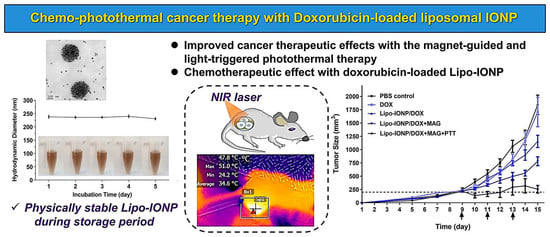
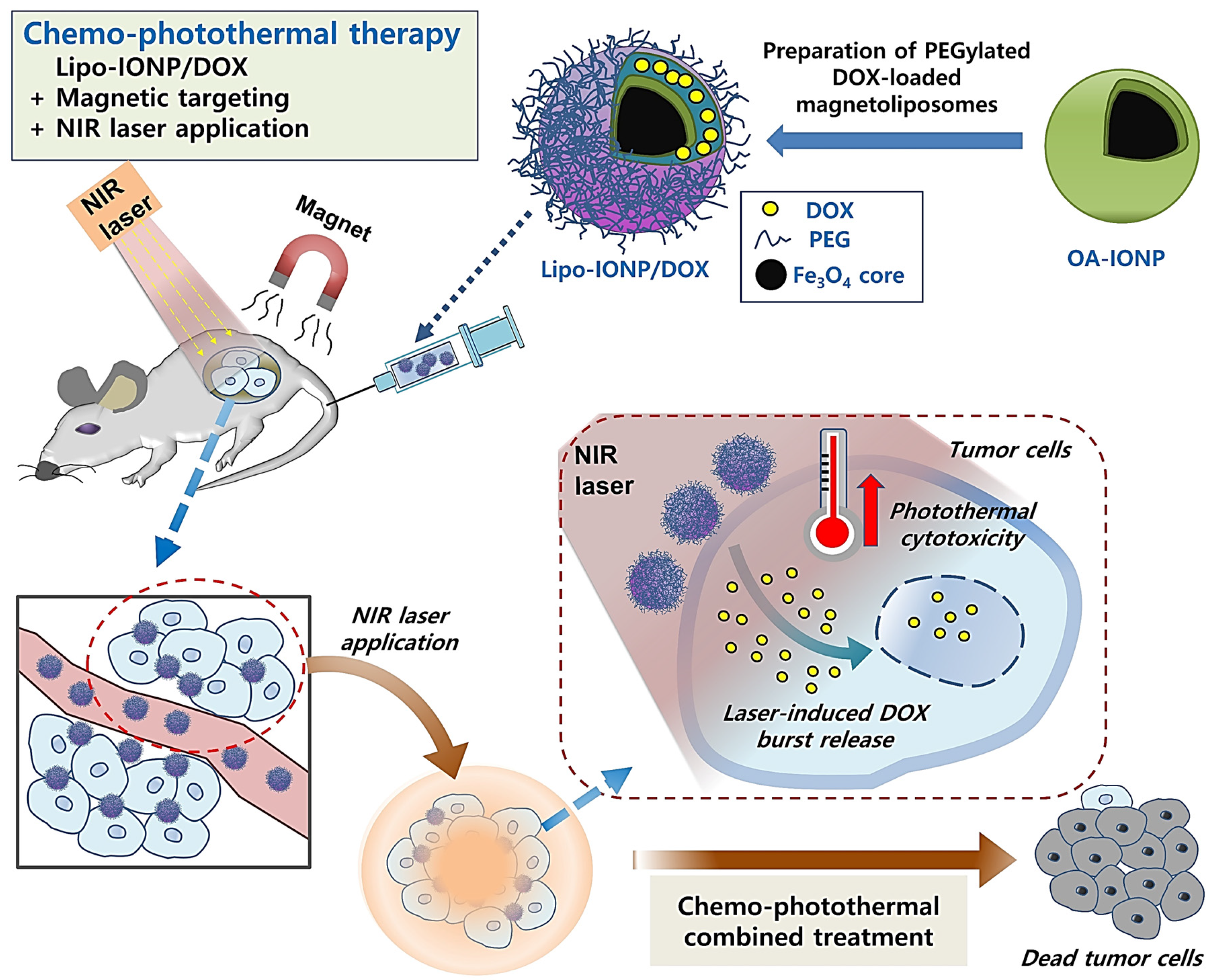
Liposomal Iron Oxide Nanoparticles Loaded with Doxorubicin for Combined Chemo-Photothermal Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Lipo-IONP
2.3. Physical Characterization of Lipo-IONP
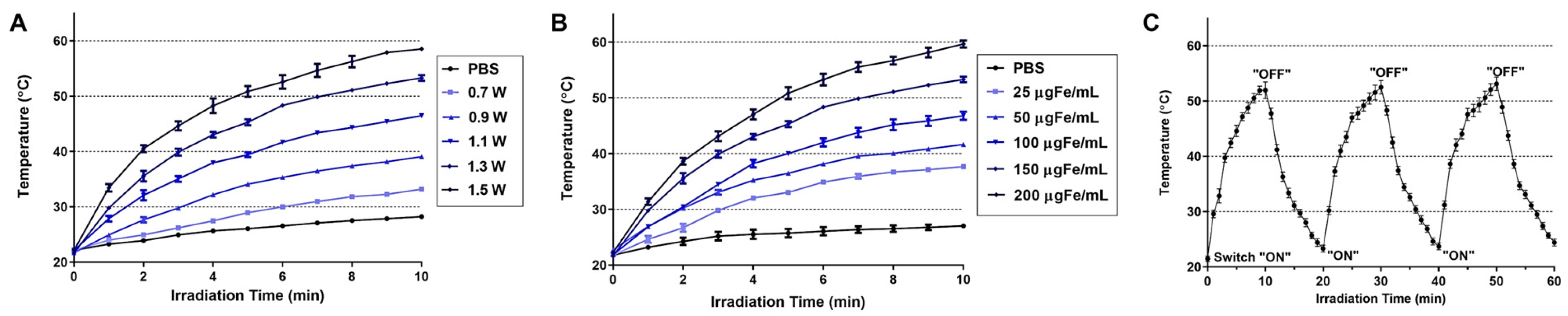
2.4. Measurement of the Photothermal Activity of Lipo-IONP
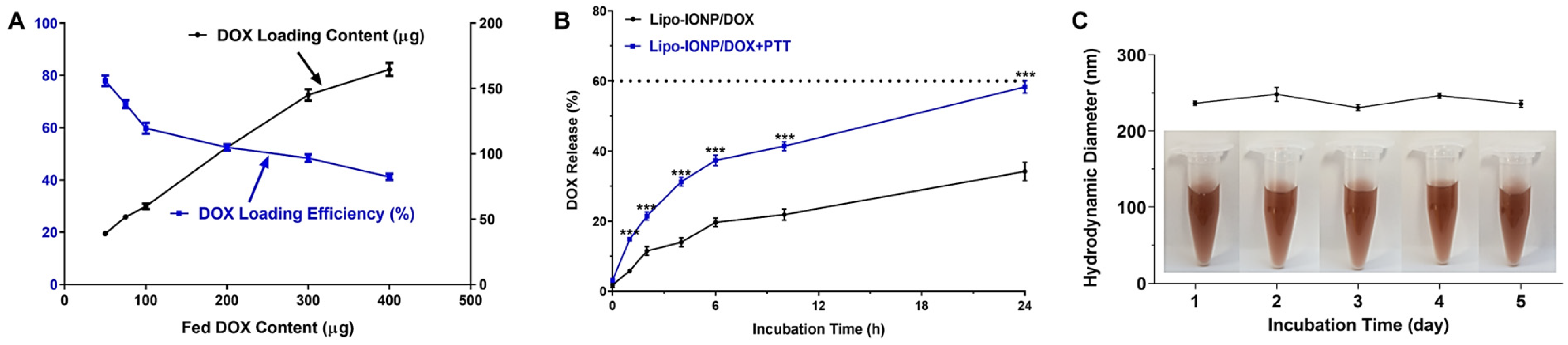
2.5. DOX Loading and Release from Lipo-IONP
2.6. Cell Culture
2.7. Cytotoxicity of Lipo-IONP/DOX
2.8. Animal Studies
2.8.1. Pharmacokinetics (PK)
2.8.2. Tissue Distribution
2.8.3. In Vivo Magnetic Tumor Targeting
2.8.4. In Vivo Photothermal Activity of Lipo-IONP/DOX
2.8.5. In vivo Evaluation of Efficacy and Toxicity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Lipo-IONP
3.2. Photothermal Activity of Lipo-IONP
3.3. DOX Loading and Release
3.4. Cellular Analyses of Lipo-IONP/DOX-Mediated Anti-Cancer Activity
3.5. Pharmacokinetic Profiles
3.6. Tissue Distribution, Magnetic Tumor Targeting, and Photothermal Effects in B16F10 S.c. Tumor-Bearing Nude Mice
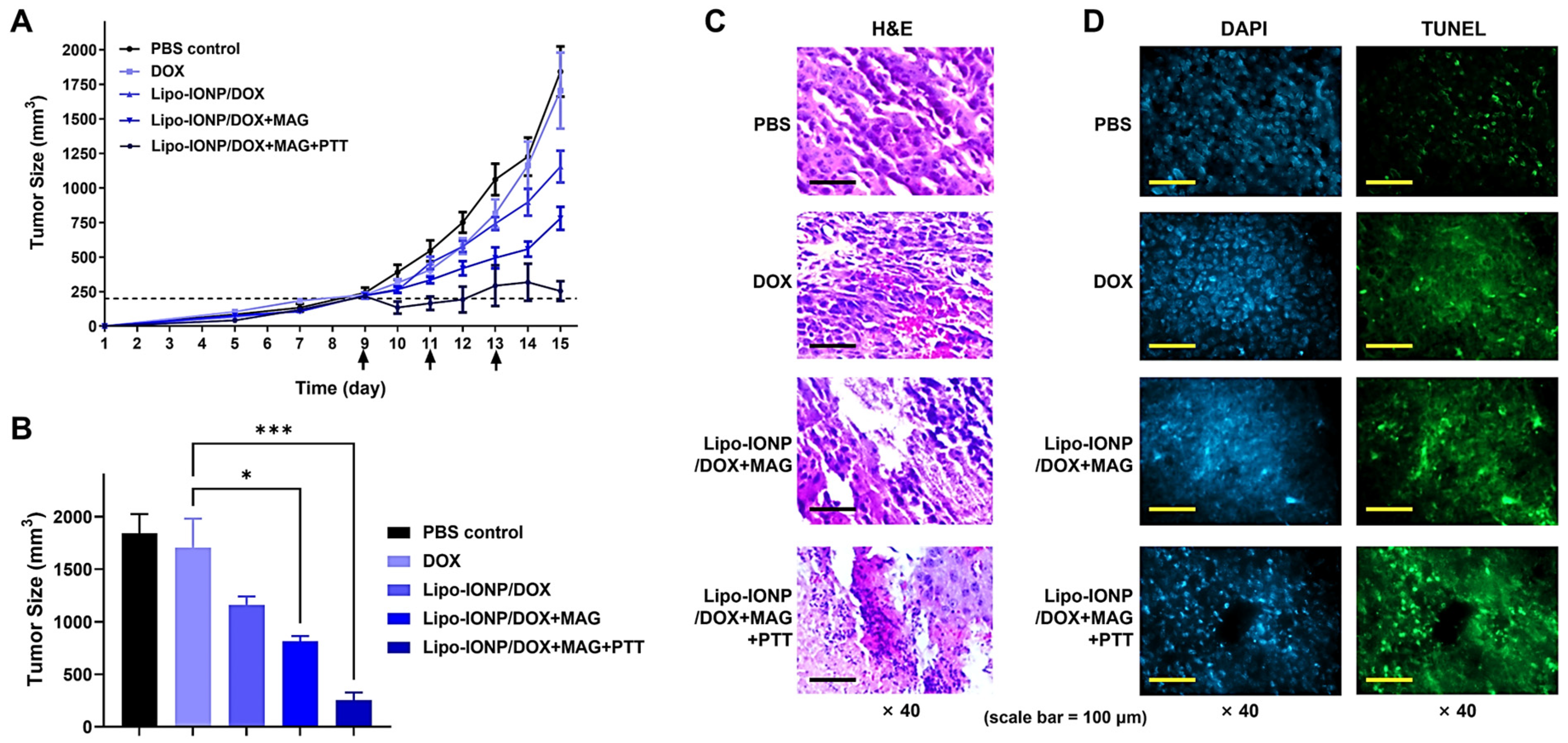
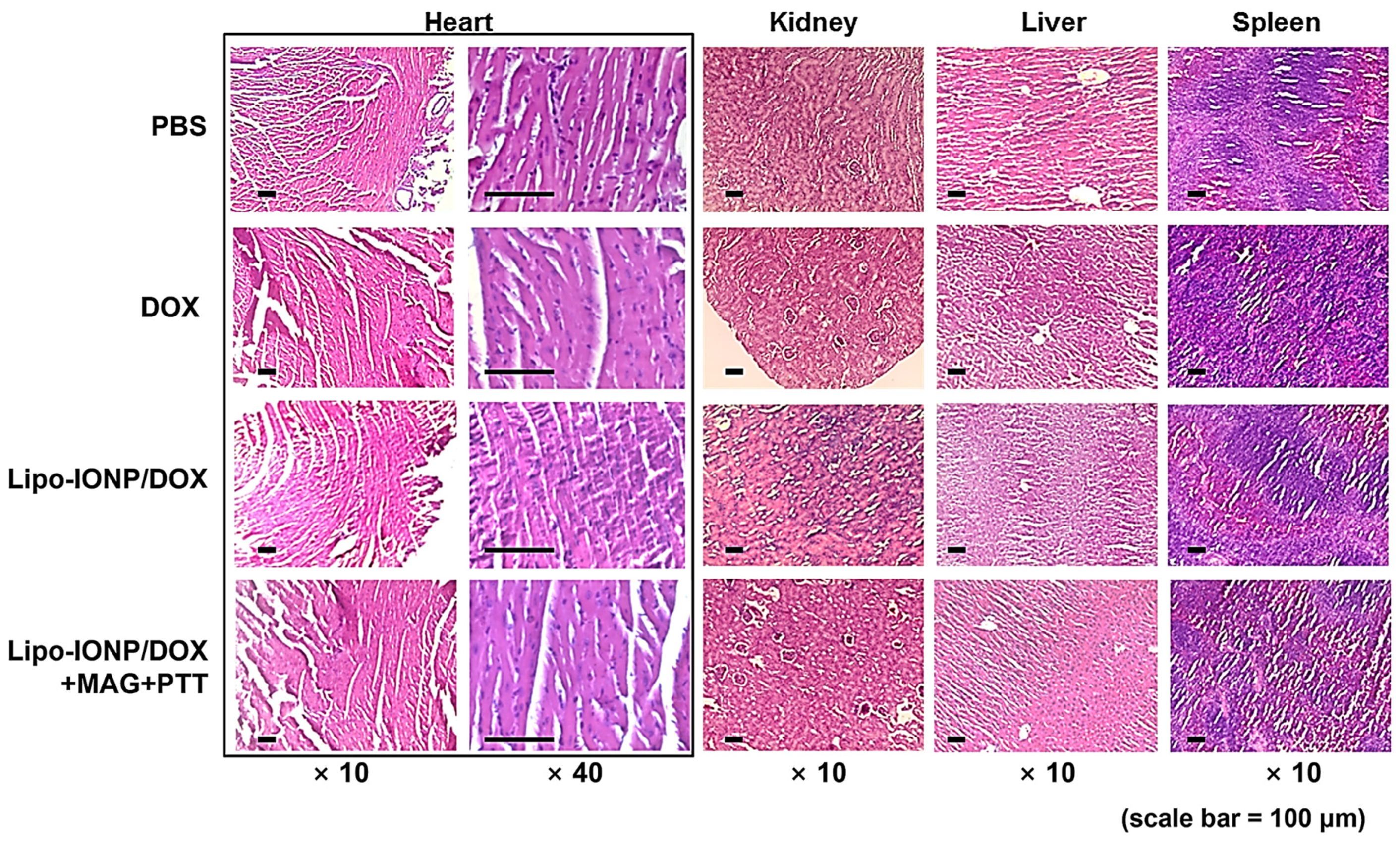
3.7. Therapeutic Efficacy and Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.; He, S.; Seare, W.J.; Almutairi, A. Review of the progress toward achieving heat confinement—The holy grail of photothermal therapy. J. Biomed. Opt. 2017, 22, 080901. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Jin, Y.; Zhen, W.; Wang, Y.; Liu, J.; Jin, L.; Zhang, S.; Zhao, Y.; Song, S. Copper (I) phosphide nanocrystals for in situ self-generation magnetic resonance imaging-guided photothermal-enhanced chemodynamic synergetic therapy resisting deep-seated tumor. Adv. Funct. Mater. 2019, 29, 1904678. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World. J. Gastroenterol. 2015, 21, 13400. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.P.; Baishya, R.; Hatiboruah, J.L.; Laloo, D.; Biswas, N. A comprehensive review on different approaches for tumor targeting using nanocarriers and recent developments with special focus on multifunctional approaches. J. Pharm. Investig. 2022, 52, 539–585. [Google Scholar] [CrossRef]
- Zhang, J.; Shin, M.C.; David, A.E.; Zhou, J.; Lee, K.; He, H.; Yang, V.C. Long-circulating heparin-functionalized magnetic nanoparticles for potential application as a protein drug delivery platform. Mol. Pharm. 2013, 10, 3892–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estelrich, J.; Busquets, M.A. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.W.; Udego, I.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems–The current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef]
- Chen, C.-S.; Yao, J.; Durst, R.A. Liposome encapsulation of fluorescent nanoparticles: Quantum dots and silica nanoparticles. J. Nanopart. Res. 2006, 8, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Shim, G.; Jeong, S.; Oh, J.L.; Kang, Y. Lipid-based nanoparticles for photosensitive drug delivery systems. J. Pharm. Investig. 2022, 52, 151–160. [Google Scholar] [CrossRef]
- Hsieh, W.-J.; Liang, C.-J.; Chieh, J.-J.; Wang, S.-H.; Lai, I.-R.; Chen, J.-H.; Chang, F.-H.; Tseng, W.-K.; Yang, S.-Y.; Wu, C.-C. In vivo tumor targeting and imaging with anti-vascular endothelial growth factor antibody-conjugated dextran-coated iron oxide nanoparticles. Int. J. Nanomedicine 2012, 7, 2833–2842. [Google Scholar]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-loaded pegylated BSA-silver nanoparticles for effective photothermal cancer therapy. Int. J. Nanomedicine 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Kim, C.-E.; Lim, S.-K.; Kim, J.-S. In vivo antitumor effect of cromolyn in PEGylated liposomes for pancreatic cancer. J. Control Release 2012, 157, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.; Kwon, N.; Lee, S.A.; Lee, Y. Smart pH-responsive nanomedicines for disease therapy. J. Pharm. Investig. 2022, 52, 427–441. [Google Scholar] [CrossRef]
- Karmacharya, P.; Patil, B.R.; Kim, J.O. Recent advancements in lipid–mRNA nanoparticles as a treatment option for cancer immunotherapy. J. Pharm. Investig. 2022, 52, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Clauson, R.M.; Chen, M.; Scheetz, L.M.; Berg, B.; Chertok, B. Size-controlled iron oxide nanoplatforms with lipidoid-stabilized shells for efficient magnetic resonance imaging-trackable lymph node targeting and high-capacity biomolecule display. ACS Appl. Mater. Interfaces 2018, 10, 20281–20295. [Google Scholar] [CrossRef] [PubMed]
- Voss, L.F.; Bazerbashi, M.F.; Beekman, C.P.; Hadad, C.M.; Allen, H.C. Oxidation of oleic acid at air/liquid interfaces. J. Geophys. Res. Atmos. 2007, 112, D06209. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Song, L.; Tian, Y.; Zhu, L.; Guo, K.; Zhang, H.; Wang, Z. Emodin-Conjugated PEGylation of Fe 3 O 4 Nanoparticles for FI/MRI Dual-Modal Imaging and Therapy in Pancreatic Cancer. Int. J. Nanomedicine 2022, 17, 711–712. [Google Scholar] [CrossRef]
- Aranda, F.J.; Villalaín, J.; Gómez-Fernández, J.C. A Fourier transform infrared spectroscopic study of the molecular interaction of ubiquinone-10 and ubiquinol-10 with bilayers of dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta Biomembr. 1986, 861, 25–32. [Google Scholar] [CrossRef]
- Mahato, M.; Sarkar, R.; Pal, P.; Talapatra, G. Formation of silver nanoparticle at phospholipid template using Langmuir–Blodgett technique and its Surface-enhanced Raman Spectroscopy application. Indian J. Phys. 2015, 89, 997–1005. [Google Scholar] [CrossRef]
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Yang, V.C. Magnetic brain tumor targeting and biodistribution of long-circulating PEG-modified, cross-linked starch-coated iron oxide nanoparticles. Biomaterials 2011, 32, 6291–6301. [Google Scholar] [CrossRef] [PubMed]





| Lipo-IONP | DPPC:DSPE-P2000 | Hydrodynamic Size (nm) | PDI | Zeta Potential (mV) | Iron Loading Content (μgFe) | Transition Temperature (°C) |
|---|---|---|---|---|---|---|
| L1 | 3:1 | 231.5 (±6.6) | 0.25 | −30.5 (±0.4). | 348 (±43) | 48.79 |
| L2 | 4:1 | 236.3 (±1.5) | 0.24 | −34.8 (±0.6) | 553 (±74) | 47.98 |
| L3 | 5:1 | 242.1 (±1.2) | 0.26 | −35.1 (±0.2) | 635 (±64) | 47.04 |
| Laser Power (W) | Surface Temperature of the Laser Irradiation Site (°C) | |||
|---|---|---|---|---|
| PBS-Control | Lipo-IONP/DOX (−Magnet) | Lipo-IONP/DOX (+Magnet) | ||
| Tumor | Contra-Lateral Normal Skin | Tumor | Tumor | |
| 0.95 | 37.2 | 37.5 | 48.8 | 59.8 |
| 0.9 | 36.1 | 36.6 | 48.1 | 59.2 |
| 0.8 | 34.8 | 35.2 | 46.2 | 54.9 |
| 0.7 | 32.5 | 33.1 | 42.7 | 50.8 |
| 0.6 | 31.6 | 32.2 | 41.6 | 45.6 |
| 0.5 | 29.3 | 29.5 | 39.5 | 41.5 |
| 0 | 29.1 | 29.2 | 29.4 | 29.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.; Amatya, R.; Min, K.A.; Shin, M.C. Liposomal Iron Oxide Nanoparticles Loaded with Doxorubicin for Combined Chemo-Photothermal Cancer Therapy. Pharmaceutics 2023, 15, 292. https://doi.org/10.3390/pharmaceutics15010292
Park T, Amatya R, Min KA, Shin MC. Liposomal Iron Oxide Nanoparticles Loaded with Doxorubicin for Combined Chemo-Photothermal Cancer Therapy. Pharmaceutics. 2023; 15(1):292. https://doi.org/10.3390/pharmaceutics15010292
Chicago/Turabian StylePark, Taehoon, Reeju Amatya, Kyoung Ah Min, and Meong Cheol Shin. 2023. "Liposomal Iron Oxide Nanoparticles Loaded with Doxorubicin for Combined Chemo-Photothermal Cancer Therapy" Pharmaceutics 15, no. 1: 292. https://doi.org/10.3390/pharmaceutics15010292
APA StylePark, T., Amatya, R., Min, K. A., & Shin, M. C. (2023). Liposomal Iron Oxide Nanoparticles Loaded with Doxorubicin for Combined Chemo-Photothermal Cancer Therapy. Pharmaceutics, 15(1), 292. https://doi.org/10.3390/pharmaceutics15010292