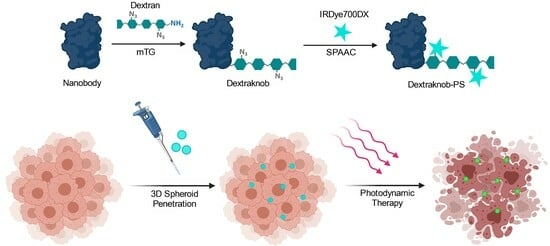
Penetration of Nanobody-Dextran Polymer Conjugates through Tumor Spheroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Protein Production and Purification
2.3. Analytical Section
2.3.1. High-Performance Liquid Chromatography
2.3.2. Liquid Chromatography–Mass Spectrometry (LC-MS)
2.3.3. Photometric Measurements
2.3.4. IR Spectroscopy
2.3.5. Quantification of Carboxyethyl Groups and Azide Groups of Modified Dextran
2.4. Synthesis of Target Compounds
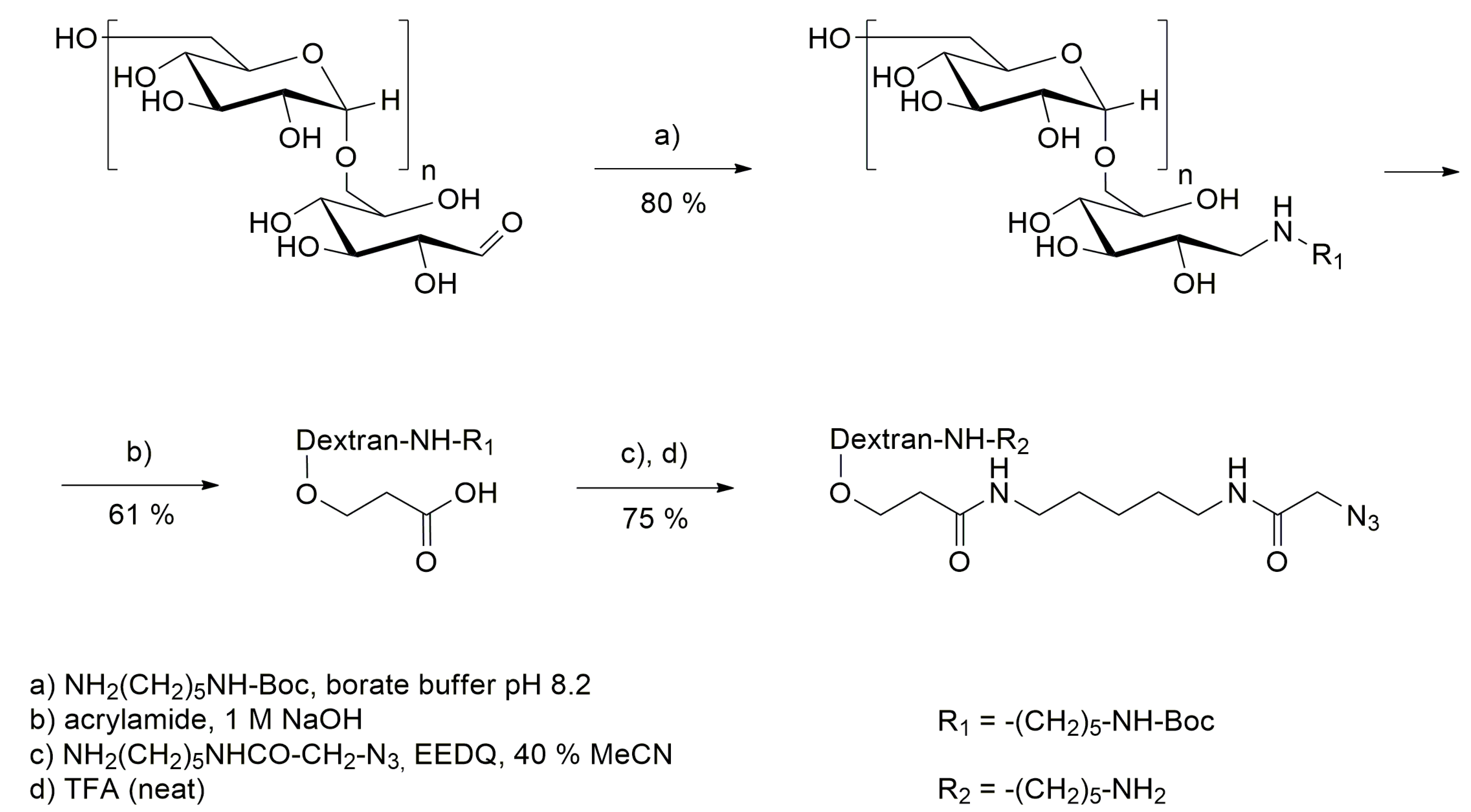
2.4.1. Modification of Dextran
2.4.2. Synthesis of BCN-IRDye700DX
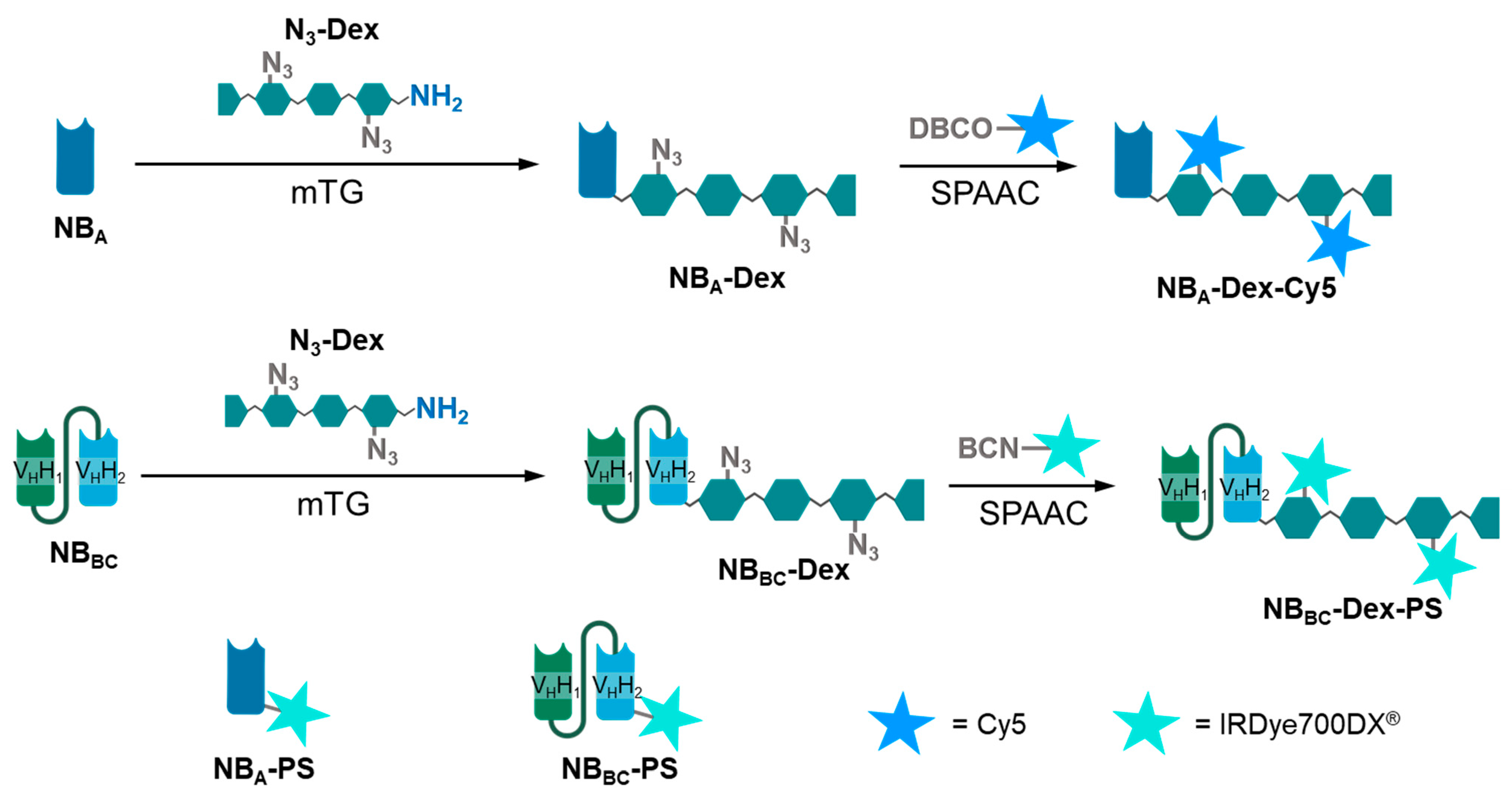
2.4.3. Generation of Nanobody–Dextran Conjugates Labelled with Cy5 and IRDye700DX
2.4.4. Generation of Directly Labelled IRDye700DX Nanobody Conjugates
2.5. Cell Culture and Cell Assays
2.5.1. Cell Culture and Spheroid Formation
2.5.2. Binding Assays with Cells
2.5.3. Treatment of Spheroids with Conjugates and Confocal Microscopy
2.5.4. Nanobody-Targeted Photodynamic Therapy (In Vitro) on 2D Monolayer Cell Culture
2.5.5. NB-Targeted PDT on 3D Spheroid Cell Culture and Cell Viability Assay
3. Results and Discussion
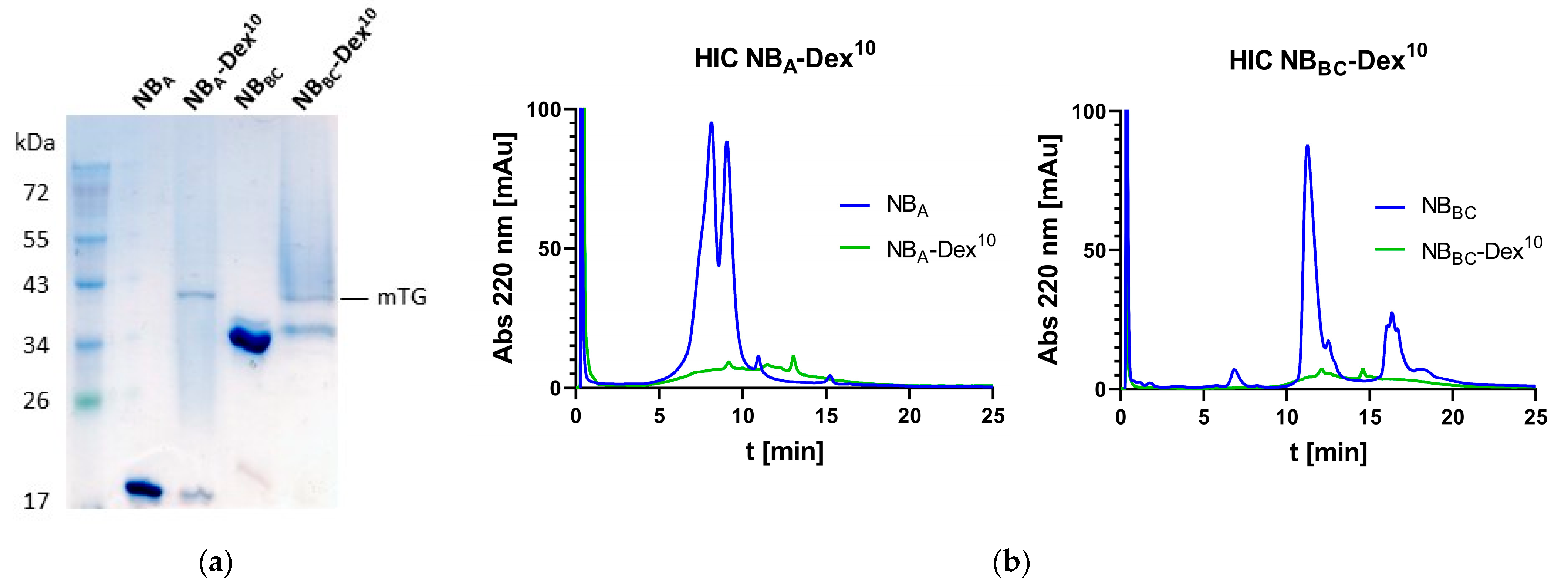
3.1. Generation of Compounds
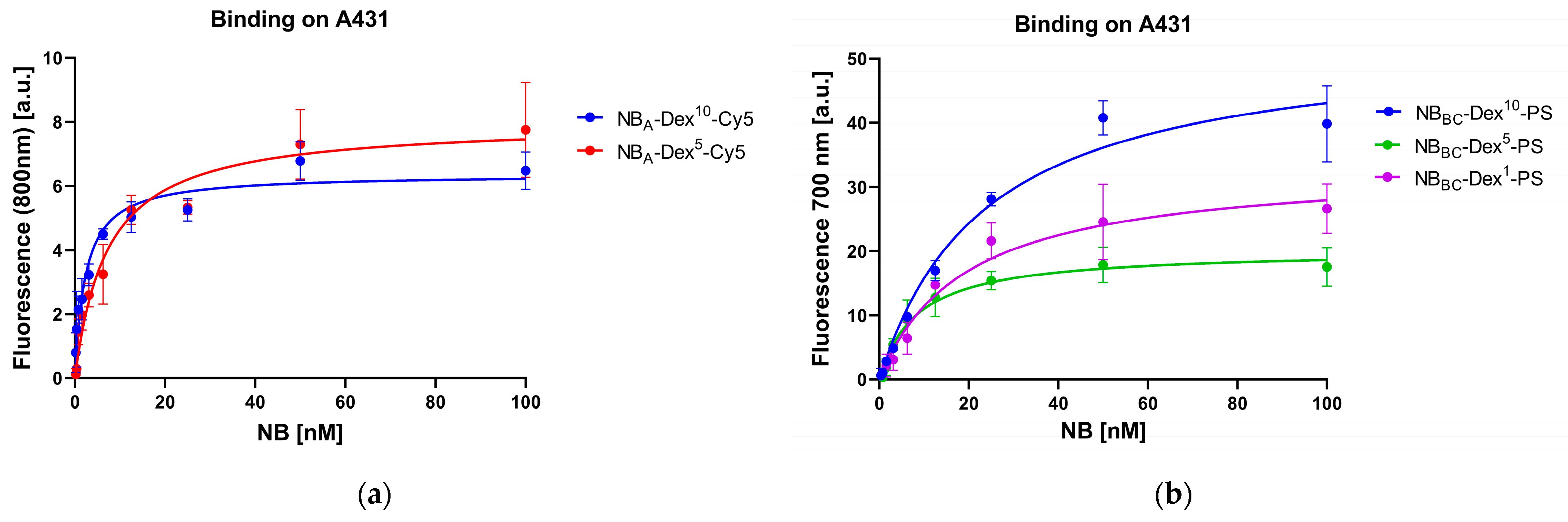
3.2. Cell Binding Studies
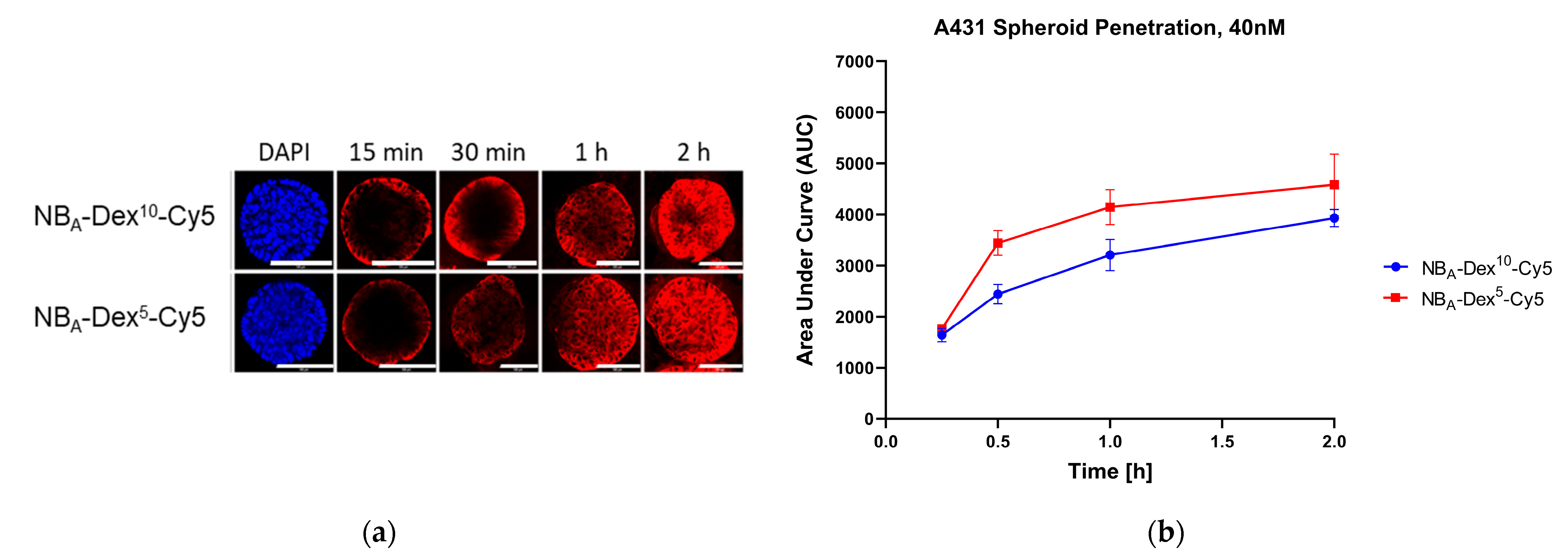
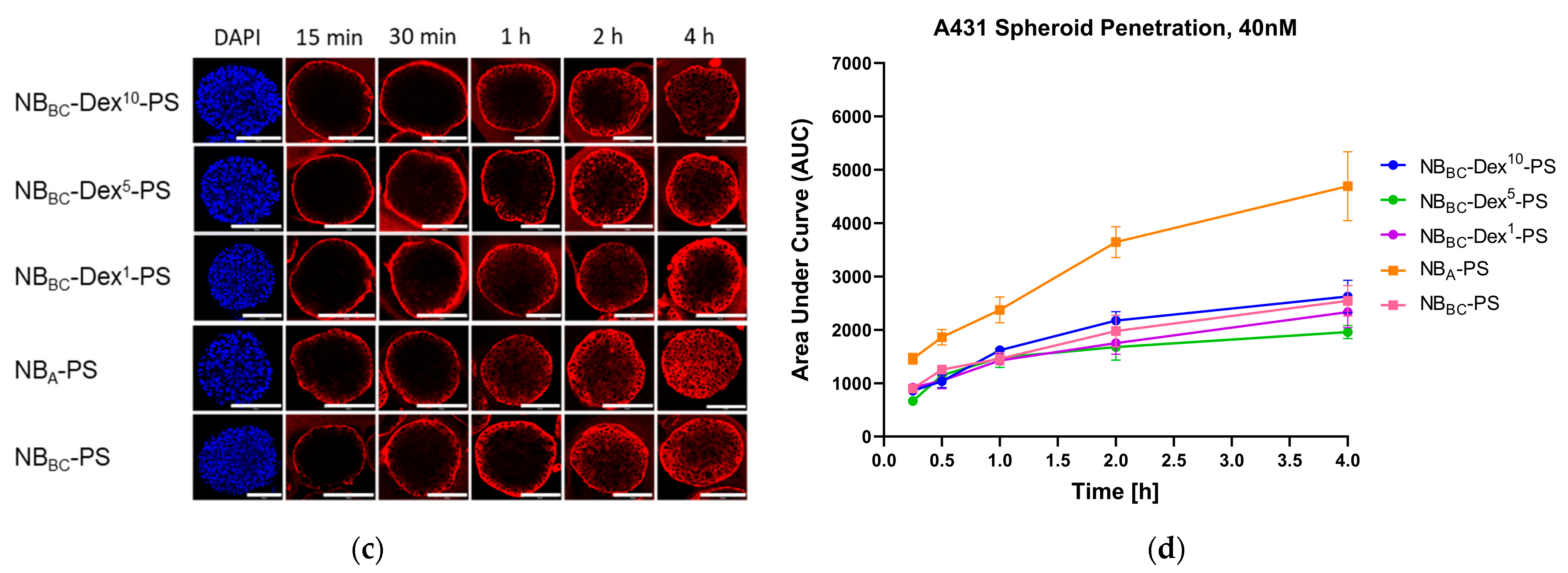
3.3. D Penetration Studies
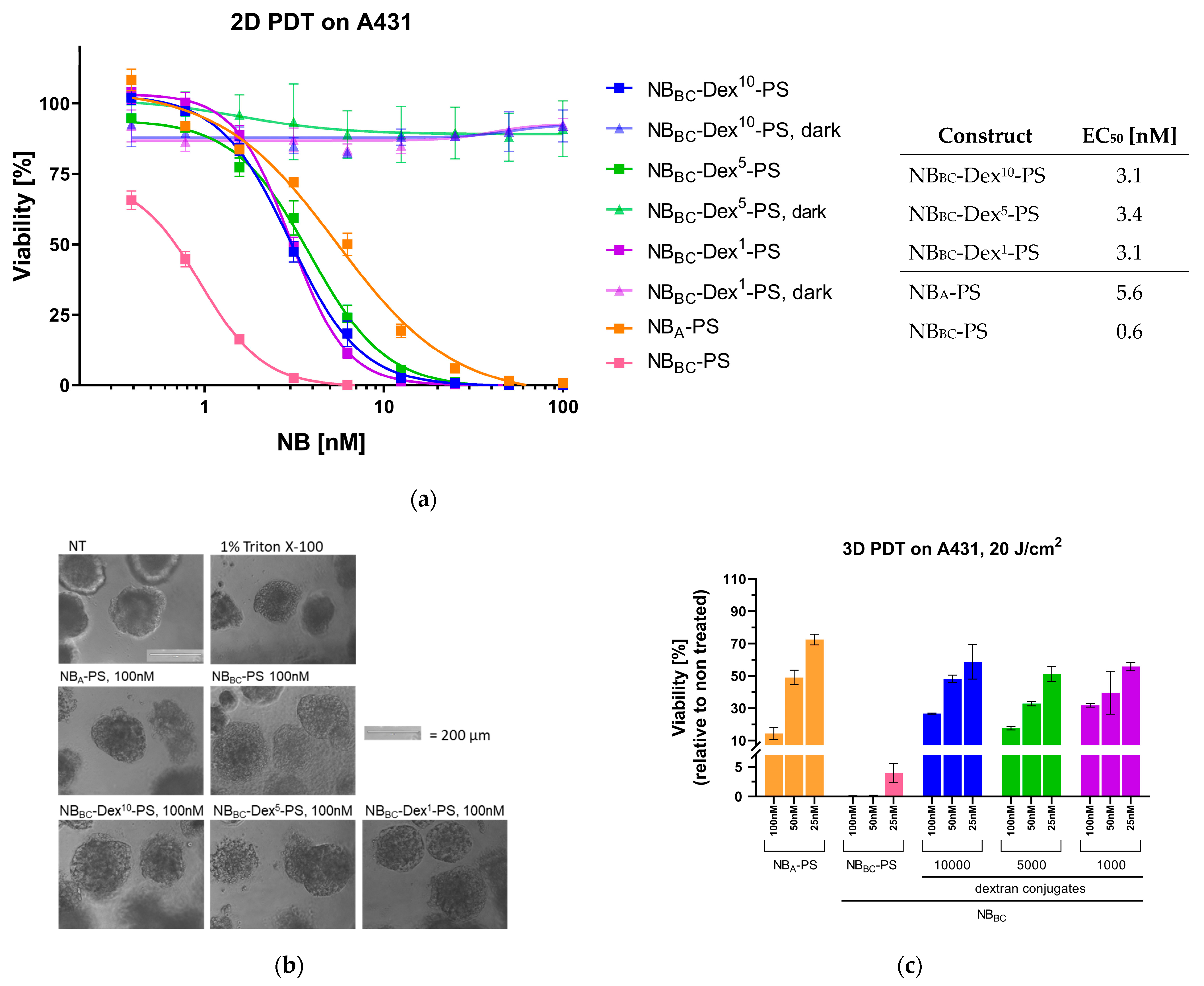
3.4. Nanobody-Targeted PDT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, E.; Gandullo-Sánchez, L.; Ocaña, A.; Pandiella, A. Novel ADCs and Strategies to Overcome Resistance to Anti-HER2 ADCs. Cancers 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody-drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Ma, P.; Jiang, Y.; Cheng, K.; Yu, Y.; Jiang, N.; Miao, H.; Tang, Q.; Liu, F.; et al. Drug conjugate-based anticancer therapy—Current status and perspectives. Cancer Lett. 2023, 552, 215969. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Chari, R.V.J. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. mAbs 2021, 13, 1951427. [Google Scholar] [CrossRef]
- Deonarain, M.P.; Yahioglu, G. Current strategies for the discovery and bioconjugation of smaller, targetable drug conjugates tailored for solid tumor therapy. Expert Opin. Drug Discov. 2021, 16, 613–624. [Google Scholar] [CrossRef]
- Kang, W.; Ding, C.; Zheng, D.; Ma, X.; Yi, L.; Tong, X.; Wu, C.; Xue, C.; Yu, Y.; Zhou, Q. Nanobody Conjugates for Targeted Cancer Therapy and Imaging. Technol. Cancer Res. Treat. 2021, 20, 15330338211010117. [Google Scholar] [CrossRef]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: Dawn of a new era? NPJ Precis. Oncol. 2023, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef]
- Kaempffe, A.; Dickgiesser, S.; Rasche, N.; Paoletti, A.; Bertotti, E.; de Salve, I.; Sirtori, F.R.; Kellner, R.; Könning, D.; Hecht, S.; et al. Effect of Conjugation Site and Technique on the Stability and Pharmacokinetics of Antibody-Drug Conjugates. J. Pharm. Sci. 2021, 110, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- Buecheler, J.W.; Winzer, M.; Tonillo, J.; Weber, C.; Gieseler, H. Impact of Payload Hydrophobicity on the Stability of Antibody-Drug Conjugates. Mol. Pharm. 2018, 15, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Buecheler, J.W.; Winzer, M.; Weber, C.; Gieseler, H. Alteration of Physicochemical Properties for Antibody-Drug Conjugates and Their Impact on Stability. J. Pharm. Sci. 2020, 109, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Beckley, N.S.; Lazzareschi, K.P.; Chih, H.-W.; Sharma, V.K.; Flores, H.L. Investigation into temperature-induced aggregation of an antibody drug conjugate. Bioconjug. Chem. 2013, 24, 1674–1683. [Google Scholar] [CrossRef]
- Sun, M.M.C.; Beam, K.S.; Cerveny, C.G.; Hamblett, K.J.; Blackmore, R.S.; Torgov, M.Y.; Handley, F.G.M.; Ihle, N.C.; Senter, P.D.; Alley, S.C. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides. Bioconjug. Chem. 2005, 16, 1282–1290. [Google Scholar] [CrossRef]
- Jackson, D.Y. Processes for Constructing Homogeneous Antibody Drug Conjugates. Org. Process Res. Dev. 2016, 20, 852–866. [Google Scholar] [CrossRef]
- Yao, H.; Jiang, F.; Lu, A.; Zhang, G. Methods to Design and Synthesize Antibody-Drug Conjugates (ADCs). Int. J. Mol. Sci. 2016, 17, 194. [Google Scholar] [CrossRef]
- Schneider, H.; Deweid, L.; Pirzer, T.; Yanakieva, D.; Englert, S.; Becker, B.; Avrutina, O.; Kolmar, H. Dextramabs: A Novel Format of Antibody-Drug Conjugates Featuring a Multivalent Polysaccharide Scaffold. Chem. Open 2019, 8, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yu, L.; Zhang, M.; He, J.; Sun, X.; Ni, P. Dextran-doxorubicin prodrug nanoparticles conjugated with CD147 monoclonal antibody for targeted drug delivery in hepatoma therapy. Colloids Surf. B Biointerfaces 2023, 228, 113400. [Google Scholar] [CrossRef] [PubMed]
- Thurber, G.M.; Schmidt, M.M.; Wittrup, K.D. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008, 60, 1421. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Yap, M.L.; Pietersz, G.; Walsh, A.P.G.; Zeller, J.; Del Rosal, B.; Wang, X.; Peter, K. In vivo fluorescence imaging: Success in preclinical imaging paves the way for clinical applications. J. Nanobiotechnol. 2022, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Odongo, S.; Radwanska, M.; Magez, S. Nanobodies: A Review of Generation, Diagnostics and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, P.B.A.A.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; de Bruijn, H.S.; van Diest, P.J.; Vahrmeijer, A.L.; van Bergen en Henegouwen, P.M.P.; et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release 2016, 229, 93–105. [Google Scholar] [CrossRef]
- Heukers, R.; van Bergen, P.M.P.; Oliveira, S. Nanobody-photosensitizer conjugates for targeted photodynamic therapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Hernández, I.; Rompen, R.; Rossin, R.; Xenaki, K.T.; Katrukha, E.A.; Nicolay, K.; van Bergen en Henegouwen, P.; Grüll, H.; Oliveira, S. Imaging of Tumor Spheroids, Dual-Isotope SPECT, and Autoradiographic Analysis to Assess the Tumor Uptake and Distribution of Different Nanobodies. Mol. Imaging Biol. 2019, 21, 1079–1088. [Google Scholar] [CrossRef]
- Oliveira, S.; van Dongen, G.A.M.S.; Stigter-van Walsum, M.; Roovers, R.C.; Stam, J.C.; Mali, W.; van Diest, P.J.; van Bergen en Henegouwen, P.M.P. Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Mol. Imaging 2012, 11, 33–46. [Google Scholar] [CrossRef]
- Bhatti, M.; Yahioglu, G.; Milgrom, L.R.; Garcia-Maya, M.; Chester, K.A.; Deonarain, M.P. Targeted photodynamic therapy with multiply-loaded recombinant antibody fragments. Int. J. Cancer 2008, 122, 1155–1163. [Google Scholar] [CrossRef]
- Kalinovsky, D.V.; Kholodenko, I.V.; Kibardin, A.V.; Doronin, I.I.; Svirshchevskaya, E.V.; Ryazantsev, D.Y.; Konovalova, M.V.; Rozov, F.N.; Larin, S.S.; Deyev, S.M.; et al. Minibody-Based and scFv-Based Antibody Fragment-Drug Conjugates Selectively Eliminate GD2-Positive Tumor Cells. Int. J. Mol. Sci. 2023, 24, 1239. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Xavier, C.; de Vos, J.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014, 25, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Tao, R.N.; De Porter, S.M.; Spiegel, D.A.; McNaughton, B.R. A Nanobody Activation Immunotherapeutic that Selectively Destroys HER2-Positive Breast Cancer Cells. Chembiochem 2016, 17, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Dennler, P.; Bailey, L.K.; Spycher, P.R.; Schibli, R.; Fischer, E. Microbial transglutaminase and c-myc-tag: A strong couple for the functionalization of antibody-like protein scaffolds from discovery platforms. Chembiochem 2015, 16, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Hernández, I.; Grinwis, G.C.; Di Maggio, A.; van Bergen en Henegouwen, P.M.P.; Hennink, W.E.; Teske, E.; Hesselink, J.W.; van Nimwegen, S.A.; Mol, J.A.; Oliveira, S. Nanobody-targeted photodynamic therapy for the treatment of feline oral carcinoma: A step towards translation to the veterinary clinic. Nanophotonics 2021, 10, 3075–3087. [Google Scholar] [CrossRef] [PubMed]
- Roovers, R.C.; Vosjan, M.J.; Laeremans, T.; el Khoulati, R.; de Bruin, R.C.; Ferguson, K.M.; Verkleij, A.J.; van Dongen, G.A.; van Bergen en Henegouwen, P.M. A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int. J. Cancer 2011, 129, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Heukers, R.; Mashayekhi, V.; Ramirez-Escudero, M.; de Haard, H.; Verrips, T.; van Bergen en Henegouwen, P.; Oliveira, S. VHH-Photosensitizer Conjugates for Targeted Photodynamic Therapy of Met-Overexpressing Tumor Cells. Antibodies 2019, 8, 26. [Google Scholar] [CrossRef]
- Yokoyama, K.I.; Nakamura, N.; Seguro, K.; Kubota, K. Overproduction of microbial transglutaminase in Escherichia coli, in vitro refolding, and characterization of the refolded form. Biosci. Biotechnol. Biochem. 2000, 64, 1263–1270. [Google Scholar] [CrossRef]
- Richter, M.; Chakrabarti, A.; Ruttekolk, I.R.; Wiesner, B.; Beyermann, M.; Brock, R.; Rademann, J. Multivalent design of apoptosis-inducing bid-bh3 peptide–oligosaccharides boosts the intracellular activity at identical overall peptide concentrations. Chem. Eur. J. 2012, 18, 16708–16715. [Google Scholar] [CrossRef]
- Jung, A.C.; Moinard-Butot, F.; Thibaudeau, C.; Gasser, G.; Gaiddon, C. Antitumor Immune Response Triggered by Metal-Based Photosensitizers for Photodynamic Therapy: Where Are We? Pharmaceutics 2021, 13, 1778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, H.; Banerjee, S.; Clarkson, G.J.; Ge, C.; Imberti, C.; Sadler, P.J. Nucleus-Targeted Organoiridium-Albumin Conjugate for Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Baalmann, M.; Best, M.; Wombacher, R. Site-Specific Protein Labeling Utilizing Lipoic Acid Ligase (LplA) and Bioorthogonal Inverse Electron Demand Diels-Alder Reaction. Methods Mol. Biol. 2018, 1728, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Huet, H.A.; Growney, J.D.; Johnson, J.A.; Li, J.; Bilic, S.; Ostrom, L.; Zafari, M.; Kowal, C.; Yang, G.; Royo, A.; et al. Multivalent nanobodies targeting death receptor 5 elicit superior tumor cell killing through efficient caspase induction. mAbs 2014, 6, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Nessler, I.; Khera, E.; Vance, S.; Kopp, A.; Qiu, Q.; Keating, T.A.; Abu-Yousif, A.O.; Sandal, T.; Legg, J.; Thompson, L.; et al. Increased Tumor Penetration of Single-Domain Antibody-Drug Conjugates Improves In Vivo Efficacy in Prostate Cancer Models. Cancer Res. 2020, 80, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, F.; Nazeer, A.; To, M.; Hammond, M.; Koski, C.; Liu, L.Y.; Yeung, V.P.; Rennerfeldt, D.A.; Henkensiefken, A.; Fox, M.; et al. Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors. Nat. Cancer 2023, 4, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C. Dextran prodrugs—Structure and stability in relation to therapeutic activity. Adv. Drug Deliv. Rev. 1989, 3, 103–154. [Google Scholar] [CrossRef]
| Abbreviation | NB | Dextran | DOC |  |
| NBA-Dex10-Cy5 | NBA | 10,000 | 0.7 | |
| NBA-Dex5-Cy5 | NBA | 5000 | 1 | |
| NBBC-Dex10-PS | NBBC | 10,000 | 1.2 | |
| NBBC-Dex5-PS | NBBC | 5000 | 0.7 | |
| NBBC-Dex1-PS | NBBC | 1000 | 1.1 | |
| NBA-PS | NBA | - | 0.9 | |
| NBBC-PS | NBBC | - | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitsch, P.; Baum, E.S.; Beltrán Hernández, I.; Bitsch, S.; Harwood, J.; Oliveira, S.; Kolmar, H. Penetration of Nanobody-Dextran Polymer Conjugates through Tumor Spheroids. Pharmaceutics 2023, 15, 2374. https://doi.org/10.3390/pharmaceutics15102374
Bitsch P, Baum ES, Beltrán Hernández I, Bitsch S, Harwood J, Oliveira S, Kolmar H. Penetration of Nanobody-Dextran Polymer Conjugates through Tumor Spheroids. Pharmaceutics. 2023; 15(10):2374. https://doi.org/10.3390/pharmaceutics15102374
Chicago/Turabian StyleBitsch, Peter, Eva S. Baum, Irati Beltrán Hernández, Sebastian Bitsch, Jakob Harwood, Sabrina Oliveira, and Harald Kolmar. 2023. "Penetration of Nanobody-Dextran Polymer Conjugates through Tumor Spheroids" Pharmaceutics 15, no. 10: 2374. https://doi.org/10.3390/pharmaceutics15102374
APA StyleBitsch, P., Baum, E. S., Beltrán Hernández, I., Bitsch, S., Harwood, J., Oliveira, S., & Kolmar, H. (2023). Penetration of Nanobody-Dextran Polymer Conjugates through Tumor Spheroids. Pharmaceutics, 15(10), 2374. https://doi.org/10.3390/pharmaceutics15102374