-Pradas, J.M.; Serra, P.; Morenza, J.L. Study of the laser-induced forward transfer of liquids for laser bioprinting. Appl. Surf. Sci. 2007, 253, 7855–7859. [Google Scholar] [CrossRef]
- Bartolo, P.; Malshe, A.; Ferraris, E.; Koc, B. 3D bioprinting: Materials, processes, and applications. CIRP Ann. 2022, 71, 577–597. [Google Scholar] [CrossRef]
- Dinca, V.; Kasotakis, E.; Catherine, J.; Mourka, A.; Ranella, A.; Ovsianikov, A.; Chichkov, B.N.; Farsari, M.; Mitraki, A.; Fotakis, C. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett. 2008, 8, 538–543. [Google Scholar] [CrossRef]
- Barron, J.A.; Spargo, B.J.; Ringeisen, B.R. Biological laser printing of three dimensional cellular structures. Appl. Phys. A 2004, 79, 1027–1030. [Google Scholar] [CrossRef]
- Koch, L.; Brandt, O.; Deiwick, A.; Chichkov, B. Laser-assisted bioprinting at different wavelengths and pulse durations with a metal dynamic release layer: A parametric study. Int. J. Bioprinting 2017, 3, 001. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D bioprinting in tissue engineering: Advantages, deficiencies, improvements, and future perspectives. J. Mater. Chem. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Szabó, G.; Kolozsvári, L.; Nogradi, A.; Kafetzopoulos, D.; Fotakis, C. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 2012, 51, 014302. [Google Scholar] [CrossRef]
- Zhigarkov, V.; Volchkov, I.; Yusupov, V.; Chichkov, B. Metal nanoparticles in laser bioprinting. Nanomaterials 2021, 11, 2584. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Bracci, R.; Maccaroni, E.; Cascinu, S. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Jacob, G.T.; Passamai, V.E.; Katz, S.; Castro, G.R.; Alvarez, V. Hydrogels for extrusion-based bioprinting: General considerations. Bioprinting 2022, 27, e00212. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Moncal, K.K.; Dey, M.; Ozbolat, I.T. Extrusion-based biofabrication in tissue engineering and regenerative medicine. In 3D Printing and Biofabrication; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–27. [Google Scholar]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Pfister, A.; Landers, R.; Laib, A.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Biofunctional rapid prototyping for tissue-engineering applications: 3D bioplotting versus 3D printing. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 624–638. [Google Scholar] [CrossRef]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Rabbani, M. Bacterial bioprinting on a flexible substrate for fabrication of a colorimetric temperature indicator by using a commercial inkjet printer. J. Med. Signals Sens. 2018, 8, 170. [Google Scholar] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, K. Stereolithography 3D bioprinting. In 3D Bioprinting; Humana: New York, NY, USA, 2020; pp. 93–108. [Google Scholar]
- Munprom, R.; Limtasiri, S. Optimization of stereolithographic 3D printing parameters using Taguchi method for improvement in mechanical properties. Mater. Today Proc. 2019, 17, 1768–1773. [Google Scholar] [CrossRef]
- Zhou, R.; Malval, J.-P.; Jin, M.; Spangenberg, A.; Pan, H.; Wan, D.; Morlet-Savary, F.; Knopf, S. A two-photon active chevron-shaped type I photoinitiator designed for 3D stereolithography. Chem. Commun. 2019, 55, 6233–6236. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [Green Version]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical properties, cytocompatibility and manufacturability of chitosan: PEGDA hybrid-gel scaffolds by stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef]
- Van Hede, D.; Liang, B.; Anania, S.; Barzegari, M.; Verlee, B.; Nolens, G.; Pirson, J.; Geris, L.; Lambert, F. 3D-printed synthetic hydroxyapatite scaffold with in silico optimized macrostructure enhances bone formation in vivo. Adv. Funct. Mater. 2022, 32, 2105002. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional hydroxyapatite composites for orthopedic applications: A review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, S.M.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Xing, J.; Liu, J.; Zhang, T.; Zhang, L.; Zheng, M.; Duan, X. A water soluble initiator prepared through host–guest chemical interaction for microfabrication of 3D hydrogels via two-photon polymerization. J. Mater. Chem. B 2014, 2, 4318–4323. [Google Scholar] [CrossRef]
- Torgersen, J.; Ovsianikov, A.; Mironov, V.; Pucher, N.; Qin, X.-H.; Li, Z.; Cicha, K.; Machacek, T.; Liska, R.; Jantsch, V.; et al. Photo-sensitive hydrogels for three-dimensional laser microfabrication in the presence of whole organisms. J. Biomed. Opt. 2012, 17, 105008. [Google Scholar] [CrossRef]
- Tseng, H.; Balaoing, L.R.; Grigoryan, B.; Raphael, R.M.; Killian, T.C.; Souza, G.R.; Grande-Allen, K.J. A three-dimensional co-culture model of the aortic valve using magnetic levitation. Acta Biomater. 2014, 10, 173–182. [Google Scholar] [CrossRef]
- Gu, Q.; Hao, J.; Lu, Y.; Wang, L.; Wallace, G.G.; Zhou, Q. Three-dimensional bio-printing. Sci. China Life Sci. 2015, 58, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Li, N.; Guo, R.; Zhang, Z.J. Bioink formulations for bone tissue regeneration. Front. Bioeng. Biotechnol. 2021, 9, 630488. [Google Scholar] [CrossRef]
- Irvine, S.A.; Venkatraman, S.S. Bioprinting and differentiation of stem cells. Molecules 2016, 21, 1188. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Liu, L.; Ouyang, J.; Li, X.; Zhang, X.; Lan, Q.; Xu, T. Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep. 2017, 7, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Jamee, R.; Araf, Y.; Naser, I.B.; Promon, S.K. The promising rise of bioprinting in revolutionalizing medical science: Advances and possibilities. Regen. Ther. 2021, 18, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, G.; Kalia, S.; Di Lisa, L.; Cicero, A.F.; Borghi, C.; Focarete, M.L. Progress towards 3D bioprinting of tissue models for advanced drug screening: In vitro evaluation of drug toxicity and drug metabolism. Bioprinting 2022, 27, e00218. [Google Scholar] [CrossRef]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue engineering and regenerative medicine: Achievements, future, and sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Thayer, P.; Martinez, H.; Gatenholm, E. History and trends of 3D bioprinting. In 3D Bioprinting; Humana: New York, NY, USA, 2020; pp. 3–18. [Google Scholar]
- Dogan, E.; Bhusal, A.; Cecen, B.; Miri, A.K. 3D Printing metamaterials towards tissue engineering. Appl. Mater. Today 2020, 20, 100752. [Google Scholar] [CrossRef]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From arteries to capillaries: Approaches to engineering human vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef]
- Gillispie, G.J.; Park, J.; Copus, J.S.; Asari AK, P.R.; Yoo, J.J.; Atala, A.; Lee, S.J. Three-dimensional tissue and organ printing in regenerative medicine. In Principles of Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 831–852. [Google Scholar]
- Richards, D.; Jia, J.; Yost, M.; Markwald, R.; Mei, Y. 3D bioprinting for vascularized tissue fabrication. Ann. Biomed. Eng. 2017, 45, 132–147. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D bioprinting of hydrogels for cartilage tissue engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Bioceramic hydroxyapatite-based scaffold with a porous structure using honeycomb as a natural polymeric porogen for bone tissue engineering. Biomater. Res. 2021, 25, 2. [Google Scholar] [CrossRef]
- Huang, G.-J.; Yu, H.-P.; Wang, X.-L.; Ning, B.-B.; Gao, J.; Shi, Y.-Q.; Zhu, Y.-J.; Duan, J.-L. Highly porous and elastic aerogel based on ultralong hydroxyapatite nanowires for high-performance bone regeneration and neovascularization. J. Mater. Chem. B 2021, 9, 1277–1287. [Google Scholar] [CrossRef]
- Black, J.D.; Tadros, B.J. Bone structure: From cortical to calcium. Orthop. Trauma 2020, 34, 113–119. [Google Scholar] [CrossRef]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef]
- Dhawan, A.; Kennedy, P.M.; Rizk, E.B.; Ozbolat, I.T. Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. JAAOS J. Am. Acad. Orthop. Surg. 2019, 27, e215–e226. [Google Scholar] [CrossRef]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Kérourédan, O.; Hakobyan, D.; Rémy, M.; Ziane, S.; Dusserre, N.; Fricain, J.C.; Delmond, S.; Thébaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication 2019, 11, 045002. [Google Scholar] [CrossRef]
- Rukavina, P.; Koch, F.; Wehrle, M.; Tröndle, K.; Stark, G.B.; Koltay, P.; Zimmermann, S.; Zengerle, R.; Lampert, F.; Strassburg, S.; et al. In vivo evaluation of bioprinted prevascularized bone tissue. Biotechnol. Bioeng. 2020, 117, 3902–3911. [Google Scholar] [CrossRef]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Castro, N.J.; Zhu, W.; Cui, H.; Aliabouzar, M.; Sarkar, K.; Zhang, L.G. Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation. Sci. Rep. 2016, 6, 32876. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.S.; Gao, G.; Cho, D.W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef]
- Quílez, C.; Aranda Izuzquiza, G.D.; García, M.; López, V.; Montero, A.; Valencia, L.; Velasco, D. Bioprinting for skin. In 3D Bioprinting; Humana: New York, NY, USA, 2020; pp. 217–228. [Google Scholar]
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng. Part A 2015, 21, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [Green Version]
- Min, D.; Lee, W.; Bae, I.H.; Lee, T.R.; Croce, P.; Yoo, S.S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, W.H.; Melnychenko, I.; Eschenhagen, T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 2004, 25, 1639–1647. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [Green Version]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.C.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [Green Version]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [Green Version]
- Skylar-Scott, M.A.; Uzel, S.G.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef] [Green Version]
- Rimann, M.; Laternser, S.; Keller, H.; Leupin, O.; Graf-Hausner, U. 3D bioprinted muscle and tendon tissues for drug development: Biotechnet Switzerland. Chimia 2015, 69, 65. [Google Scholar] [CrossRef]
- Boyd-Moss, M.; Fox, K.; Brandt, M.; Nisbet, D.; Williams, R. Bioprinting and biofabrication with peptide and protein biomaterials. In Peptides and Peptide-Based Biomaterials and Their Biomedical Applications; Springer: Cham, Switzerland, 2017; pp. 95–129. [Google Scholar]
- Farina, M.F.; Chua, C.Y.X.; Ballerini, A.; Thekkedath, U.; Alexander, J.F.; Rhudy, J.R.; Torchio, G.; Fraga, D.; Pathak, R.R.; Villanueva, M.; et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials 2018, 177, 125–138. [Google Scholar] [CrossRef]
- Cho, D.W.; Kim, B.S.; Jang, J.; Gao, G.; Han, W.; Singh, N.K. Various applications of 3D-bioprinted tissues/organs using tissue-specific bioinks. In 3D Bioprinting; Springer: Cham, Switzerland, 2019; pp. 53–108. [Google Scholar]
- Turunen, S.; Kaisto, S.; Skovorodkin, I.; Mironov, V.; Kalpio, T.; Vainio, S.; Rak-Raszewska, A. 3D bioprinting of the kidney—Hype or hope? AIMS Cell Tissue Eng. 2018, 2, 119–162. [Google Scholar] [CrossRef]
- Foerster, A.; Cantu, L.R.; Wildman, R.; Tuck, C. Current market for biomedical implants. In Polymer-Based Additive Manufacturing; Springer: Cham, Switzerland, 2019; pp. 97–119. [Google Scholar]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of fused deposition modeling process parameters for dimensional accuracy using I-optimality criterion. Measurement 2016, 81, 174–196. [Google Scholar] [CrossRef]
- Galliger, Z.; Vogt, C.D.; Panoskaltsis-Mortari, A. 3D bioprinting for lungs and hollow organs. Transl. Res. 2019, 211, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Osagie, J.; Syeda, S.; Krueger, A.; Turner-Brannen, E.; West, A.R. Validation of a 3D bioprinted model of airway smooth muscle—A novel tool to study the effects of airway stiffening in asthma. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Fucile, P.; Onofrio, I.; Papallo, I.; Gallicchio, V.; Rega, A.; D’Antò, V.; Improta, G.; De Santis, R.; Gloria, A.; Russo, T. Strategies for the design of additively manufactured nanocomposite scaffolds for hard tissue regeneration. Acta IMEKO 2020, 9, 53–59. [Google Scholar] [CrossRef]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.H.; Park, J.; Kim, W.D.; Park, S.A. Fabrication of 3D printing scaffold with porcine skin decellularized bio-ink for soft tissue engineering. Materials 2020, 13, 3522. [Google Scholar] [CrossRef]
- Zou, Q.; Grottkau, B.E.; He, Z.; Shu, L.; Yang, L.; Ma, M.; Ye, C. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater. Sci. Eng. C 2020, 108, 110205. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Zhu, W.; Harris, B.T.; Zhang, L.G. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; IEEE: New York, NY, USA, 2016; pp. 4185–4188. [Google Scholar]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Ahn, M.; Cho, W.W.; Kim, B.S.; Cho, D.W. 3D printing of pharmaceutical application: Drug screening and drug delivery. Pharmaceutics 2021, 13, 1373. [Google Scholar] [CrossRef]
- Nam, K.H.; Smith, A.S.; Lone, S.; Kwon, S.; Kim, D.H. Biomimetic 3D tissue models for advanced high-throughput drug screening. J. Lab. Autom. 2015, 20, 201–215. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Senna, J.P.; Barradas, T.N.; Cardoso, S.; Castiglione, T.C.; Serpe, M.J.; e Silva, K.G.D.H.; Mansur, C.R.E. Dual alginate-lipid nanocarriers as oral delivery systems for amphotericin B. Colloids Surf. B Biointerfaces 2018, 166, 187–194. [Google Scholar] [CrossRef]
- Miao, T.; Rao, K.S.; Spees, J.L.; Oldinski, R.A. Osteogenic differentiation of human mesenchymal stem cells through alginate-graft-poly (ethylene glycol) microsphere-mediated intracellular growth factor delivery. J. Control. Release 2014, 192, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Guan, T.; Zhang, X.; Wang, Z.; Wang, M.; Zhong, W.; Feng, H.; Xing, M.; Kong, J. The effect of layer-by-layer assembly coating on the proliferation and differentiation of neural stem cells. ACS Appl. Mater. Interfaces 2015, 7, 3018–3029. [Google Scholar] [CrossRef]
- Thakur, G.; Rodrigues, F.C.; Singh, K. Crosslinking biopolymers for advanced drug delivery and tissue engineering applications. Cut. Edge Enabling Technol. Regen. Med. 2018, 1078, 213–231. [Google Scholar]
- Genina, N.; Boetker, J.P.; Colombo, S.; Harmankaya, N.; Rantanen, J.; Bohr, A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. J. Control. Release 2017, 268, 40–48. [Google Scholar] [CrossRef]
- Ozbolat, I.T. 3D Bioprinting: Fundamentals, Principles and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Organ-on-a-chip platforms for drug delivery and cell characterization: A review. Sens. Mater. 2015, 27, 487–506. [Google Scholar]
- Nie, J.; Gao, Q.; Fu, J.; He, Y. Grafting of 3D bioprinting to in vitro drug screening: A review. Adv. Healthc. Mater. 2020, 9, 1901773. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Lotz, M.; D’Lima, D. Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol. Bioeng. 2012, 109, 2357–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Lee, J.Y.; Hwang, C.; Shin, J.H.; Park, Y. Inhibition of rho-associated protein kinase increases the angiogenic potential of mesenchymal stem cell aggregates via paracrine effects. Tissue Eng. Part A 2016, 22, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.G.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PloS ONE 2016, 11, e0158674. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.S.; Morris, K.V. Transcriptional gene silencing in humans. Nucleic Acids Res. 2016, 44, 6505–6517. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.P.; Tiong, D.; Berry, R.M.; Tam, K.C. Comparative release studies of two cationic model drugs from different cellulose nanocrystal derivatives. Eur. J. Pharm. Biopharm. 2014, 88, 207–215. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Syverud, K. Pretreatment-dependent surface chemistry of wood nanocellulose for pH-sensitive hydrogels. J. Biomater. Appl. 2014, 29, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Koçak, E.; Yıldız, A.; Acartürk, F. Three dimensional bioprinting technology: Applications in pharmaceutical and biomedical area. Colloids Surf. B Biointerfaces 2021, 197, 111396. [Google Scholar] [CrossRef]
- Sachdev, A., IV; Acharya, S.; Gadodia, T.; Shukla, S.; Harshita, J.; Akre, C.; Khare, M.; Huse, S. A review on techniques and biomaterials used in 3D bioprinting. Cureus 2022, 14, e28463. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Seol, Y.J.; Kang, H.W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting technology and its applications. Eur. J. Cardio Thorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Frejo, L.; Grande, D.A. 3D-bioprinted tracheal reconstruction: An overview. Bioelectron. Med. 2019, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Hosseinabadi, H.G.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S.; et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv. Mater. 2018, 30, 1800242. [Google Scholar] [CrossRef]



| Biomaterials | Pros | Cons | Ref. |
|---|---|---|---|
| Synthetic Polymers | |||
| PGA | Chemical adaptability; processing simplicity; biocompatibility; and biological characteristics. | Bulk erosion resulting in scaffold collapsing, thereby liberating acidic degradation products that affect the body. | [102] |
| PLA | Biocompatibility; processability; and printing capability. | Releases acidic by-products; brittleness. | [31] |
| PCL | Less costly; possesses rigidity, biocompatibility, and degradability. | Longer biological half-life develops secondary obstacle in scaffolds; low bioactivity caused by higher hydrophobicity features. | [35,36] |
| PEG | Good when combined with other components. | Low cell proliferation and adhesion; poor mechanical strength; UV causes cell damage. | [103,104] |
| PBT | Exhibits high flexibility, simple processing, and allowable strength and resilience. | Breaks down in aqueous media via oxidation or hydrolysis; non-biodegradable essence. | [37,105] |
| PU | Great biocompatibility, thermosetting tendency, and mechanical strength. | - | [60] |
| PVA | Hydrophilicity and chemical stability. | Water solubility that bears adversity in controlling. | [39,106] |
| Natural Polymers | |||
| Alginate | Fast gelation; low cost; good stability. | Poor cell attachment; easily clogs at high concentrations. | [107,108] |
| Collagen | Promotes cell attachment; good printing abilities; has an RGD sequence. | Poor mechanical stability; slow gelation; easily clogs; soluble in acid. | [36] |
| Chitosan | Antibacterial and antifungal. | Slow gelation rate; poor mechanical properties. | [109] |
| Gelatin | Reversible; promotes cell adhesion. | Unstable/fragile; poor abilities without modification; low rigidity; poor shape stability. | [50,110] |
| Hyaluronic acid (HA) | Promotes proliferation and Angiogenesis; fast gelation. | Rapid degradation; poor mechanical strength and structural stability. | [111] |
| dECM | Ability to apply materials from the same tissue of interest; the complex biomolecular and physical cues in the ECM are preserved and can support cell growth and viability. | Residual DNA or nuclear materials; poor mechanical qualities; low construction resolution, surprising form-shrinking; quick degradation rate. | [45,94] |
| Bio-Printing Technique | Pros | Cons | Viscosity & Resolution | Cell Viability | Price | Ref. |
|---|---|---|---|---|---|---|
| Inkjet | High speed; availability; low cost; high efficiency; the capability to bioprint multiple bio-inks at a time. | Lack of precision in droplet placement and size; need for low viscosity bio-ink; heat damage to cell behaviors; difficult to operate and maintain; frequent nozzle clogging. | <15 mPa/s 50–100 μm | >85% | Low | [15,191] |
| Micro- extrusion | Ability to use high-viscosity bio-ink at the same time and print at high cell density; capability to generate high-freedom degree motion; versatility; cost-effectiveness; user-friendly; sterilization possible. | Distortion of cell structures; low resolution; low printing speed. | <6 mPa/s 100 μm | >45% | Medium | [66,192] |
| Laser-assisted | High degree of precision and resolution; absence of nozzle; accurate and fast printing; the ability to use high-viscosity bio-ink and print at high cell density. | Complicated preparation process; time consuming; high cost; trace metallic residues; low-flow rate, bio-ink restriction; very high temperature required (up to 1400 °C). | <300 mPa/s 20 μm | >95% | High | [135,149] |
| Stereolitho-graphy | High degree of fabrication accuracy; low printing time; creation of smooth surfaces. | Use of high-intensity UV light; lengthy post-processing; lack of compatible materials; bio-inks must be photopolymers; utilized photo-cross-linkers are toxic; difficult to bioprint multi-material constructs. | No limitation 100 μm | >90% | Medium | [133,193] |
| Tissue/Organ | Polymer | Technique | Cell Source | Outcome | Ref. |
|---|---|---|---|---|---|
| Bone | Alginate/PVA | Extrusion Bio-printing | Bone-marrow stem cells | This study demonstrates that bone tissue could be bio-printed using alginate and polyvinyl alcohol bio-inks in appropriate amounts. | [59] |
| Cartilage | Cellulose/alginate | Extrusion Bio-printing | Human nasal chondrocytes, mesenchymal stem cells | The therapeutic significance and cartilage synthesis in constructs with high fidelity and good mechanical characteristics are revealed in this study. | [243] |
| Skin | Alginate | Extrusion Bio-printing | Mouse embryonic fibroblasts | The research demonstrates that the PSP-ink employed was non-toxic, and the suggested skin dermis decellularized bio-ink is discovered to be a good contender for tissue engineering applications. | [244] |
| Heart | Alginate | Extrusion Bio-printing | H9c2 cells, human umbilical-vein endothelial cells | This study reveals that valentine-like constructions with a self-defined height and appropriate mechanical properties may be created utilising 3D bio-printing employing sacrificial and hydrogel materials. | [245] |
| Vascular Grafts | poly(ethylene glycol) diacrylate | SLA Bio-printing | Human red blood cells | This study reveals the possibility of simultaneous and orthogonal control of tissue architecture and biomaterials for the creation of regenerated tissues. | [246] |
| Neural tissue | Gelatin methacrylamide | SLA Bio-printing | Mouse neural stem cells | These results demonstrate that, after two weeks of culture, neural stem cells demonstrated neuron differentiation and neurite extension within the printed construct, indicating the 3D-bio-printed neural construct has tremendous promise for regenerating neural tissue. | [247] |
| Liver | Gelatin methacrylate, glycidyl methacrylate-hyaluronic acid | SLA Bio-printing | Human-induced pluripotent-stem-cell-derived hepatic progenitor cells, human umbilical-vein endothelial cells, adipose-derived stem cells | This study demonstrates that, throughout weeks of in vitro development, the hiPSC-HPCs exhibit phenotypic and functional improvements in the 3D triculture paradigm. | [248] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assad, H.; Assad, A.; Kumar, A. Recent Developments in 3D Bio-Printing and Its Biomedical Applications. Pharmaceutics 2023, 15, 255. https://doi.org/10.3390/pharmaceutics15010255
Assad H, Assad A, Kumar A. Recent Developments in 3D Bio-Printing and Its Biomedical Applications. Pharmaceutics. 2023; 15(1):255. https://doi.org/10.3390/pharmaceutics15010255
Chicago/Turabian StyleAssad, Humira, Arvina Assad, and Ashish Kumar. 2023. "Recent Developments in 3D Bio-Printing and Its Biomedical Applications" Pharmaceutics 15, no. 1: 255. https://doi.org/10.3390/pharmaceutics15010255
APA StyleAssad, H., Assad, A., & Kumar, A. (2023). Recent Developments in 3D Bio-Printing and Its Biomedical Applications. Pharmaceutics, 15(1), 255. https://doi.org/10.3390/pharmaceutics15010255







