Am80-Encapsulated Lipid Nanoparticles, Developed with the Aim of Achieving Alveolar Regeneration, Have an Improvement Effect on Pulmonary Emphysema
Abstract
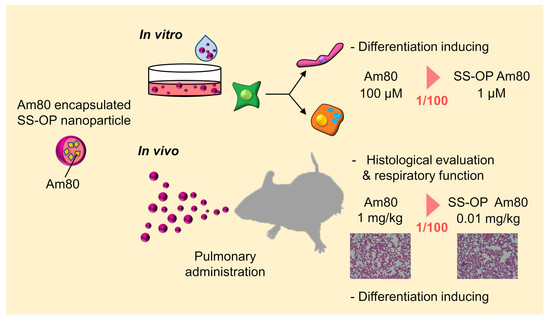
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Species
2.3. Model Mouse Preparation and Drug Administration
2.4. Nanoparticle Preparation
2.5. Evaluation of Physical Properties and Measurement of the Encapsulation Rate
2.6. Differentiation Inducibility
2.7. Tissue Immunofluorescence Staining
2.8. Alveolar Repair Effect
2.9. Improvement of Respiratory Function
2.10. Statistical Analysis
3. Results
3.1. Evaluation of the Physical Properties and Differentiation Induction Potency of SS-OP Nanoparticles
3.1.1. Evaluation of the Physical Properties of SS-OP Nanoparticles
3.1.2. Differentiation Induction Potency of SS-OP Nanoparticles
3.2. Alveolar Repair Effect and Tissue Immunostaining
3.2.1. Evaluation by the Mean Linear Intercept (Lm)
3.2.2. Tissue Immunostaining
3.3. Improvement of Respiratory Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauwels, R.A.; Buist, A.S.; Ma, P.; Jenkins, C.R.; Hurd, S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): Executive summary. Respir. Care 2001, 46, 798–825. [Google Scholar] [PubMed]
- Svanes, C.; Sunyer, J.; Plana, E.; Dharmage, S.; Heinrich, J.; Jarvis, D.; de Marco, R.; Norbäck, D.; Raherison, C.; Villani, S.; et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010, 65, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postma, D.S.; Kerkhof, M.; Boezen, H.M.; Koppelman, G.H. Asthma and chronic obstructive pulmonary disease: Common genes, common environments? Am. J. Respir. Crit. Care Med. 2011, 183, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Aoshiba, K.; Tsuji, T.; Yamaguchi, K.; Itoh, M.; Nakamura, H. The danger signal plus DNA damage two-hit hypothesis for chronic inflammation in COPD. Eur. Respir. J. 2013, 42, 1689–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Diab, N.; Gershon, A.S.; Sin, D.D.; Tan, W.C.; Bourbeau, J.; Boulet, L.P.; Aaron, S.D. Underdiagnosis and Overdiagnosis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1130–1139. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Fujita, M. New therapies for chronic obstructive pulmonary disease, lung regeneration. World J. Respirol. 2015, 5, 34. [Google Scholar] [CrossRef]
- Hind, M.; Maden, M. Is a regenerative approach viable for the treatment of COPD? J. Cereb. Blood Flow Metab. 2011, 163, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Fujino, N.; Kubo, H.; Suzuki, T.; Ota, C.; Hegab, A.E.; He, M.; Suzuki, S.; Suzuki, T.; Yamada, M.; Kondo, T.; et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab. Investig. 2010, 91, 363–378. [Google Scholar] [CrossRef]
- Wang, J.; Edeen, K.; Manzer, R.; Chang, Y.; Wang, S.; Chen, X.; Funk, C.J.; Cosgrove, G.P.; Fang, X.; Mason, R.J. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 2007, 36, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruberte, E.; Friederich, V.; Morriss-Kay, G.; Chambon, P. Differential distribution patterns of CRABP I and CRABP II transcripts during mouse embryogenesis. Development 1992, 115, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Massaro, G.D.; Massaro, D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat. Med. 1997, 3, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Malpel, S.; Mendelsohn, C.; Cardoso, W.V. Regulation of retinoic acid signaling during lung morphogenesis. Development 2000, 127, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
- Maden, M.; Hind, M. Retinoic acid in alveolar development, maintenance and regeneration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.T.; Goldin, J.G.; Dermand, J.; Ibrahim, G.; Brown, M.S.; Emerick, A.; McNitt-Gray, M.F.; Gjertson, D.W.; Estrada, F.; Tashkin, D.P.; et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am. J. Respir. Crit. Care Med. 2002, 165, 718–723. [Google Scholar] [CrossRef]
- Roth, M.D.; Connett, J.E.; D’Armiento, J.M.; Foronjy, R.F.; Friedman, P.J.; Goldin, J.G.; Louis, T.A.; Mao, J.T.; Muindi, J.R.; O’Connor, G.T.; et al. Feasibility of retinoids for the treatment of emphysema study. Chest 2006, 130, 1334–1345. [Google Scholar] [CrossRef]
- Ohnishi, K. PML-RARalpha inhibitors (ATRA, tamibaroten, arsenic troxide) for acute promyelocytic leukemia. Int. J. Clin. Oncol. 2007, 12, 313–317. [Google Scholar] [CrossRef]
- Osanai, M.; Petkovich, M. Expression of the retinoic acid-metabolizing enzyme CYP26A1 limits programmed cell death. Mol. Pharm. 2005, 67, 1808–1817. [Google Scholar] [CrossRef] [Green Version]
- Sakai, H.; Horiguchi, M.; Ozawa, C.; Akita, T.; Hirota, K.; Shudo, K.; Terada, H.; Makino, K.; Kubo, H.; Yamashita, C. Pulmonary administration of Am80 regenerates collapsed alveoli. J. Control. Release 2014, 196, 154–160. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research: Rockville, MD, USA, 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-startingdose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 12 December 2022).
- Budhu, A.; Gillilan, R.; Noy, N. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J. Mol. Biol. 2001, 305, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, H.; Nakagomi, M.; Yamagata, N.; Katsuki, H.; Kawahara, K.; Kitaoka, K.; Miki, T.; Shudo, K. Tamibarotene: A candidate retinoid drug for Alzheimer’s disease. Biol. Pharm. Bull. 2012, 35, 1206–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Takahashi, T.; Konishi, M.; Takata, N.; Gomi, M.; Shirane, D.; Miyama, R.; Hagiwara, S.; Yamasaki, Y.; Sakurai, Y.; et al. Self-Degradable Lipid-Like Materials Based on “Hydrolysis accelerated by the intra-Particle Enrichment of Reactant (HyPER)” for Messenger RNA Delivery. Adv. Funct. Mater. 2020, 30, 1910575. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Marchand-Adam, S.; Antico Arciuch, V.G.; Boyer, L.; De Coster, C.; Marchal, J.; Bachoual, R.; Mailleux, A.; Boczkowski, J.; Crestani, B. Keratinocyte growth factor protects against elastase-induced pulmonary emphysema in mice. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L1230–L1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, M.P.; Parham, A.R.; Waldrep, J.C.; McKenzie, W.N.; Dhand, R. Alveolar fractal box dimension inversely correlates with mean linear intercept in mice with elastase-induced emphysema. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suraya, R.; Nagano, T.; Ryanto, G.R.T.; Effendi, W.I.; Hazama, D.; Katsurada, N.; Yamamoto, M.; Tachihara, M.; Emoto, N.; Nishimura, Y.; et al. Budesonide/glycopyrronium/formoterol fumarate triple therapy prevents pulmonary hypertension in a COPD mouse model via NFkappaB inactivation. Respir. Res. 2022, 23, 173. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fujinawa, R.; Ota, F.; Kobayashi, S.; Angata, T.; Ueno, M.; Maeno, T.; Kitazume, S.; Yoshida, K.; Ishii, T.; et al. A single dose of lipopolysaccharide into mice with emphysema mimics human chronic obstructive pulmonary disease exacerbation as assessed by micro-computed tomography. Am. J. Respir. Cell Mol. Biol. 2013, 49, 971–977. [Google Scholar] [CrossRef]
- Horiguchi, M.; Kojima, H.; Sakai, H.; Kubo, H.; Yamashita, C. Pulmonary administration of integrin-nanoparticles regenerates collapsed alveoli. J. Control. Release 2014, 187, 167–174. [Google Scholar] [CrossRef]
- Horiguchi, M.; Oiso, Y.; Sakai, H.; Motomura, T.; Yamashita, C. Pulmonary administration of phosphoinositide 3-kinase inhibitor is a curative treatment for chronic obstructive pulmonary disease by alveolar regeneration. J. Control. Release 2015, 187, 112–119. [Google Scholar] [CrossRef]
- Horiguchi, M.; Hirokawa, M.; Abe, K.; Kumagai, H.; Yamashita, C. Pulmonary administration of 1,25-dihydroxyvitamin D3 to the lungs induces alveolar regeneration in a mouse model of chronic obstructive pulmonary disease. J. Control. Release 2016, 233, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Oiso, Y.; Akita, T.; Kato, D.; Yamashita, C. Method for Pulmonary Administration Using Negative Pressure Generated by Inspiration in Mice. Pharmaceutics 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Ye, Q.; Ouchi, H.; Nakashima, N.; Hamada, N.; Hagimoto, N.; Kuwano, K.; Mason, R.J.; Nakanishi, Y. Retinoic acid fails to reverse emphysema in adult mouse models. Thorax 2004, 59, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uniyal, S.; Tyagi, A.K.; Muyal, J.P. All Trans Retinoic Acid (ATRA) progresses alveolar epithelium regeneration by involving diverse signalling pathways in emphysematous rat. Biomed. Pharmacother. 2020, 131, 110725. [Google Scholar] [CrossRef]
- March, T.H.; Cossey, P.Y.; Esparza, D.C.; Dix, K.J.; McDonald, J.D.; Bowen, L.E. Inhalation administration of all-trans-retinoic acid for treatment of elastase-induced pulmonary emphysema in Fischer 344 rats. Exp. Lung Res. 2004, 30, 383–404. [Google Scholar] [CrossRef]
- Frankenberger, M.; Heimbeck, I.; Moller, W.; Mamidi, S.; Kassner, G.; Pukelsheim, K.; Wjst, M.; Neiswirth, M.; Kroneberg, P.; Lomas, D.; et al. Inhaled all-trans retinoic acid in an individual with severe emphysema. Eur. Respir. J. 2009, 34, 1487–1489. [Google Scholar] [CrossRef] [Green Version]
- Probst, S.; Kraemer, C.; Demougin, P.; Sheth, R.; Martin, G.R.; Shiratori, H.; Hamada, H.; Iber, D.; Zeller, R.; Zuniga, A. SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling. Development 2011, 138, 1913–1923. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.; Barlow, H.R.; Sutton, G.I.; Gu, X.; Htike, Y.; Cowdin, M.A.; Cleaver, O. Cyp26b1 is an essential regulator of distal airway epithelial differentiation during lung development. Development 2020, 147, dev181560. [Google Scholar] [CrossRef]
- Saito, K.; Cagle, P.; Berend, N.; Thurlbeck, W.M. The “destructive index” in nonemphysematous and emphysematous lungs. Morphologic observations and correlation with function. Am. Rev. Respir. Dis. 1989, 139, 308–312. [Google Scholar] [CrossRef]
- Tantucci, C.; Modina, D. Lung function decline in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 95–99. [Google Scholar] [CrossRef]
- Muralidharan, P.; Hayes, D., Jr.; Mansour, H.M. Dry powder inhalers in COPD, lung inflammation and pulmonary infections. Expert Opin. Drug Deliv. 2014, 12, 947–962. [Google Scholar] [CrossRef] [PubMed]






| Particle | Am80 Concentration (mM) | Am80-Encapsulated Percent (%) | Particle Size | P.I. | Zeta Potential (mV) |
|---|---|---|---|---|---|
| SS-OP (Placebo) | - | - | 126.3 ± 2.32 | 0.07 ± 0.03 | −8.63 ± 4.72 |
| SS-OP (Am80) | 1.78 ± 0.21 | 71.3 ± 8.49 | 125.7 ± 2.40 | 0.10 ± 0.02 | −4.07 ± 1.54 |
| Group | Time after Am80 Treatment (Week) | ||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | |
| Untreated | 32.8 ± 0.7 | 36.7 ± 0.9 | 39.5 ± 1.1 | 40.8 ± 1.2 | 43.3 ± 1.2 |
| Control | 32.4 ± 0.4 | 37.0 ± 0.9 | 39.3 ± 0.9 | 40.1 ± 0.9 | 42.4 ± 1.0 |
| 0.1 mg/kg Am80 -Free | 31.7 ± 0.6 | 36.3 ± 1.1 | 38.8 ± 1.2 | 39.2 ± 1.2 | 41.5 ± 1.0 |
| 1.0 mg/kg Am80 -Free | 32.2 ± 0.5 | 36.8 ± 0.7 | 39.5 ± 0.8 | 40.8 ± 0.5 | 43.2 ± 1.0 |
| 0 mg/kg Am80 -SS-OP | 33.0 ± 0.4 | 37.9 ± 0.6 | 40.7 ± 0.8 | 41.7 ± 0.7 | 44.0 ± 0.8 |
| 0.01 mg/kg Am80 -SS-OP | 32.6 ± 0.5 | 39.6 ± 0.7 | 42.0 ± 0.8 | 42.8 ± 0.8 | 45.4 ± 0.8 |
| 0.1 mg/kg Am80 -SS-OP | 33.2 ± 0.7 | 38.0 ± 0.7 | 40.7 ± 0.7 | 41.2 ± 0.8 | 43.8 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, T.; Morita, Y.; Kawai, T.; Oda, K.; Tange, K.; Nakai, Y.; Yamashita, C. Am80-Encapsulated Lipid Nanoparticles, Developed with the Aim of Achieving Alveolar Regeneration, Have an Improvement Effect on Pulmonary Emphysema. Pharmaceutics 2023, 15, 37. https://doi.org/10.3390/pharmaceutics15010037
Akita T, Morita Y, Kawai T, Oda K, Tange K, Nakai Y, Yamashita C. Am80-Encapsulated Lipid Nanoparticles, Developed with the Aim of Achieving Alveolar Regeneration, Have an Improvement Effect on Pulmonary Emphysema. Pharmaceutics. 2023; 15(1):37. https://doi.org/10.3390/pharmaceutics15010037
Chicago/Turabian StyleAkita, Tomomi, Yuki Morita, Takehiro Kawai, Kazuaki Oda, Kota Tange, Yuta Nakai, and Chikamasa Yamashita. 2023. "Am80-Encapsulated Lipid Nanoparticles, Developed with the Aim of Achieving Alveolar Regeneration, Have an Improvement Effect on Pulmonary Emphysema" Pharmaceutics 15, no. 1: 37. https://doi.org/10.3390/pharmaceutics15010037
APA StyleAkita, T., Morita, Y., Kawai, T., Oda, K., Tange, K., Nakai, Y., & Yamashita, C. (2023). Am80-Encapsulated Lipid Nanoparticles, Developed with the Aim of Achieving Alveolar Regeneration, Have an Improvement Effect on Pulmonary Emphysema. Pharmaceutics, 15(1), 37. https://doi.org/10.3390/pharmaceutics15010037