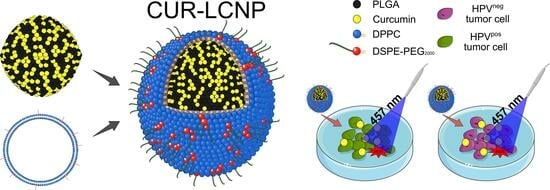
Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Light Source
2.3. Nanoparticle Preparation
2.4. Liposome (LIPO) Preparation
2.5. Preparation of Lipid-Coated Polymeric Nanoparticles (LCNPs)
2.6. Particle Size and ζ-Potential
2.7. Encapsulation Efficiency
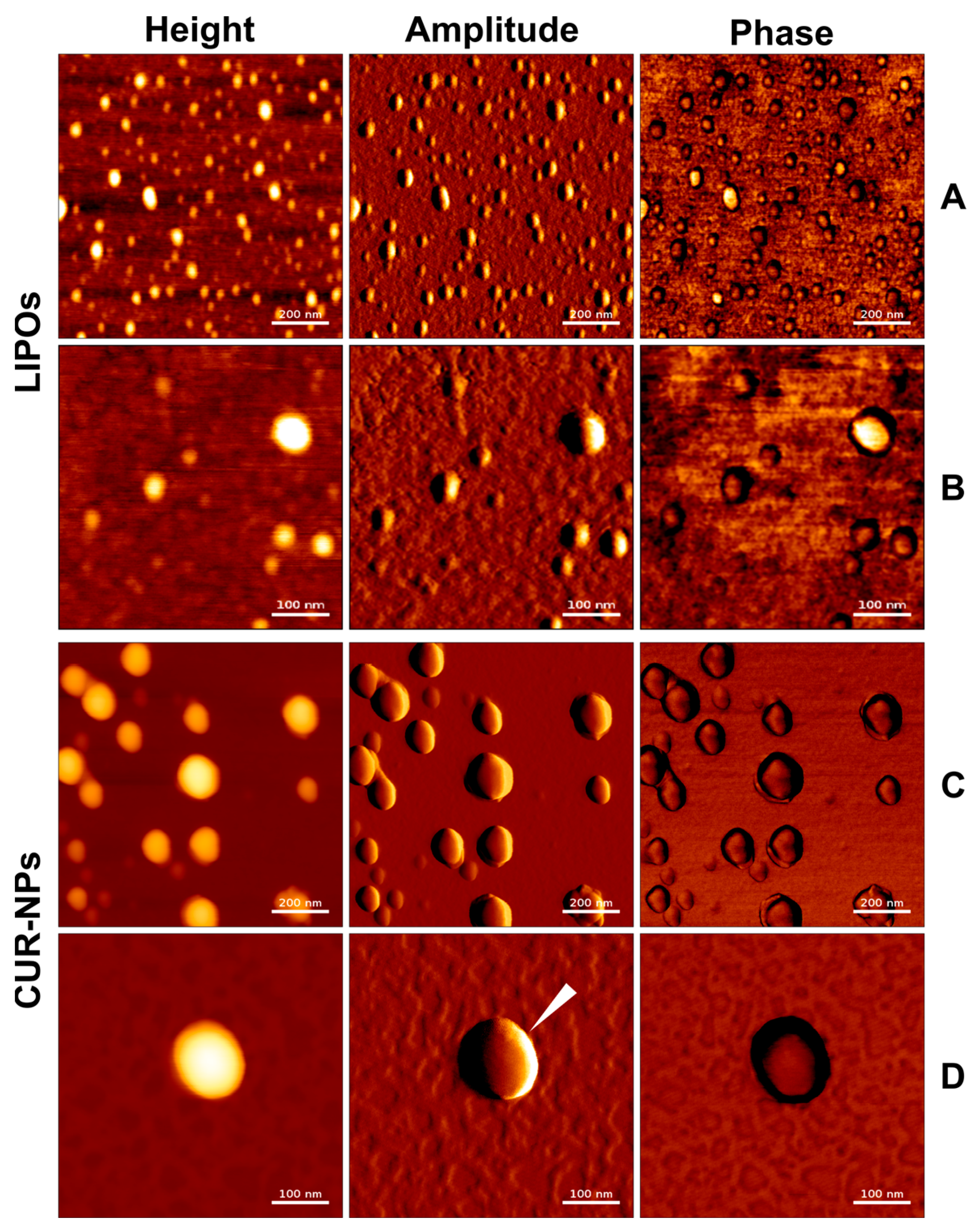
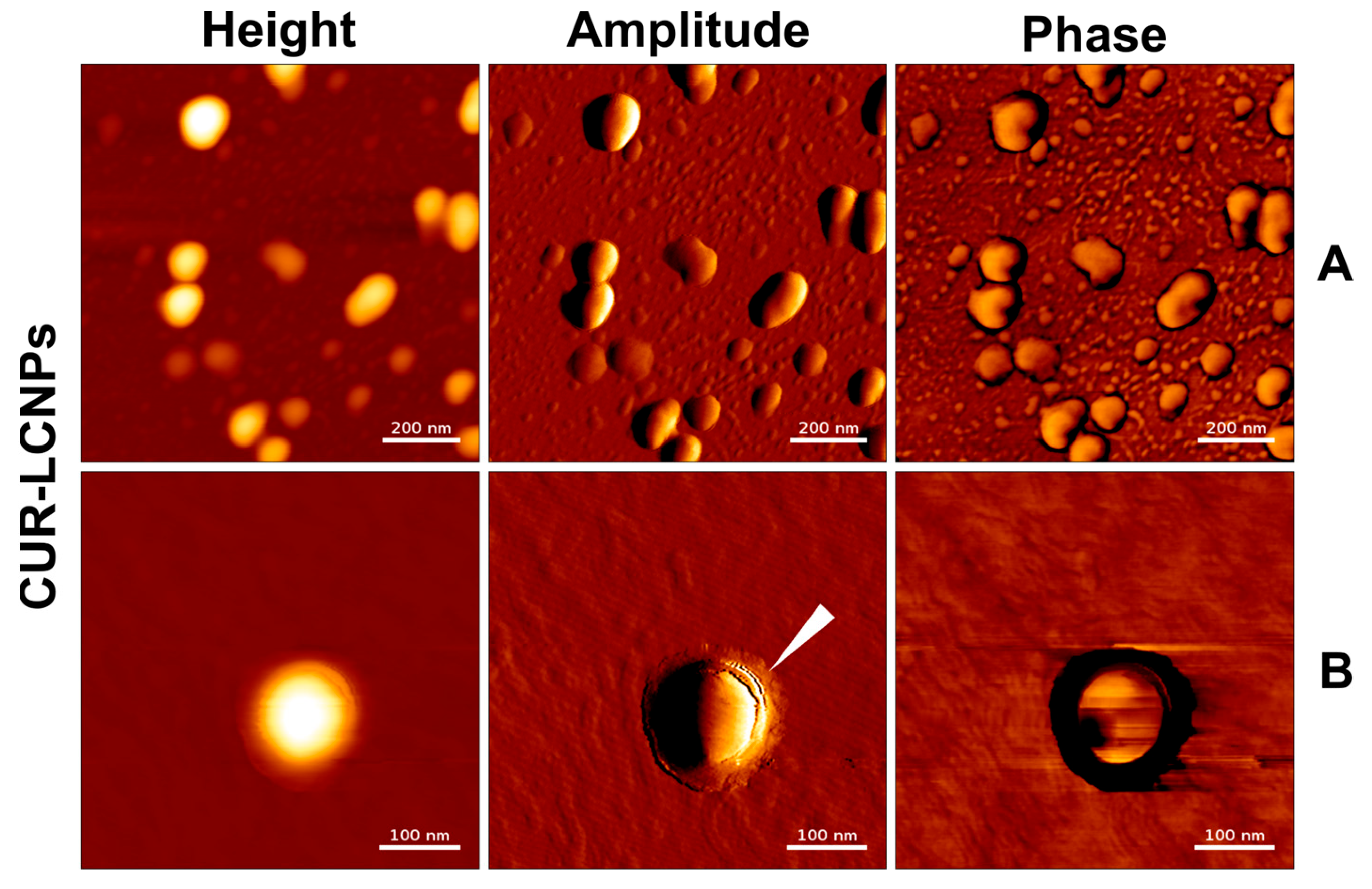
2.8. Atomic Force Microscopy
2.9. Cell Culture
2.10. Drug Uptake Studies
2.10.1. Confocal Laser Scanning Microscopy (CLSM)
2.10.2. Flow Cytometry
2.11. In Vitro Cell Viability
2.12. Biocompatibility Studies
2.13. Mitochondrial Membrane Potential
2.14. Cellular Reactive Oxygen Species (cROS)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterizations
3.2. Atomic Force Microscopy
3.3. Drug Uptake Studies
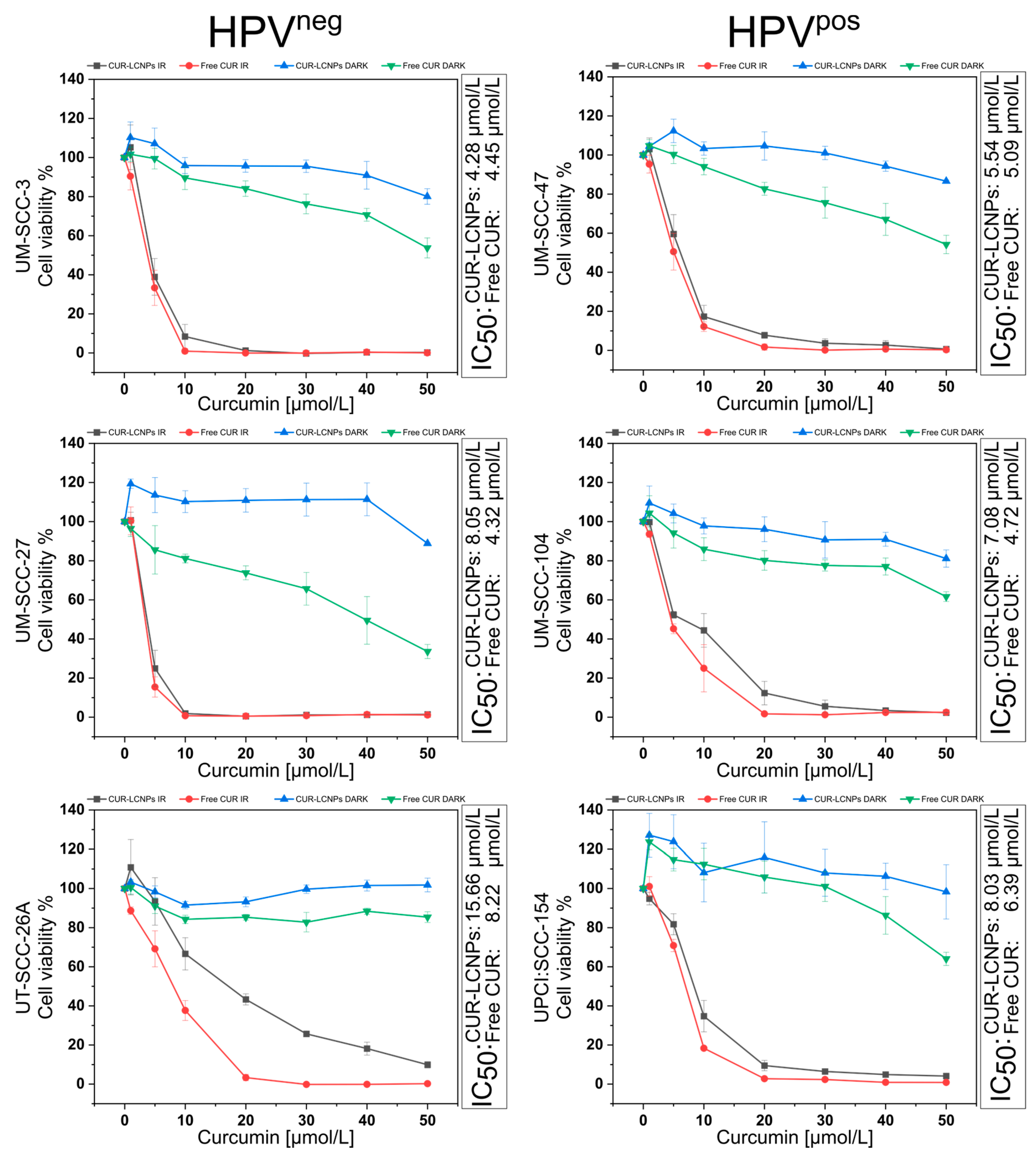
3.4. Effect of Photodynamic Therapy on Cell Viability
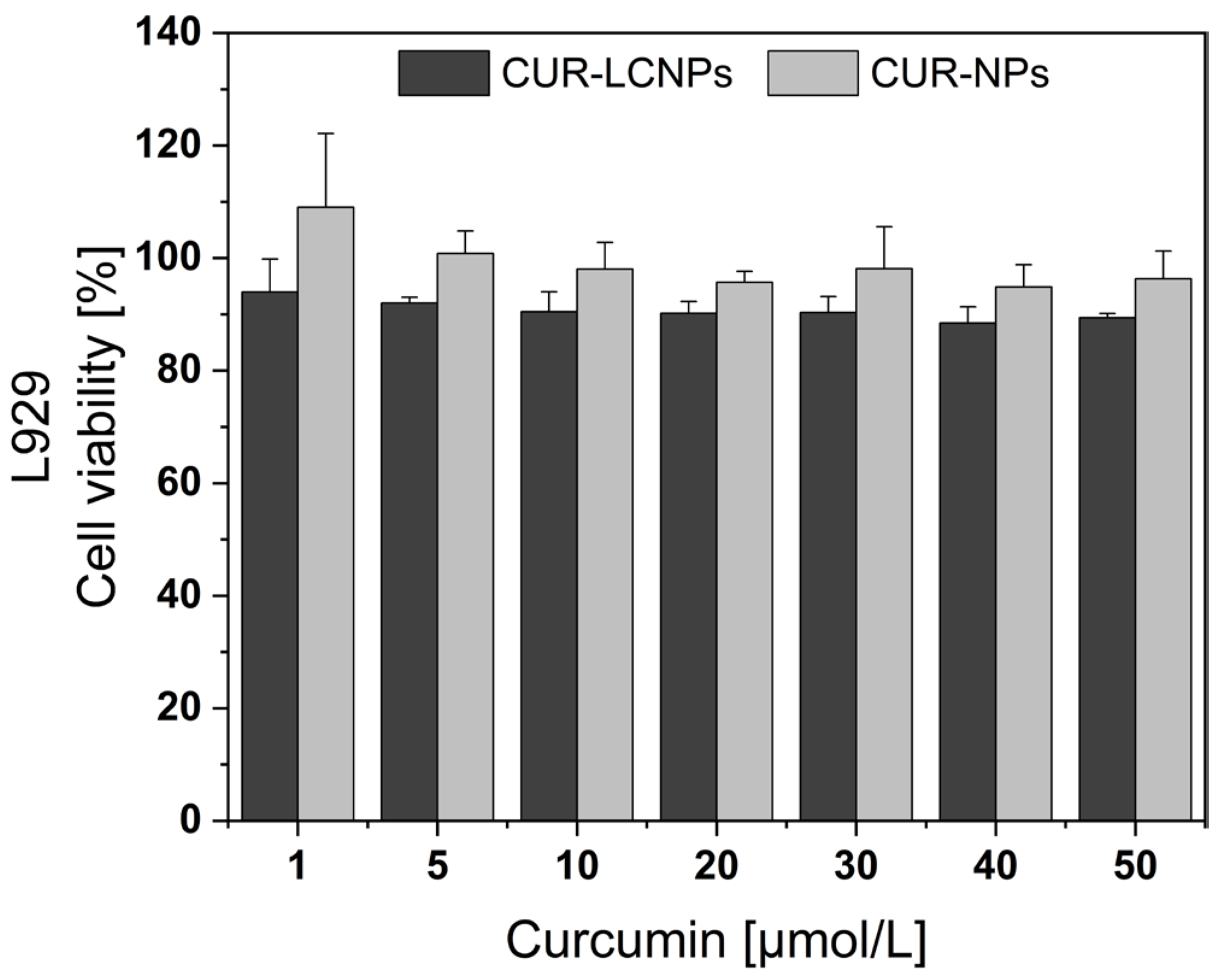
3.5. Biocompatibility
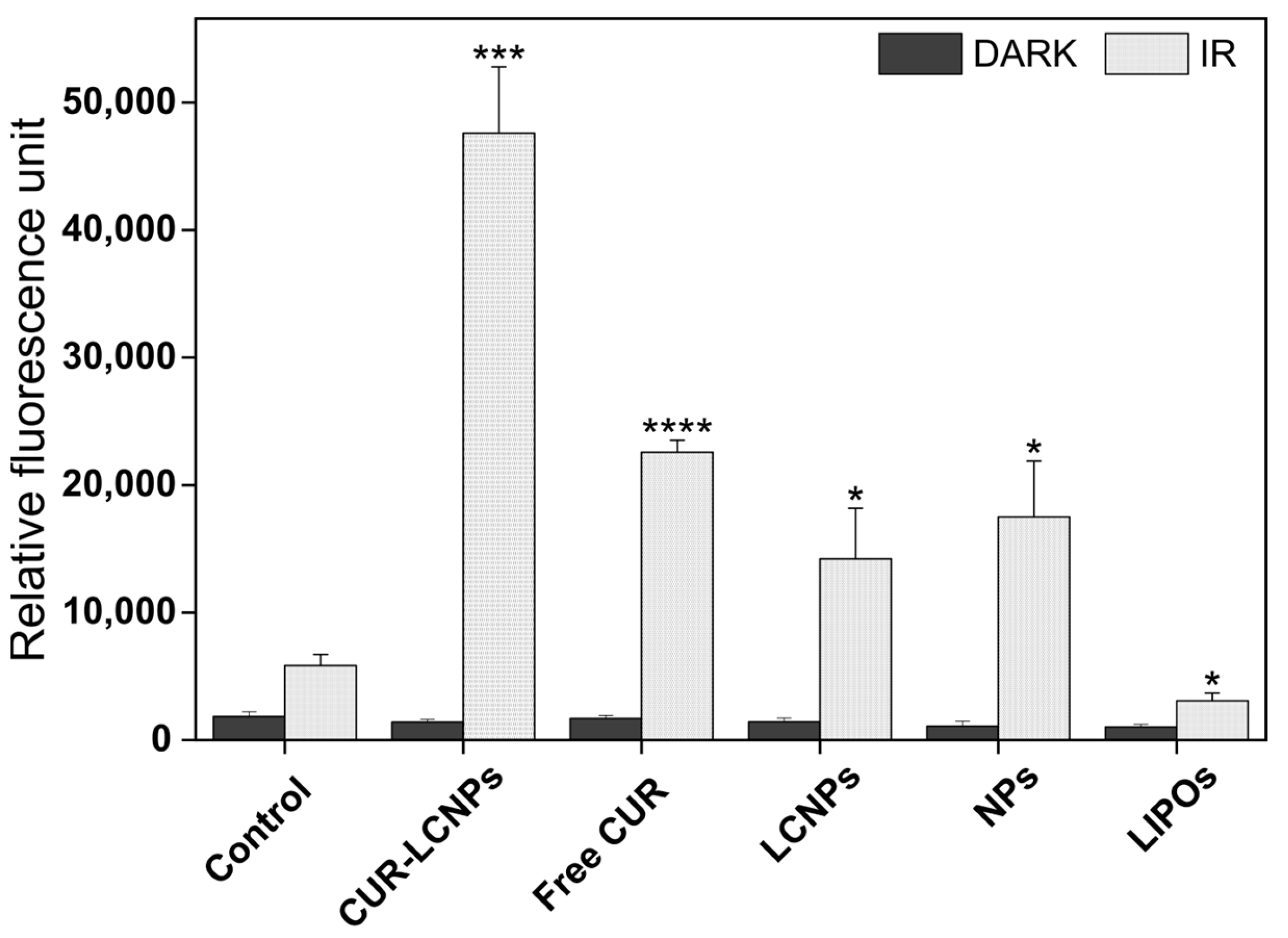
3.6. cROS
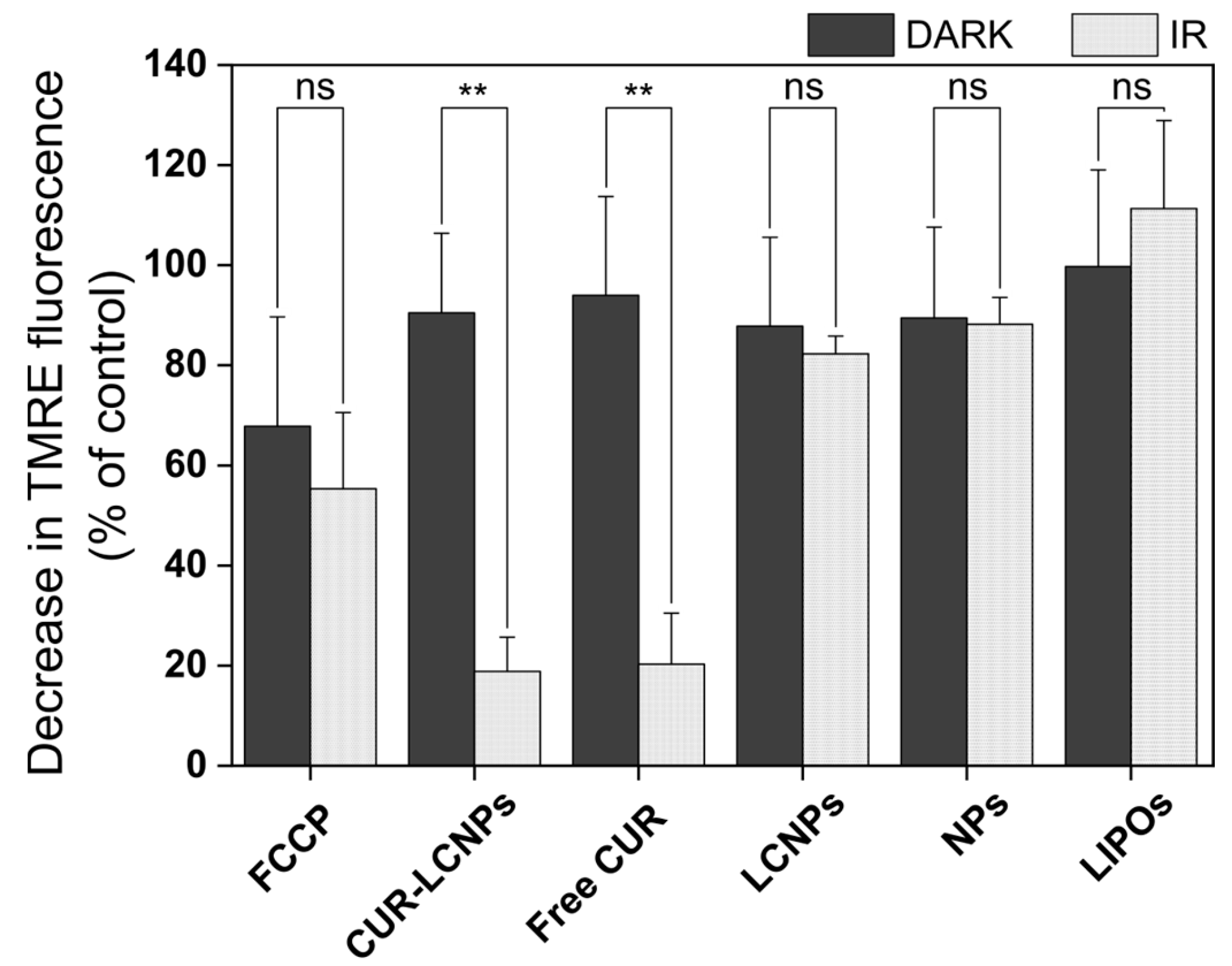
3.7. Mitochondrial Membrane Potential (MMP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marziliano, A.; Teckie, S.; Diefenbach, M.A. Alcohol-related head and neck cancer: Summary of the literature. Head Neck 2020, 42, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.T.; Bhambhani, C.; Brummel, C.; Jewell, B.; Bellile, E.; Heft Neal, M.E.; Sandford, E.; Spengler, R.M.; Bhangale, A.; Spector, M.E.; et al. Human papilloma virus circulating tumor DNA assay predicts treatment response in recurrent/metastatic head and neck squamous cell carcinoma. Oncotarget 2021, 12, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Panwar, A.; Batra, R.; Lydiatt, W.M.; Ganti, A.K. Human papilloma virus positive oropharyngeal squamous cell carcinoma: A growing epidemic. Cancer Treat. Rev. 2014, 40, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-I.; Francoeur, A.A.; Kapp, D.S.; Caesar, M.A.P.; Huh, W.K.; Chan, J.K. Trends in Human Papillomavirus-Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001–2017. JAMA Netw. Open 2022, 5, e222530. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Specenier, P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann. Oncol. 2010, 21 (Suppl. 7), vii252–vii261. [Google Scholar] [CrossRef]
- Ayan, S.; Gunaydin, G.; Yesilgul-Mehmetcik, N.; Gedik, M.E.; Seven, O.; Akkaya, E.U. Proof-of-principle for two-stage photodynamic therapy: Hypoxia triggered release of singlet oxygen. Chem. Commun. 2020, 56, 14793–14796. [Google Scholar] [CrossRef]
- Yoo, J.-O.; Ha, K.-S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int. Rev. Cell Mol. Biol. 2012, 295, 139–174. [Google Scholar] [CrossRef]
- Gutberlet, B.; Preis, E.; Roschenko, V.; Bakowsky, U. Photothermally Controlled Drug Release of Poly(d,l-lactide) Nanofibers Loaded with Indocyanine Green and Curcumin for Efficient Antimicrobial Photodynamic Therapy. Pharmaceutics 2023, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Das, M.; Sahoo, S.K. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin. Drug Deliv. 2012, 9, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Martens-de Kemp, S.R.; Buijze, M.; Jacobs, G.; Braakhuis, B.J.M.; Brakenhoff, R.H. Treatment response of HPV-positive and HPV-negative head and neck squamous cell carcinoma cell lines. Oral Oncol. 2013, 49, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.J.; Kessel, D.; Rakowski, J.; Loughery, B.; Najy, A.J.; Pham, T.; Kim, S.; Kwon, Y.T.; Kato, I.; Kim, H.E.; et al. Photodynamic Therapy as a Potent Radiosensitizer in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 1193. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; McNeel, T.S.; Fakhry, C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. 2017, 28, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Machiels, J.-P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Wilhelm Romero, K.; Quirós, M.I.; Vargas Huertas, F.; Vega-Baudrit, J.R.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and In Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles. Polymers 2021, 13, 4207. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, B.; Xia, G.; Choi, S.H. FDA’s Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Broniatowski, M.; Mach, M.; Płachta, Ł.; Wydro, P. The effect of the polyethylene glycol chain length of a lipopolymer (DSPE-PEGn) on the properties of DPPC monolayers and bilayers. J. Mol. Liq. 2021, 335, 116529. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Zhang, Y.; Wang, L.; Zhang, Z.; Zhang, L. Comparison of two methods for tumour-targeting peptide modification of liposomes. Acta Pharmacol. Sin. 2023, 44, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Dayyih, A.A.; Gutberlet, B.; Preis, E.; Engelhardt, K.H.; Amin, M.U.; Abdelsalam, A.M.; Bonsu, M.; Bakowsky, U. Thermoresponsive Liposomes for Photo-Triggered Release of Hypericin Cyclodextrin Inclusion Complex for Efficient Antimicrobial Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 31525–31540. [Google Scholar] [CrossRef] [PubMed]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. [Google Scholar] [CrossRef]
- Raschpichler, M.; Preis, E.; Pinnapireddy, S.R.; Baghdan, E.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Photodynamic inactivation of circulating tumor cells: An innovative approach against metastatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 38–46. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Tariq, I.; Sohail, M.F.; Ali, M.Y.; Preis, E.; Ambreen, G.; Pinnapireddy, S.R.; Jedelská, J.; Schäfer, J.; et al. Lipoparticles for Synergistic Chemo-Photodynamic Therapy to Ovarian Carcinoma Cells: In vitro and in vivo Assessments. Int. J. Nanomed. 2021, 16, 951–976. [Google Scholar] [CrossRef]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.A.S.; Santos, P.P.d.; Da Silva, M.M.; Paese, K.; Guterres, S.S.; Costa, T.M.H.; Pohlmann, A.R.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Lutein-loaded lipid-core nanocapsules: Physicochemical characterization and stability evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 477–484. [Google Scholar] [CrossRef]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Mandic, R.; Schamberger, C.J.; Müller, J.F.; Geyer, M.; Zhu, L.; Carey, T.E.; Grénman, R.; Dünne, A.A.; Werner, J.A. Reduced cisplatin sensitivity of head and neck squamous cell carcinoma cell lines correlates with mutations affecting the COOH-terminal nuclear localization signal of p53. Clin. Cancer Res. 2005, 11, 6845–6852. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Hauff, S.J.; Owen, J.H.; Graham, M.P.; Czerwinski, M.J.; Park, J.J.; Walline, H.; Papagerakis, S.; Stoerker, J.; McHugh, J.B.; et al. UM-SCC-104: A new human papillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck 2012, 34, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Göttgens, E.-L.; Ansems, M.; Leenders, W.P.J.; Bussink, J.; Span, P.N. Genotyping and Characterization of HPV Status, Hypoxia, and Radiosensitivity in 22 Head and Neck Cancer Cell Lines. Cancers 2021, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, Y.-X.; Pan, W.; Lei, W.; Feng, B.; Wang, X.-J. Enhancement of Anti-Inflammatory Activity of Curcumin Using Phosphatidylserine-Containing Nanoparticles in Cultured Macrophages. Int. J. Mol. Sci. 2016, 17, 969. [Google Scholar] [CrossRef]
- Punfa, W.; Yodkeeree, S.; Pitchakarn, P.; Ampasavate, C.; Limtrakul, P. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol. Sin. 2012, 33, 823–831. [Google Scholar] [CrossRef]
- Escobar, P.; Vera, A.M.; Neira, L.F.; Velásquez, A.O.; Carreño, H. Photodynamic therapy using ultradeformable liposomes loaded with chlorine aluminum phthalocyanine against L. (V.) braziliensis experimental models. Exp. Parasitol. 2018, 194, 45–52. [Google Scholar] [CrossRef]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef]
- Guo, L.; Chen, B.; Liu, R.; Xia, G.; Wang, Y.; Li, X.; Wei, C.; Wang, X.; Jiang, H. Biocompatibility Assessment of Polyethylene Glycol-Poly L-Lysine-Poly Lactic-Co-Glycolic Acid Nanoparticles In Vitro and In Vivo. J. Nanosci. Nanotechnol. 2015, 15, 3710–3719. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, M.; Magarkar, A.; Koivuniemi, A.; Róg, T.; Bunker, A. Mechanistic Insight into How PEGylation Reduces the Efficacy of pH-Sensitive Liposomes from Molecular Dynamics Simulations. Mol. Pharm. 2021, 18, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Nosova, A.S.; Koloskova, O.O.; Nikonova, A.A.; Simonova, V.A.; Smirnov, V.V.; Kudlay, D.; Khaitov, M.R. Diversity of PEGylation methods of liposomes and their influence on RNA delivery. Medchemcomm 2019, 10, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Sitterberg, J.; Ehrhardt, C.; Kumar, M.R.; Bakowsky, U. A New Drug Vehicle—Lipid Coated Biodegradable Nanoparticles. In Biomedical Applications of Smart Materials; CIMTEC 2008; Trans Tech Publications Ltd.: Zurich, Switzerland, 2008; pp. 148–153. [Google Scholar]
- Liu, J.; Xu, L.; Liu, C.; Zhang, D.; Wang, S.; Deng, Z.; Lou, W.; Xu, H.; Bai, Q.; Ma, J. Preparation and characterization of cationic curcumin nanoparticles for improvement of cellular uptake. Carbohydr. Polym. 2012, 90, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Mol, I.M.; Kerrebijn, J.D.F.; van Driel, P.B.A.A.; Xie, B.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Löwik, C.W.G.M. Targeting integrins and enhanced permeability and retention (EPR) effect for optical imaging of oral cancer. J. Surg. Oncol. 2012, 105, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, P.; Yang, L. The role of nanotherapy in head and neck squamous cell carcinoma by targeting tumor microenvironment. Front. Immunol. 2023, 14, 1189323. [Google Scholar] [CrossRef] [PubMed]
- Mandic, R.; Rodgarkia-Dara, C.J.; Krohn, V.; Wiegand, S.; Grénman, R.; Werner, J.A. Cisplatin resistance of the HNSCC cell line UT-SCC-26A can be overcome by stimulation of the EGF-receptor. Anticancer Res. 2009, 29, 1181–1187. [Google Scholar] [PubMed]
- Tonigold, M.; Rossmann, A.; Meinold, M.; Bette, M.; Märken, M.; Henkenius, K.; Bretz, A.C.; Giel, G.; Cai, C.; Rodepeter, F.R.; et al. A cisplatin-resistant head and neck cancer cell line with cytoplasmic p53(mut) exhibits ATP-binding cassette transporter upregulation and high glutathione levels. J. Cancer Res. Clin. Oncol. 2014, 140, 1689–1704. [Google Scholar] [CrossRef]
- Padilla, L.A.; Leung, B.S.; Carson, L.F. Evidence of an association between human papillomavirus and impaired chemotherapy-induced apoptosis in cervical cancer cells. Gynecol. Oncol. 2002, 85, 59–66. [Google Scholar] [CrossRef]
- Reid, P.; Staudacher, A.H.; Marcu, L.G.; Olver, I.; Moghaddasi, L.; Brown, M.P.; Bezak, E. Influence of the human papillomavirus on the radio-responsiveness of cancer stem cells in head and neck cancers. Sci. Rep. 2020, 10, 2716. [Google Scholar] [CrossRef]
- Spanos, W.C.; Nowicki, P.; Lee, D.W.; Hoover, A.; Hostager, B.; Gupta, A.; Anderson, M.E.; Lee, J.H. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Mamidi, M.K.; Das, A.K.; Bhonde, R. Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells. Arch. Toxicol. 2012, 86, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Healthc. Mater. 2021, 10, e2002153. [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Hanafi-Bojd, M.Y.; Aghili, S.H.; Iranshahi, M.; Nabavi, S.M.; Saberi, S.; Filosa, R.; Nezhad, I.F.; Hasanpour, M. Curcumin-loaded mesoporous silica nanoparticles for drug delivery: Synthesis, biological assays and therapeutic potential—A review. RSC Adv. 2023, 13, 22250–22267. [Google Scholar] [CrossRef] [PubMed]
- Turan, I.S.; Yildiz, D.; Turksoy, A.; Gunaydin, G.; Akkaya, E.U. A Bifunctional Photosensitizer for Enhanced Fractional Photodynamic Therapy: Singlet Oxygen Generation in the Presence and Absence of Light. Angew. Chem. Int. Ed. 2016, 55, 2875–2878. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.R. An in vivo model for squamous cell carcinoma of the head and neck. Laryngoscope 1985, 95, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Grenman, R.; Virolainen, E.; Shapira, A.; Carey, T. In vitro effects of tamoxifen on UM-SCC head and neck cancer cell lines: Correlation with the estrogen and progesterone receptor content. Int. J. Cancer 1987, 39, 77–81. [Google Scholar] [CrossRef]
- Platel, A.; Carpentier, R.; Becart, E.; Mordacq, G.; Betbeder, D.; Nesslany, F. Influence of the surface charge of PLGA nanoparticles on their in vitro genotoxicity, cytotoxicity, ROS production and endocytosis. J. Appl. Toxicol. 2016, 36, 434–444. [Google Scholar] [CrossRef]
- Ricci-Júnior, E.; Marchetti, J.M. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int. J. Pharm. 2006, 310, 187–195. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Palmeira, C.M.; Rolo, A.P. Mitochondrial Membrane Potential (ΔΨ) Fluctuations Associated with the Metabolic States of Mitochondria. In Mitochondrial Bioenergetics; Humana Press: New York, NY, USA, 2018; pp. 109–119. [Google Scholar]
- Gu, X.; Shen, C.; Li, H.; Goldys, E.M.; Deng, W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J. Nanobiotechnol. 2020, 18, 87. [Google Scholar] [CrossRef]


| Formulation | Particle Size [nm] | PdI | ζ-Potential [mV] | EE [%] |
|---|---|---|---|---|
| CUR-NPs | 149.51 ± 4.35 | 0.16 ± 0.06 | −2.72 ± 0.31 | |
| CUR-LCNPs | 153.37 ± 1.58 | 0.11 ± 0.01 | −3.74 ± 0.31 | 92.69 ± 0.03 |
| Blank NPs | 132.87 ± 0.85 | 0.06 ± 0.01 | −3.10 ± 0.23 | |
| LCNP | 134.40 ± 2.78 | 0.08 ± 0.02 | −4.17 ± 0.43 | |
| LIPOs | 108.08 ± 4.48 | 0.36 ± 0.02 | −23.03 ± 2.17 |
| Cell Line | HPV-Status | Mean IC50 CUR-LCNPs [µmol/L] | Mean IC50 Free CUR [µmol/L] |
|---|---|---|---|
| UM-SCC-3 | |||
| UM-SCC-27 | HPVneg | 9.34 ± 4.73 | 5.66 ± 1.81 |
| UT-SCC-26A | |||
| UM-SCC-47 | |||
| UM-SCC-104 | HPVpos | 6.88 ± 1.03 | 5.40 ± 0.72 |
| UPCI:SCC-154 |
| CUR-LCNP/PDT Sensitivity | Cell Line | HPV-Status | IC50 CUR-LCNPs [µmol/L] |
|---|---|---|---|
| Highest | UM-SCC-3 | HPVneg | 4.28 |
 | UM-SCC-47 | HPVpos | 5.54 |
| UM-SCC-104 | HPVpos | 7.08 | |
| UPCI:SCC-154 | HPVpos | 8.03 | |
| UM-SCC-27 | HPVneg | 8.05 | |
| Lowest | UT-SCC-26A | HPVneg | 15.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roschenko, V.; Ayoub, A.M.; Engelhardt, K.; Schäfer, J.; Amin, M.U.; Preis, E.; Mandic, R.; Bakowsky, U. Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas. Pharmaceutics 2023, 15, 2412. https://doi.org/10.3390/pharmaceutics15102412
Roschenko V, Ayoub AM, Engelhardt K, Schäfer J, Amin MU, Preis E, Mandic R, Bakowsky U. Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas. Pharmaceutics. 2023; 15(10):2412. https://doi.org/10.3390/pharmaceutics15102412
Chicago/Turabian StyleRoschenko, Valeri, Abdallah M. Ayoub, Konrad Engelhardt, Jens Schäfer, Muhammad Umair Amin, Eduard Preis, Robert Mandic, and Udo Bakowsky. 2023. "Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas" Pharmaceutics 15, no. 10: 2412. https://doi.org/10.3390/pharmaceutics15102412
APA StyleRoschenko, V., Ayoub, A. M., Engelhardt, K., Schäfer, J., Amin, M. U., Preis, E., Mandic, R., & Bakowsky, U. (2023). Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas. Pharmaceutics, 15(10), 2412. https://doi.org/10.3390/pharmaceutics15102412