Multifunctional Mesoporous Silica-Coated Gold Nanorods Mediate Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of siRNAs and Primers
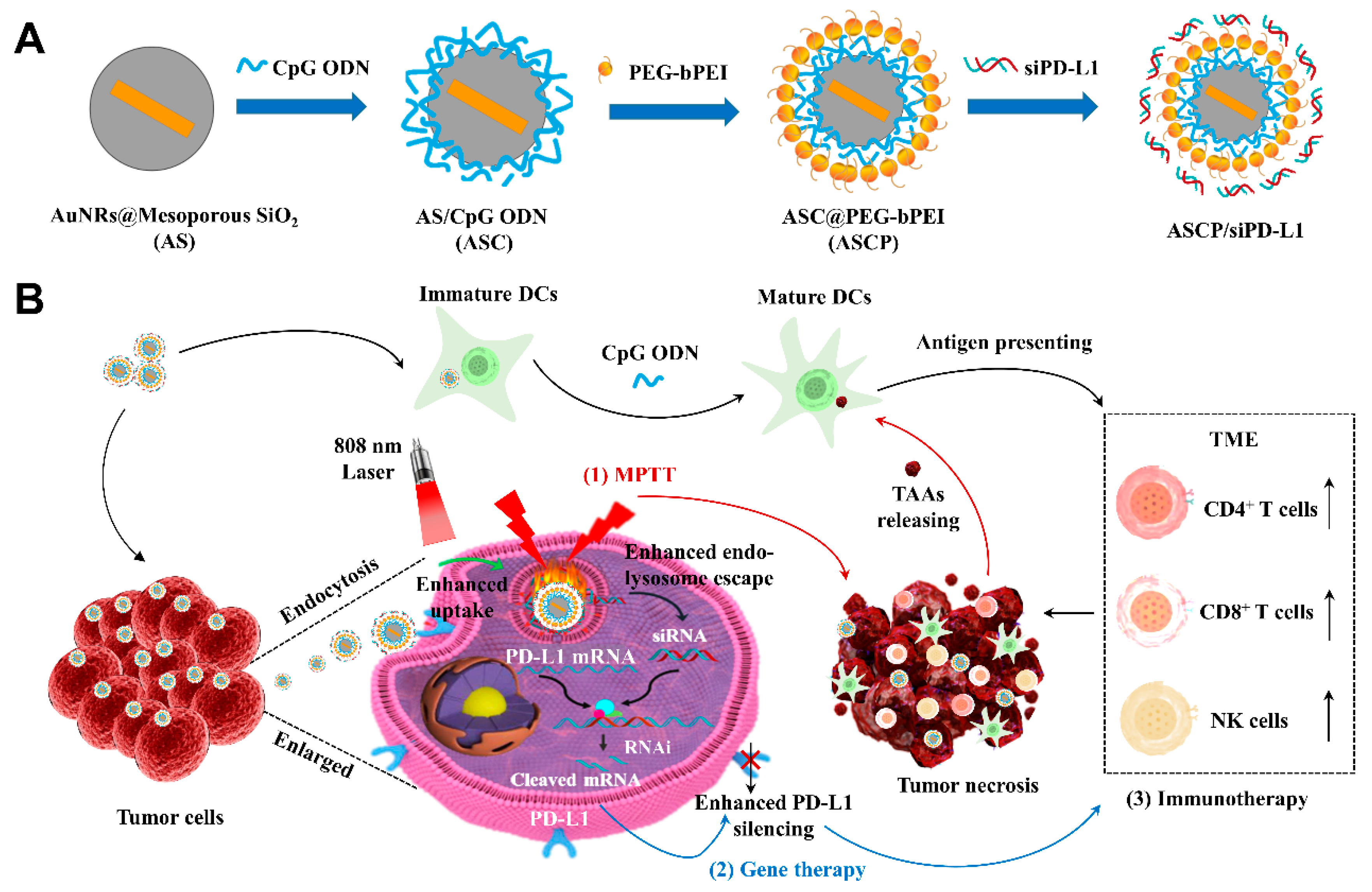
2.3. Preparation of AS NPs
2.4. Modification from AS NPs to ASCP NPs
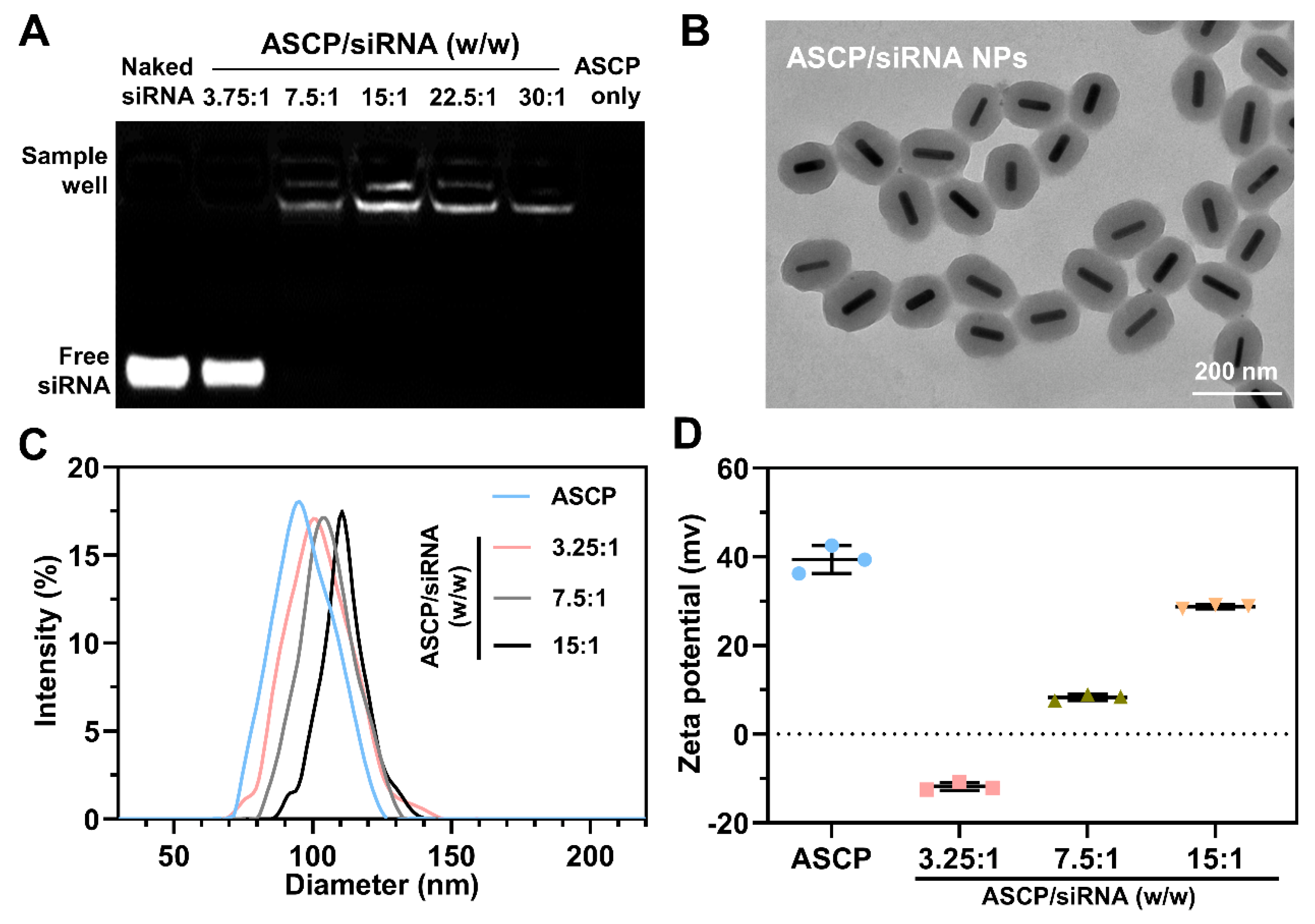
2.5. Preparation of ASCP/siRNA NPs
2.6. Characterization
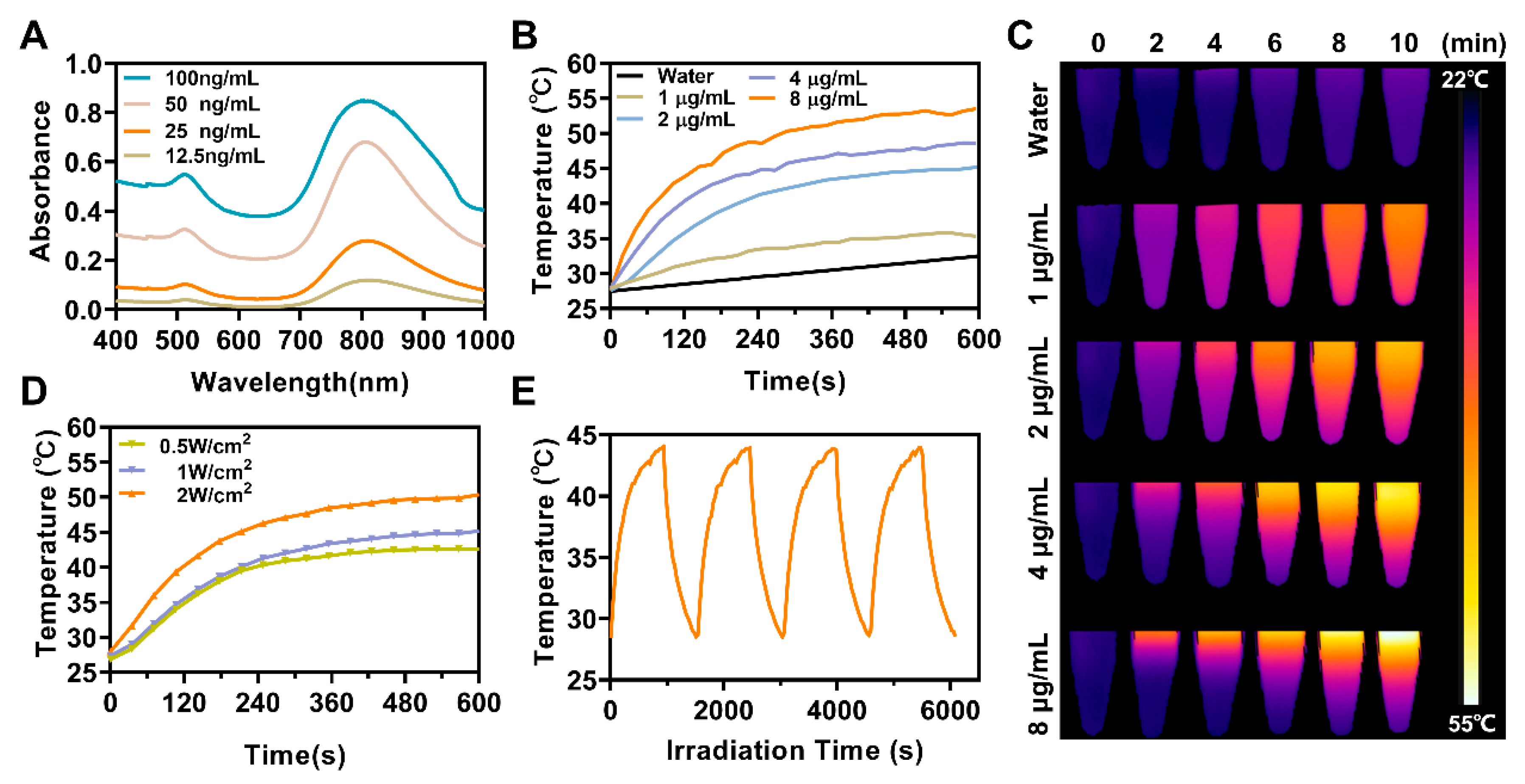
2.7. In Vitro Photothermal Properties Measurement
2.8. Cells and Culture
2.9. Mice and Feeding
2.10. Hemolysis Assay
2.11. In Vitro Cell Cytotoxicity
2.12. Preparation of BMDCs
2.13. Transfection Efficiency of ASCP/siRNA NPs
2.14. Intracellular Uptake
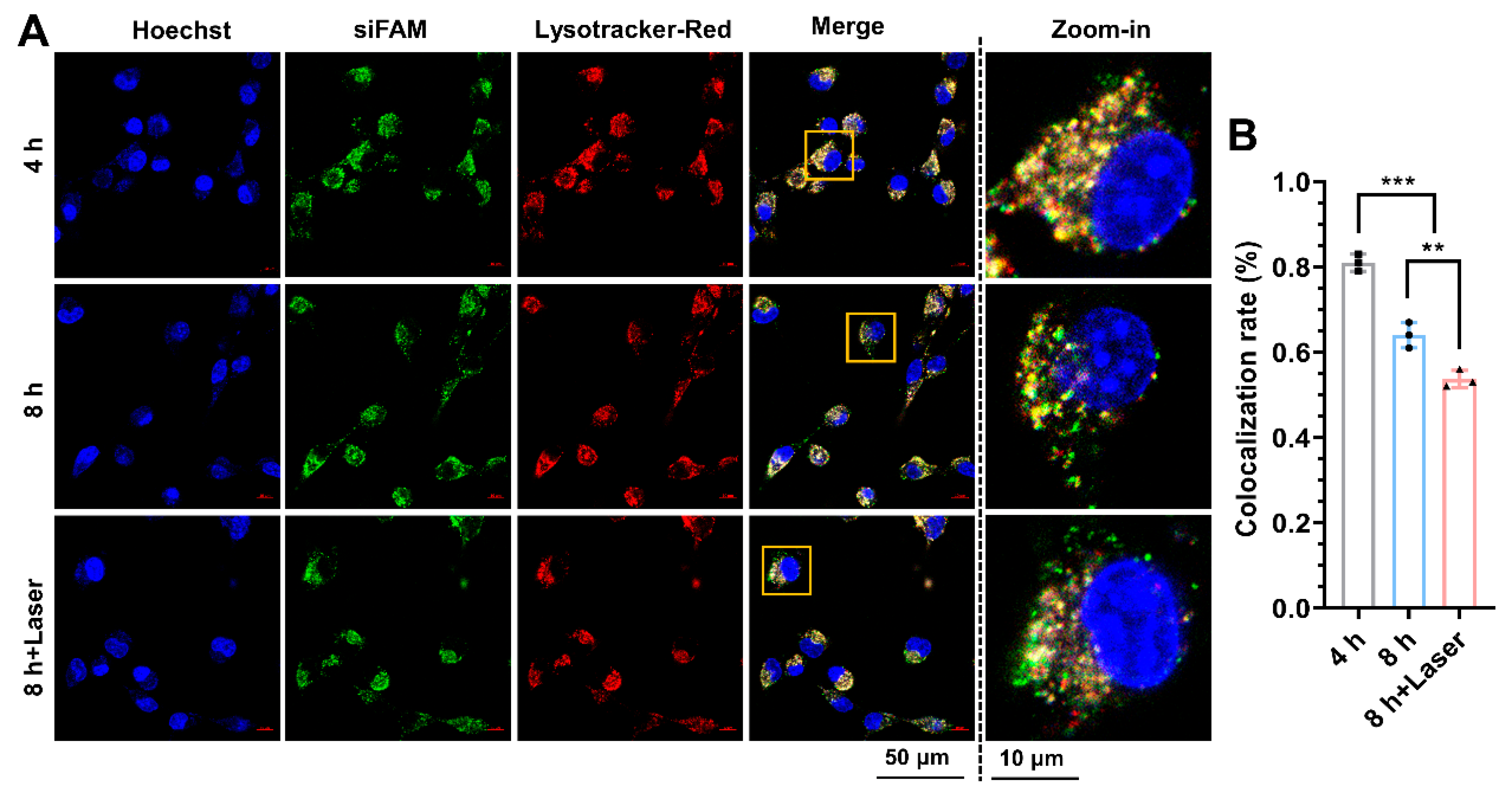
2.15. Endo-Lysosome Escape Observation
2.16. In Vitro Immune Activation Effect Induced by ASCP-Based NPs Only
2.17. Fluorescence Images of Live–Dead Staining
2.18. Proliferation Inhibition Analysis with or without Irradiation
2.19. Gene Expression Assay
2.20. In Vitro Killing Effect of Co-Incubation on MC38 Cells
2.21. In Vitro Immune Activation Effect Induced by Co-Incubation
2.22. Statistical Analysis Methods
3. Results and Discussion
3.1. Preparation and Characterization of ASCP/siRNA NPs
3.2. In Vitro Photothermal Effect of ASCP NPs
3.3. In Vitro Biosafety Evaluation of ASCP NPs
3.4. Mild Photothermal Heating-Enhanced Transfection of ASCP/siRNA NPs
3.5. Mild Photothermal Heating-Enhanced Endo-Lysosomal Escape of ASCP/siRNA NPs
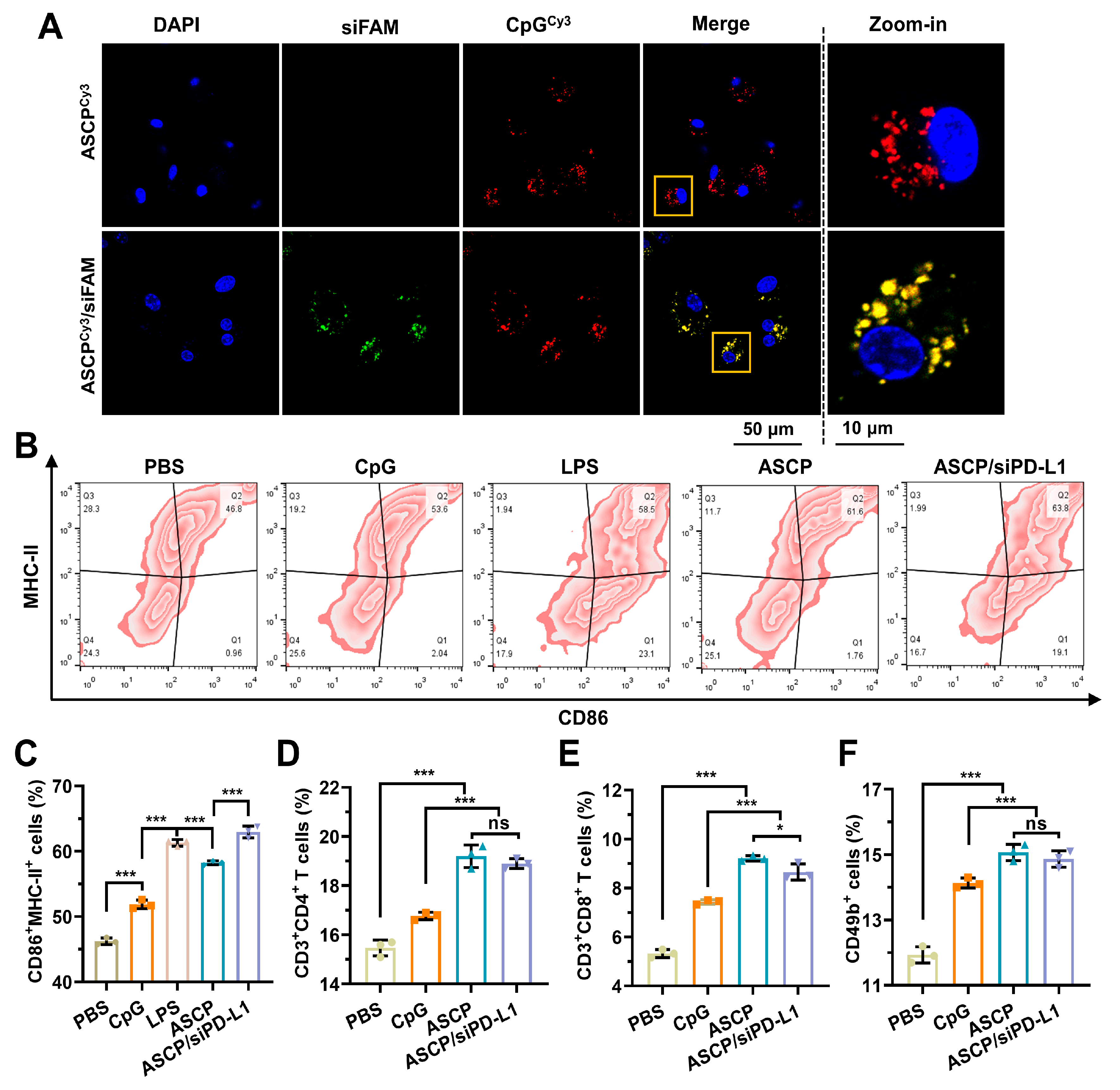
3.6. In Vitro Immune Activation Effect of ASCP/siRNA NPs
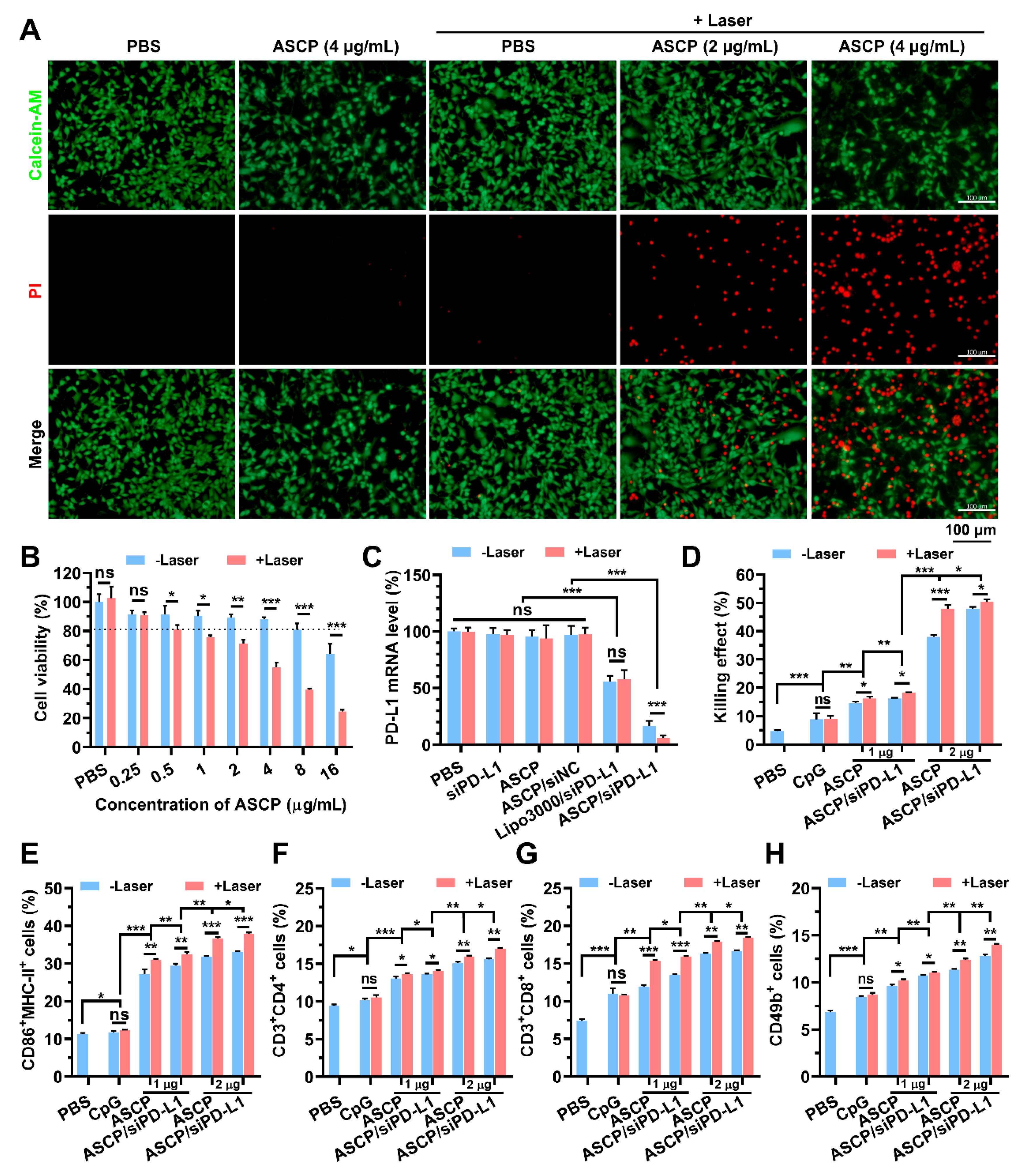
3.7. In Vitro Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2022, 72, 338–344. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccin. Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, Y.; Zhang, P.; Chu, Q. Acquired Resistance to Immune Checkpoint Blockades: The Underlying Mechanisms and Potential Strategies. Front. Immunol. 2021, 12, 693609. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Won, J.E.; Byeon, Y.; Wi, T.I.; Lee, C.M.; Lee, J.H.; Kang, T.H.; Lee, J.W.; Lee, Y.; Park, Y.M.; Han, H.D. Immune checkpoint silencing using RNAi-incorporated nanoparticles enhances antitumor immunity and therapeutic efficacy compared with antibody-based approaches. J. Immunother. Cancer 2022, 10, e003928. [Google Scholar] [CrossRef]
- Jung, J.Y.; Ryu, H.J.; Lee, S.H.; Kim, D.Y.; Kim, M.J.; Lee, E.J.; Ryu, Y.M.; Kim, S.Y.; Kim, K.P.; Choi, E.Y.; et al. siRNA Nanoparticle Targeting PD-L1 Activates Tumor Immunity and Abrogates Pancreatic Cancer Growth in Humanized Preclinical Model. Cells 2021, 10, 2734. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, Y.; Qiao, S.; Liu, M.; Ji, Q.; Zhang, B.L.; Mei, Q.B.; Zhou, S. Nano-Codelivery of Temozolomide and siPD-L1 to Reprogram the Drug-Resistant and Immunosuppressive Microenvironment in Orthotopic Glioblastoma. ACS Nano 2022, 16, 7409–7427. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Lu, X.; Wang, G.; Chi, P. Antitumor effects of the silencing of programmed cell death ligand 1 in colorectal cancer via immunoregulation. Oncol. Rep. 2018, 40, 3370–3380. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Wang, G.X.; Lin, B.A.; Huang, J.S. MicroRNA-93-5p expression in tumor tissue and its tumor suppressor function via targeting programmed death ligand-1 in colorectal cancer. Cell. Biol. Int. 2020, 44, 1224–1236. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Lin, L.; Chen, J.; Hu, Y.; Sun, P.; Tian, H.; Maruyama, A.; Chen, X. Efficient PD-L1 gene silence promoted by hyaluronidase for cancer immunotherapy. J. Control Release 2019, 293, 104–112. [Google Scholar] [CrossRef]
- Tsuruta, N.; Tsuchihashi, K.; Ohmura, H.; Yamaguchi, K.; Ito, M.; Ariyama, H.; Kusaba, H.; Akashi, K.; Baba, E. RNA N6-methyladenosine demethylase FTO regulates PD-L1 expression in colon cancer cells. Biochem. Biophys. Res. Commun. 2020, 530, 235–239. [Google Scholar] [CrossRef]
- Ling, X.; Han, W.; Jiang, X.; Chen, X.; Rodriguez, M.; Zhu, P.; Wu, T.; Lin, W. Point-source burst of coordination polymer nanoparticles for tri-modality cancer therapy. Biomaterials 2021, 270, 120690. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.H.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery. Adv. Mater. 2019, 31, e1903637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, M.; Sun, M.; Wu, S.; Zhang, H.; Dai, Y.; Wang, D. Multifunctional Nanoparticles Boost Cancer Immunotherapy Based on Modulating the Immunosuppressive Tumor Microenvironment. ACS Appl. Mater. Interfaces 2020, 12, 50734–50747. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, M.; Yu, W.; Xu, Z.; Liu, X.; Jia, Q.; Guan, X.; Zhang, W. CpG-Based Nanovaccines for Cancer Immunotherapy. Int. J. Nanomed. 2021, 16, 5281–5299. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.; Shim, G.; Oh, Y.K. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J. Control. Release 2021, 330, 540–553. [Google Scholar] [CrossRef]
- Zhan, G.; Xu, Q.; Zhang, Z.; Wei, Z.; Yong, T.; Bie, N.; Zhang, X.; Li, X.; Li, J.; Gan, L.; et al. Biomimetic sonodynamic therapy-nanovaccine integration platform potentiates Anti-PD-1 therapy in hypoxic tumors. Nano Today 2021, 38, 101195. [Google Scholar] [CrossRef]
- Wang, S.; Campos, J.; Gallotta, M.; Gong, M.; Crain, C.; Naik, E.; Coffman, R.L.; Guiducci, C. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8(+) T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7240–E7249. [Google Scholar] [CrossRef] [Green Version]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, Y.; Kim, J. Mesoporous Silica as a Versatile Platform for Cancer Immunotherapy. Adv. Mater. 2019, 31, e1803953. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Ahmad, R.; Fu, J.; He, N.; Li, S. Advanced Gold Nanomaterials for Photothermal Therapy of Cancer. J. Nanosci. Nanotechnol. 2016, 16, 67–80. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, J.H.; Colas, M.; Kim, D.H.; Lee, H. Gold-based hybrid nanomaterials for biosensing and molecular diagnostic applications. Biosens. Bioelectron. 2016, 80, 543–559. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, Y.; Ye, E.; Li, Z.; Wu, Y.L. Recent Progress in Macromolecule-Anchored Hybrid Gold Nanomaterials for Biomedical Applications. Macromol. Rapid Commun. 2019, 40, e1800029. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Peng, Y.; Wang, F.; Deng, L. Gold Nanomaterials as a Promising Integrated Tool for Diagnosis and Treatment of Pathogenic Infections—A Review. J. Biomed. Nanotechnol. 2021, 17, 744–770. [Google Scholar] [CrossRef]
- Yougbare, S.; Chou, H.L.; Yang, C.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Shahidi, M.; Abazari, O.; Dayati, P.; Bakhshi, A.; Zavarreza, J.; Modarresi, M.H.; Haghiralsadat, F.; Rahmanian, M.; Naghib, S.M.; Tofighi, D. Multicomponent siRNA/miRNA-loaded modified mesoporous silica nanoparticles targeted bladder cancer for a highly effective combination therapy. Front. Bioeng. Biotechnol. 2022, 10, 949704. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Z.; Fang, J.; Wang, B.; Lin, Z.; Chen, Z.S.; Chen, Y.; Zhang, N.; Yang, X.; Gao, W. A multi-functionalized nanocomposite constructed by gold nanorod core with triple-layer coating to combat multidrug resistant colorectal cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110224. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Jiang, Y.; Gao, W.; Wang, Y.; Yang, X.; Yang, X.; Liu, Z. Gold Nanorods with Silica Shell and PAMAM Dendrimers for Efficient Photothermal Therapy and Low Toxic Codelivery of Anticancer Drug and siRNA. Adv. Mater. Interfaces 2017, 4, 1701166. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, T.; Cheng, H.; Zhang, F.; Yang, X.; Wang, S.; Zhou, J.; Ding, Y. Nanomedicine potentiates mild photothermal therapy for tumor ablation. Asian J. Pharm. Sci. 2021, 16, 738–761. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, B.; Dai, X.; Zhao, N.; Xu, F.J. Biomineralized calcium carbonate nanohybrids for mild photothermal heating-enhanced gene therapy. Biomaterials 2021, 274, 120885. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Yu, X.; Han, S.; Yang, B. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater. Sci. 2020, 8, 5241–5259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Li, S.; Miao, D.; Qian, G.; Su, G. Engineering of Porous Silica Coated Gold Nanorods by Surface-Protected Etching and Their Applications in Drug Loading and Combined Cancer Therapy. Langmuir 2019, 35, 14238–14247. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Kou, X.; Yang, Z.; Wang, J. Tailoring longitudinal surface plasmon wavelengths, scattering and absorption cross sections of gold nanorods. ACS Nano 2008, 2, 677–686. [Google Scholar] [CrossRef]
- Tang, H.; Xie, H.; Wang, Z.; Peng, S.; Ni, W.; Guo, L. Economical and Efficient Protocol for Isolating and Culturing Bone Marrow-derived Dendritic Cells from Mice. J. Vis. Exp. 2022, 185, e63125. [Google Scholar] [CrossRef]
- Yang, C.; Mo, X.; Lv, J.; Liu, X.; Yuan, M.; Dong, M.; Li, L.; Luo, X.; Fan, X.; Jin, Z.; et al. Lipopolysaccharide enhances FcepsilonRI-mediated mast cell degranulation by increasing Ca2+ entry through store-operated Ca2+ channels: Implications for lipopolysaccharide exacerbating allergic asthma. Exp. Physiol. 2012, 97, 1315–1327. [Google Scholar] [CrossRef]
- Gupta, G.K.; Agrawal, D.K. CpG oligodeoxynucleotides as TLR9 agonists: Therapeutic application in allergy and asthma. BioDrugs 2010, 24, 225–235. [Google Scholar] [CrossRef]
- Siu, K.S.; Zhang, Y.; Zheng, X.; Koropatnick, J.; Min, W.P. Non-Covalently Functionalized of Single-Walled Carbon Nanotubes by DSPE-PEG-PEI for SiRNA Delivery. Methods Mol. Biol. 2016, 1364, 151–163. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, S.; Ma, M.; Zhang, Y. Fluorinated PEG-PEI Coated Magnetic Nanoparticles for siRNA Delivery and CXCR4 Knockdown. Nanomaterials 2022, 12, 1692. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Wu, Y.; Dong, Y.; Du, L.; Yang, T.; Wang, Y.; Guo, S.; Zhang, M.; Hussain, A.; et al. Core Role of Hydrophobic Core of Polymeric Nanomicelle in Endosomal Escape of siRNA. Nano Lett. 2021, 21, 3680–3689. [Google Scholar] [CrossRef]
- Alnasser, S.M. Review on mechanistic strategy of gene therapy in the treatment of disease. Gene 2021, 769, 145246. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell. Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef]
- Lee, Y.S.; Radford, K.J. The role of dendritic cells in cancer. Int. Rev. Cell. Mol. Biol. 2019, 348, 123–178. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; He, W.; Xiong, S.; Zhang, L.; Wu, Y.; Gao, Y.; Tian, Z.; Wu, C.; Zhang, Y.; Sheng, N.; et al. Medical Immunology, 3rd ed.; Cao, X., He, W., Eds.; People’s Medical Press: Beijing, China, 2015; pp. 29–30. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yang, J.; Yao, X.; Li, X.; Xu, Z.; Tang, S.; Sun, B.; Lin, S.; Yang, C.; Liu, J. Multifunctional Mesoporous Silica-Coated Gold Nanorods Mediate Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer. Pharmaceutics 2023, 15, 854. https://doi.org/10.3390/pharmaceutics15030854
Li M, Yang J, Yao X, Li X, Xu Z, Tang S, Sun B, Lin S, Yang C, Liu J. Multifunctional Mesoporous Silica-Coated Gold Nanorods Mediate Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer. Pharmaceutics. 2023; 15(3):854. https://doi.org/10.3390/pharmaceutics15030854
Chicago/Turabian StyleLi, Meirong, Jingyu Yang, Xinhuang Yao, Xiang Li, Zhourui Xu, Shiqi Tang, Bangxu Sun, Suxia Lin, Chengbin Yang, and Jia Liu. 2023. "Multifunctional Mesoporous Silica-Coated Gold Nanorods Mediate Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer" Pharmaceutics 15, no. 3: 854. https://doi.org/10.3390/pharmaceutics15030854
APA StyleLi, M., Yang, J., Yao, X., Li, X., Xu, Z., Tang, S., Sun, B., Lin, S., Yang, C., & Liu, J. (2023). Multifunctional Mesoporous Silica-Coated Gold Nanorods Mediate Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer. Pharmaceutics, 15(3), 854. https://doi.org/10.3390/pharmaceutics15030854





