Abstract
Photodynamic therapy (PDT) is an approved therapeutic procedure that exerts cytotoxic activity towards tumor cells by activating photosensitizers (PSs) with light exposure to produce reactive oxygen species (ROS). Compared to traditional treatment strategies such as surgery, chemotherapy, and radiation therapy, PDT not only kills the primary tumors, but also effectively suppresses metastatic tumors by activating the immune response. However, the anti-tumor immune effects induced by PDT are influenced by several factors, including the localization of PSs in cells, PSs concentration, fluence rate of light, oxygen concentration, and the integrity of immune function. In this review, we systematically summarize the influence factors of anti-tumor immune effects mediated by PDT. Furthermore, an update on the combination of PDT and other immunotherapy strategies are provided. Finally, the future directions and challenges of anti-tumor immunity induced by PDT are discussed.
1. Introduction
Photodynamic therapy (PDT) utilizes an administered photosensitizer (PS) activated by light to achieve localized cytotoxicity for treatment of various indications (Figure 1). At present, PDT has been proven to be an effective strategy in various cancer treatment, including cervical cancer, skin cancer, nasopharyngeal carcinoma, etc. [,]. There are three mechanisms of PDT-induced tumor destruction. Firstly, ROS generated by PDT directly kill the primary tumor cells. Secondly, ROS disrupt the tumor vascular system by inducing the release of vasoconstrictors and the form of blood clots. Finally, the immune system is activated by PDT, which can not only eliminate primary tumors, but also effectively destroy metastatic lesions through the activation of T cells []. However, the PDT-mediated immune effects are influenced by several factors, such as the localization of PSs in cells, PS concentration, fluence rate of light, oxygen concentration, and the integrity of immune function. Therefore, comprehensive consideration of these factors to achieve the optimal outcome of PDT is important for the physician to make a treatment protocol. In addition, the combination of PDT with immune checkpoints, DC vaccines, and chemotherapy have been reported to effectively improve the immune effects of PDT [,]. Herein, we first analyze the mechanism and influence factors of anti-tumor immunity mediated by PDT. Then, the recent advances on combination of PDT with other immunotherapies are also summarized. Finally, the future direction and challenges of anti-tumor immunity induced by PDT are discussed.
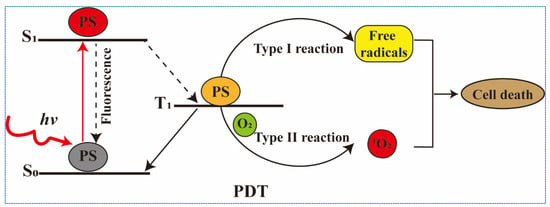
Figure 1.
The mechanism of action of photodynamic therapy.
2. Immunological Effects of PDT
Many studies have demonstrated that innate and specific immunity can be activated by PDT. Tumor cell necrosis induced by PDT is frequently accompanied by an acute inflammatory response and infiltration of inflammatory cytokines, which can trigger innate immunity []. Meanwhile, immunogenic cell death (ICD) induced by PDT leads to the release of damage-associated molecular patterns (DAMPs) and the activation of specific immune response [].
2.1. Activation of Innate Immunity
The mechanism of PDT-mediated innate immunity has been widely studied, which mainly include three parts [,,]. Firstly, nuclear factor κB (NF-κB) and activator protein 1 (AP-1) in the tumor cells can be activated after PDT and contribute to the activation of acute inflammatory response [,]. Secondly, the activation of inflammatory signaling pathway results in the secretion of inflammatory cytokines and chemokines, such as interleukin (IL), tumor necrosis factor (TNF), and interferon (IFN) []. Interleukin-1β (IL-1β) plays an important role in neutrophils infiltration of tumors. The increased activation of neutrophils is an essential symbol for innate immune response induced by PDT []. Kousis et al. reported that the activation and proliferation of CD8+ T cells is influenced by exhaust of neutrophils []. It is widely known that CD8+ T cells can preferentially attack tumor cells by recognizing antigens. Thirdly, the activation of the complement system is conducive to clearance of tumor cells after PDT. Stott et al. have demonstrated that C3, C5, and C9 have significantly increased in Lewis lung carcinoma (LLC) cells after Photofrin-PDT []. Cecic et al. also discovered that the cure rate of LLC tumors after PDT can be influenced by complement antagonists of C3aR or C5aR []. These studies suggested that the complement system is a pivotal part of PDT-mediated anti-tumor immunity.
2.2. Activation of Specific Immunity
PDT stimulates specific immune effects by inducing the immunogenic death (ICD) of tumor cells. ICD is a form of cell death to activate immune system, which provokes specific immune response by eliciting danger signals released from dying tumor cells. The occurrence of ICD is one precondition of anti-tumor immunity. Therefore, the development of ICD inducers has become a research hotspot. PSs are considered an efficient ICD inducer under irradiation, especially Hypericin (Hyp) (Scheme 1, Compound 1), which can target endoplasmic reticulum (ER) [] and trigger ER stress by producing ROS, resulting in the release of a variety of damage-associated molecular patterns (DAMPs) and the activation of specific immune response [,,].
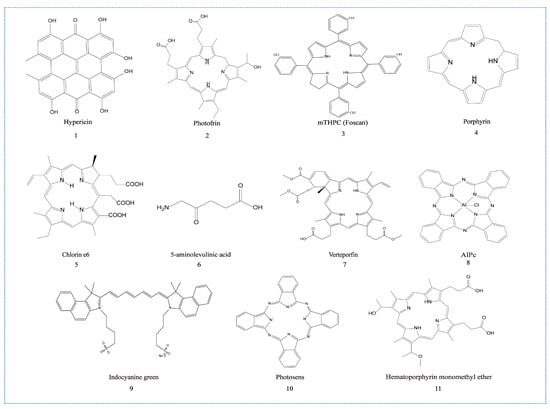
Scheme 1.
Chemical structure of the PSs that can induce immune effects.
ER stress is an adaptive response of cells to the over-accumulation of damaged proteins. ER receptors are activated when ER is under stress. In order to relieve ER stress and achieve self-help, signaling pathways such as PERK, ATF6 and IRE1 are activated []. Among them, PERK is an important regulatory molecule in immunogenicity. It can incite the release of DAMPs such as calreticulin (CRT), high-mobility group protein 1 (HMGB1), and ATP []. DAMPs are a kind of dangerous signal released into the extracellular space during tumor cell death []. The characteristics of PSs known to trigger ICD and their immune activation effects are summarized in Table 1.
The process of anti-tumor immune activation of PDT is briefly introduced as follows. Firstly, CRT, as an “eat me” signal for APCs, is the most important DAMP that triggers ICD. It can be translocated to the outer surface of the plasma membrane of dying tumor cells after PDT, and then recognized by the low-density lipoprotein receptor-related protein 1 (LRP1) receptor on dendritic cells (DCs). Then, DCs become matured and boost anti-tumor immune response by sparking antigen presentation [,,]. Extracellular ATP, as a “find me” signal, can bind to the purinergic receptor P2Y2 (P2Y2R) and purinergic receptor P2X7 (P2X7R) on DCs. It is worth mentioning that ATP can attract the recruitment of monocytes by recognizing P2Y2 receptors, as well as promote formation of the inflammasome and secretion of inflammatory stimulating factors by combining P2X7 receptors []. Additionally, heat shock protein (HSP) and HMGB1 are also important DAMPs which can be triggered by PDT. HSPs can effectively promote DC maturation and stimulate T cells by binding to Toll-like receptor 2 (TLR2) and Toll-like receptor 4 (TLR4) [,].
Overall, PDT, as an ICD inducer, generates ROS in tumor cells to stimulate massive exposure of DAMPs, which in turn promotes maturation of DCs and activation of cytotoxic T lymphocytes. Finally, the activated DCs and T cells mediate patient-specific immune effects for the elimination of primary and metastatic lesions (Figure 2). The existing PSs capable of triggering the ICD effects are summarized and listed in the Table 2. The structural formulas of classic PSs are shown in Scheme 1.
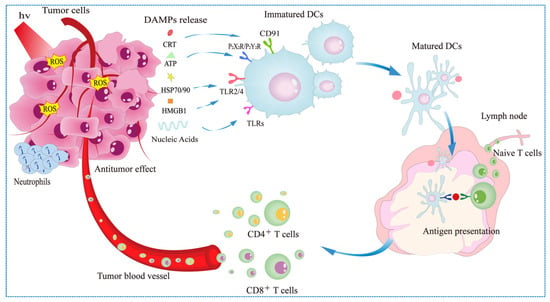
Figure 2.
Mechanism of photodynamic therapy-induced immune effects.

Table 1.
Common DAMPs and their functions.
Table 1.
Common DAMPs and their functions.
| DAMP | PRR Receptor | Function | References | |
|---|---|---|---|---|
| 1 | CRT | LRP1 (CD91) | As a pro-phagocytic signal and promoting antigen presentation | [,] |
| 2 | ATP | P2RX7 | Activate inflammatory bodies and promote the secretion of inflammatory factors | [,] |
| P2RY2 | Attract recruitment of monocytes | |||
| 3 | HMGB1 | TLR2, TLR4, TLR9 | Promote DC maturation (especially its metastasis to lymph nodes) and activate T cells | [,] |
| 4 | HSP70 | TLR2, TLR4 | Induce DC expression and maturation, and promote cytokine release, especially IL-12 and TNF-α | [,,] |
| HSP90 | ||||
| 5 | Annexin A1 | FPR1 | Help DC move to dying cells | [] |
| 6 | CpG DNA | TLR9 | Expression of high levels of MHCII and costimulatory molecules (CD80, CD86) and production of IL-12, interleukin, and other cytokines to promote DC maturation and activation | [,] |
| 7 | CXCL10 | CXCR3 | Induction of DC activation and T cell infiltration | [] |
| 8 | ExRNA | TLR3 | Release TNF-α, IL-1β, or IL-6 and other inflammatory cytokines | [,,] |
| 9 | dsDNA | TLR3, RIG-I | Promote the expression of proinflammatory cytokines type I IFN, etc. | [] |
| 10 | dsRNA | TLR3 | Promote the expression of proinflammatory cytokines type I IFN, etc. | [] |
| 11 | Type I IFNs | IFNAR1/IFNAR2 | Enhance the function of CTL and NK cells and promote the secretion of CXCL10 | [,,] |
| 12 | ssRNA | TLR7, TLR8 | Promote the release of other DAMPs, release cytokines, and promote DC maturation | [,] |
CRT: Calreticulin; HMGB1: High-mobility group protein box 1; HSP: Heat shock protein; DC: Dendritic cell; TNF: Tumor necrosis factor; IFN: Interferon; IL: Interleukin; MHC: Major histocompatibility complex; CTL: Cytotoxic T lymphocyte.

Table 2.
PS-induced ICD.
Table 2.
PS-induced ICD.
| Photosensitizer | Cell Line | Cell Death Type | Subcellular Localization | DAMP | Immunological Effects of Tumor Cells In Vitro | Immunological Effects of Tumor Cells In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| Indocyanine green (Scheme 1, Compound 9) | CT26 | Apoptosis | ER | CRT | N/D | Maturation of DCs; CD8+T cells ↑; TNF-α, IFN-γ ↑; Tregs cells ↓; | [] |
| B16 | N/D | Maturation of DCs (CD11c+/CD80+/CD86+↑); CD4+ T cells, CD8+ T cells ↑; IL-6 ↑, TNF-α, IFN-γ ↑; Tregs cells ↓; | |||||
| TCPP-TER | 4T1 | N/D | ER | CRT, HMGB1 | Maturation of DCs (CD80+CD86+ ↑); IL-12, TNF-α ↑; | CD8+ T cells ↑; IL-12, TNF-α, INF-γ ↑; | [] |
| Hypericin (Scheme 1, Compound 1) | T24 | Apoptosis | ER | CRT, ATP | Phenotypic maturation of DCs (MHC II, CD80+, CD83+ and CD86+ ↑); | DC phenotype maturation (CD80+, CD83+, CD86+, MHC II ↑); IL-1β ↑, IL-10 ↓; | [] |
| 5-aminolevulinic acid (Scheme 1, Compound 6) | PECA | Apoptosis | ER | CRT, HSP70, and HMGB1 | Phenotypic maturation of DCs (CD80+, CD86+ and MHC II↑); IFN-γ, IL-12 ↑; | N/D | [] |
| Porphyrazines (Pz I and Pz III) | MCA205 | Apoptosis | Pz-I: GA and Lys | ATP, HMGB1 | Maturation of DCs (CD80+, CD86+ ↑); | N/D | [] |
| Ferroptosis, Necrosis | Pz-III: ER and Lys | ||||||
| Verteporfin (Scheme 1, Compound 7) | CT26 | Apoptosis, Necrosis | N/D | CRT, HSP70, and HMGB1 | Maturation of DCs (CD11c+CD40+CD86+ ↑); | Phenotypic maturation of DCs (CD86+↑); CTL ↑; IFN-γ ↑; Tregs ↓; | [] |
| TPE-PR-FFKDEL | 4T1 | N/D | ER | CRT, ATP, HMGB1, and HSP70 | N/D | DC phenotype maturation (CD80+CD86+ ↑); CD8+ T cells, NK cells ↑; | [] |
| Photosens (Scheme 1, Compound 10) | GL261, MCA205 | Apoptosis, Ferroptosis | Lys | CRT, HMGB1, and ATP | Maturation of DCs (CD86+ ↑); IL-6 ↑; | N/D | [] |
| Photodithazine | GL261, MCA205 | Apoptosis | ER and GA | CRT, HMGB1, and ATP | DC phenotype maturation (CD86+ ↑); IL-6 ↑; | N/D | [] |
| Photofrin (Scheme 1, Compound 2) | C-26 | Apoptosis, Necrosis | N/D | HSP | Maturation of DCs (IL-2 ↑); | CD8+ T cells, NK cells ↑; | [] |
| Cu-TBP nMOF | B16F10 | Apoptosis | N/D | CRT | N/D | Maturation of DCs (CD11+ ↑); IFN-β, CD4+ T cells and CD8+ T cells ↑; | [] |
| Chlorin e6 (Scheme 1, Compound 5) | 4T1 | Apoptosis | N/D | CRT | Phenotypic maturation of DCs (CD80+, CD86+ ↑); | DC phenotype maturation (CD86 ↑); CD8+ T cells, CD4+ T cells ↑; | [] |
| Chlorin e6 (Scheme 1, Compound 5) | B16 | Apoptosis | N/D | CRT, HSP90, HMGB1, and ATP | MI macrophage activation (GBP5, iNOS and MHC-II ↑); IFN-β ↑; | N/D | [] |
| Indocyanine green (Scheme 1, Compound 9) | MC38 | Apoptosis | N/D | CRT, HSP70, and ATP | Maturation of DCs (CD86+ ↑); IL-12-p40 ↑; | Significantly inhibited tumor growth; | [] |
| CT26 | |||||||
| Pyrolipid | 4T1 | Apoptosis, Necrosis | N/D | CRT | N/D | TNF-α, IL-6 and IFN-γ ↑; B cells, CD8+ T cells ↑; Significantly inhibited tumor growth; | [] |
| Chlorin e6 (Scheme 1, Compound 5) | 4T1 | Apoptosis | N/D | CRT | DC phenotype maturation (CD80+CD86+ ↑); | DC phenotype maturation (CD80+CD86+ ↑); CD8+ T cells ↑; | [] |
| Chlorin e6 (Scheme 1, Compound 5) | 4T1 | Apoptosis | N/D | CRT, HMGB1, and ATP | Phenotypic maturation of DCs (MHC II, CD86 ↑); | CD8+ T cells, CD4+ T cells and NK cells ↑; | [] |
| Pyropheophorbide | CT26 | Apoptosis, Necrosis | N/D | CRT | N/D | TNF-α, IL-6 and IFN-γ ↑; | [] |
| Porphyrin (Scheme 1, Compound 4) | CT26 | Apoptosis | N/D | CRT, ATP, and HMGB1 | N/D | DC phenotype maturation (CD80+CD86+ ↑); CD8+ T cells ↑; | [] |
| Chlorin e6 (Scheme 1, Compound 5) | LLC or A549 | Apoptosis | N/D | CRT, HSP 90, and HMGB1 | MHC I ↑ | Ce6-PDT showed excellent anti-tumor efficacy; MHC I ↑; | [] |
| Indocyanine green (Scheme 1, Compound 9) | 4T1 | N/D | N/D | CRT | N/D | Phenotypic maturation of DCs (CD86, CD80 ↑); IL-10 and IFN-γ ↑; CD8+ T cells, CD4+ T cells and NK cells ↑; TGF-β ↓; | [] |
| Core-shell gold nanocages coated with manganese dioxide (AuNC@MnO2) | 4T1 | Apoptosis | N/D | CRT, ATP, and HMGB1 | DC phenotype maturation (CD83, CD86 ↑); IL-12 ↑; | Maturation of DCs (CD86+ ↑); NK cells, CD8+ T cells and CD4+ T cells ↑; Treg cells↓; | [] |
| Chlorin e6 (Scheme 1, Compound 5) | 4T1 | Apoptosis, Necrosis | Cytoplasm | CRT, ATP | Maturation of DCs (CD80+CD86+ ↑); | Significantly inhibited tumor growth; CD4+ T cells, CD8+ T cells ↑; | [] |
| 2-(1-hexyloxyethyl)-2-devinyl pyropheophor-bide-a (HPPH) | B16F10 | Apoptosis | Endo/Lys then in ER | CRT | N/D | IL-6, TNF-α ↑; CD8+ T cells ↑; | [] |
| Zinc-phthalocyanine | MC38 | Apoptosis | Mitochondria | CRT | N/D | Significantly inhibited tumor growth | [] |
| Zinc-phthalocyanine | TC-1 | Pyroptosis | Mitochondria | CRT, HMGB1 | N/D | Significantly inhibited the growth of primary tumors and metastatic tumors | [] |
| IR780 | CT26 | N/D | Mitochondria | CRT, ATP, HMGB1, and HSP90 | Phenotypic maturation of DCs (CD80+CD86+ ↑); | CD4+T cells and CD8+T cells ↑; Significantly inhibited the growth of primary tumors and metastatic tumors | [] |
| TPE-DPA-TCyP | 4T1 | N/D | Mitochondria | CRT, ATP, HMGB1, and HSP70 | N/D | DC phenotype maturation (CD80+ CD86+ ↑); Significantly inhibited the growth of primary tumors and metastatic tumors; CD4+ T cells, NK cells ↑; | [] |
N/D: Not detected; ER: Endoplasmic Reticulum; GA: Golgi Apparatus; Lys: Lysosomes; CRT: Calreticulin; HMGB1: High-mobility group protein box 1; HSP: Heat shock protein; DC: Dendritic cell; TNF: Tumor necrosis factor; IFN: Interferon; IL: Interleukin; MHC: Major histocompatibility complex; Tregs: Regulatory T cells; ↑: Increase in proportion; ↓: Reduction in proportion.
3. Influence Factors of the Anti-Tumor Immunity Induced by PDT
The three elements of PDT are PSs, light, and oxygen, which determine the efficacy of PDT and the death mode of cancer cells. Hence, the immunity induced by PDT relies on several factors, including the localization and dose of PSs, light fluence, and concentration of oxygen in tumors. Understanding and regulation of these influencing factors are important to improve the anti-tumor immune effects of PDT.
3.1. Localization of PSs
The immune effects induced by PDT is highly related to the intracellular localization of PSs. The mitochondria, ER, Golgi apparatus, and lysosomes are the binding sites of PSs in the cells []. Different PSs mainly bind to the different organelles. Among these organelles, ER maintains the intracellular calcium homeostasis and protein folding, which plays a central role in immunogenic cell death [,]. Many studies have examined the relationship between ER stress and the efficiency of induction of ICD. Garg et al. found that Hyp-PDT could trigger CRT exposure, but not Photofrin-PDT under the same conditions because Hyp is primarily located in the ER, whereas Photofrin (Scheme 1, Compound 2) is dispersed within the cells and binds to the ER, mitochondria, and Golgi apparatus []. Brodin [] and Alzeibak []’s studies highlight the role of ER stress response for incited ICD. Therefore, ER targeting of PSs is a decisive factor for the anti-tumor immunity of PDT. Currently, a few PSs can target the ER have been reported, including mTHPC (Foscan) (Scheme 1, Compound 3) [], Benzoporphyrin derivative (BPD) [], and Hyp []. However, there is still a lack of ER-targeted PSs in the clinic. Turubanova et al. developed a new PS porphyrins III (Pz III) [], which targets to ER and causes ER stress and DAMP release under 630 nm laser irradiation (Figure 3A). Particularly, there was a significant increase in HMGB1 and ATP release (Figure 3B,C). In addition, the activation and maturation of BMDCs were induced by MCA205 dying cells treated with Pz III-PDT (Figure 3D). Similarly, in C57BL/6J mice model, the inhibitory effects on tumor growth of the Pz III-PDT group were more pronounced than PBS-injected group (Figure 3E). These results indicated that tumor dying cells after Pz III-PDT can be immunogenic, which would contribute to the stimulation of an adaptive immune response.
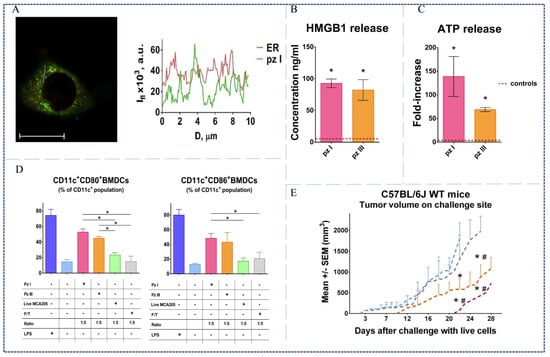
Figure 3.
(A) Confocal microscopy showed co-localization of porphyrins III with ER, scale bar 20 μm. (B) Quantitative analyses of HMGB1 release. (C) Quantitative analyses of ATP release. (D) Flow cytometry analysis showed maturation of BMDCs in vitro induced by different treatment killed cancer cells. (E) Volume changes of tumors after different treatments in vivo, *, # p < 0.05 []. Copyright, 2021, published by the Author(s).
Li et al. designed a new ER-targeted PS, TPE-PR-FFKDEL, which has been proven to be effective in inducing ICD []. The exposure of CRT and the release of HSP70, HMGB1, and ATP were detected in 4T1 cells after TPE-PR-FFKDEL-PDT. This marked preponderance of released DAMPs can be attributed to the ability of TPE-PR-FFKDEL targeting ER. Furthermore, TPE-PR-FFKDEL-PDT observably led to higher numbers of CD8+ T cells and NK cells in the spleen of mice, indicating the effective provoking of specific and innate immunity. All these results indicate that designing ER-targeted PSs are an effective strategy to induce ICD and adaptive anti-tumor immune response.
In addition, transporting PSs with nanoparticles (NPs) to the subcellular organelles is another strategy for improving immunity of PDT. Zhang et al. obtained Ce6-IMDQ NPs via self-assembly, which combined chlorin e6 (Ce6) (Scheme 1, Compound 5) with TLR7 agonists (IMDQ) (Figure 4A) []. Upon 660 nm laser irradiation, Ce6-IMDQ NPs could induce tumor cell death and antigen release, which effectively upregulated CRT exposure and induced DC maturation (Figure 4D). Flow cytometric analyses confirmed that the Ce6-IMDQ NPs group induced higher level of CD8+ T cells and CD4+ T cells in the spleen and the distant tumors. In particular, the numbers of CD8+ T cells increased from 7.85% to 14.14% (Figure 4E). Therefore, the stronger anti-tumor effects of PDT were elicited by Ce6-IMDQ NPs through promoting the infiltration of cytotoxic T lymphocytes. Similar results were also obtained in primary and distant animal tumor models. Tumor growth was more effectively inhibited in the Ce6-IMDQ NPs treatment group compared to the other treatment groups (Figure 4B,C).
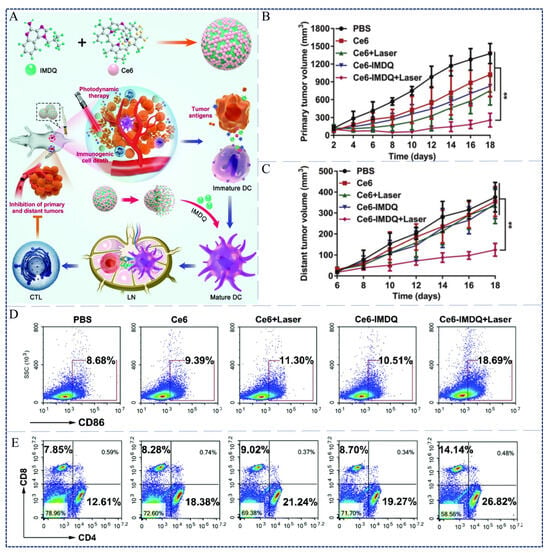
Figure 4.
(A) Schematic of PDT immune-enhanced based on Ce6-IMDQ NPs. (B) Volume changes of tumors in primary tumors of mice after different treatments in the 4T1 tumor models. (C) Volume changes of tumors in distant tumors of mice after different treatments in the 4T1 tumor models. (D) Flow cytometric analysis of DC maturation (CD86) in tumor-draining lymph nodes after different treatments. (E) Flow cytometric analysis of the percentage of CD4+ and CD8+ T cells in spleen []. ** p < 0.01. Copyright, 2021, published by Royal Society of Chemistry.
Deng et al. [] developed new NPs, Ds-sP/TCPP-TER, which consist of reduction-sensitive Ds-sP NPs (PEG-s-s-1,2distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] NPs) and an efficient ER-targeting PS TCPP-TER (Figure 5A). Ds-sP/TCPP-TER NPs could selectively accumulate in the ER. Upon 670 nm laser irradiation, ICD was induced by the expression of ecto-CRT and HMGB1 (Figure 5B,C). The immunogenic effects of Ds-sP/TCPP-TER-PDT were further demonstrated in vivo (Figure 5D–F). Therefore, PSs modified by NPs with ER-targeting function can effectively boost immune effects induced by PDT and show extraordinary performance in eliminating primary and metastatic tumors.
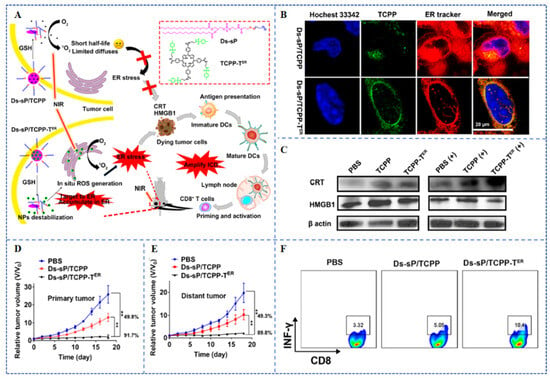
Figure 5.
(A) The preparation and principle of ER-targeting Ds-sP/TCPP-TER NPs. (B) CLSM showed co-localization of Ds-sP/TCPP-TER and TCPP-TER with the ER in 4T1 cells. (C) Western blot showed the effects of different treatments on the expression levels of HMGB1 and CRT in 4T1 cells. (D) Growth curves of primary tumors in mice after different treatments of the 4T1 tumor models. (E) Growth curves of distal tumors in mice after different treatments of the 4T1 tumor models. (F) Flow cytometry showed the proportion of IFN-γ+ CD8+ T cells in mice tumor tissues []. ** p < 0.01. Copyright, 2020, published by American Chemical Society.
In addition to the ER, other organelles targeted by PSs can also induce the release of DAMPs under laser irradiation. For instance, mitochondria are the primary site for aerobic respiration and participate in energy supply, signal transduction, and apoptosis []. Wang et al. have demonstrated that 5-aminolevulinic acid (5-ALA) (Scheme 1, Compound 6), as a mitochondrial-targeted PS, was able to substantially increase the expression of CRT, HSP70, and HMGB1, followed by maturation of DCs []. Another important organelle is the lysosome which, as the main recycling organelle of the cell, is associated with digestion and autophagy []. When cells are damaged, the lysosomal membrane will rupture to trigger cell death by initiating the release of tissue proteases and ROS []. Therefore, lysosome-mediated cell death in various forms has received wide attention in recent years. Turubanova et al. [] found that a PS-targeting lysosome named Photosens (Scheme 1, Compound 10) can induce CRT exposure and HMGB1 and ATP release after 635 nm laser irradiation. MCA205 tumor growth was significantly inhibited when the mice were injected with the dead MCA205 cells treated by Photosens-PDT. These results indicated that the specific immune response was successfully triggered. In a word, mitochondria play a more important role in cell death, especially in apoptosis induced by PDT than lysosomes because pro-apoptotic cytochrome c is located in the mitochondria. However, lysosomes have a greater contribution in the immune activation of PDT.
3.2. Dose of PSs
In addition to subcellular localization, the dose of PS also influences PDT-mediated antitumor immunity. For example, PDT-treated of SCC cells with 1 µM OR141 can evoke more release of HSP90 and HMGB1 compared to 10 µM OR141, which indicates that a stronger ICD is induced by low dose of OR141 []. It was further confirmed by in vivo experiments that the growth of SCC tumors is more efficiently inhibited after treatment with 4 mg/kg OR141 in comparison with 40 mg/kg. In addition, Morais et al. showed that the AlPc (Scheme 1, Compound 8) can cause the release of HMGB1 at concentrations of 4.3 nM, 7.8 nM, and 12.2 nM under the same dose of laser irradiation, but the release of ATP only happened at a concentration of 12.2 nM []. It is hypothesized that this is due to a non-linear correlation between PSs dose and ICD effects. Therefore, the dose of PSs is an important parameter for PDT-induced ICD effects. The dose of PSs should be selected to provide maximum activation of the immune effects while minimizing damage to normal cells.
3.3. Light Fluence Rate
The laser energy used in PDT is another factor that may affect the anti-tumor immune response. The excitation of PSs is influenced by the energy density and power density of the laser. These two parameters are capable of influencing PDT-induced inflammatory response and tumor suppression by altering the oxygen concentration in tumor tissues. Henderson et al. compared the inflammatory response induced by different energy density (48 J/cm2, 128 J/cm2) and power density (14 mW/cm2, 112 mW/cm2). They revealed that low power density and low energy density could trigger the stronger inflammatory response []. However, this PDT regimen had poor local tumor suppression. In contrast, the strongest anti-tumor effects as well as the smallest inflammatory response was obtained by high energy density and low power density. In a subsequent study, it was found that high energy density (128 J/cm2) and high power density (224 mW/cm2) displayed the worst tumor suppression effects [], because the oxygen in the tumor tissues were seriously depleted by the higher light energy. The sharp decrease in oxygen concentration within tumor tissues may lead to remarkable reduction in ROS production, which ultimately severely limited the therapeutic efficacy of PDT. To solve this problem, a two-step combination therapy was designed, which combined low energy density (48 J/cm2) and low power density (14 mW/cm2) with high energy density (132 J/cm2) and low power density (14 mW/cm2) []. This combination therapy has significant advantages in enhancing anti-tumor immunity, especially in promoting the recruitment of CD8+ T cells and inhibiting the growth of murine colon and mammary tumors. Therefore, the treatment protocol formulated with optimal light parameters not only significantly inhibits the growth of primary tumors, but also effectively activates anti-tumor immunity.
3.4. Oxygen Content
Recently, many studies have revealed the association between specific immunity of PDT and oxygen content in tumors []. The tumors will develop a hypoxic microenvironment due to the continuous consumption of oxygen during PDT, which can cause the immune suppression of tumors. Therefore, alleviating the hypoxic state of tumors is one of the effective strategies to improve the anti-tumor efficacy of PDT.
Strategy one: constructing a hypoxia-reverse nanosystem to alleviate tumor hypoxia during PDT. As an example, Li et al. developed novel NPs with double ER targeting function []. The NPs consisting of indocyanine green (ICG) (Scheme 1, Compound 9) conjugated-hollow gold nanospheres (named: fAL-ICG-HAuNS) and oxygen-delivering hemoglobin (named: FAL-Hb lipo) are endowed with the ability to target ER after modification with pardaxin (FAL) peptides (Figure 6A). Under hypoxic conditions, the ICG-HAuNS plus Hb-lipo group resulted in approximately 46% cell death compared to only 20% in free ICG, HAuNS, and ICG-HAuNS groups (Figure 6B). Correspondingly, in mice with B16 tumors, fAL-ICG-HAuNS plus FAL-Hb lipo treatment dramatically reduced the proliferation rate of tumors and effectively extended the life of mice. It can be speculated that the ICG-HAuNS plus FAL-Hb lipo treatment can induce 33.1% DC maturation (MHC I+/MHC II+) in the tumors and activate specific effector cells (CD8+ T cells high) (Figure 6C). Therefore, this double ER-targeting strategy was demonstrated to induce anti-tumor immunity and eliminate primary tumors.
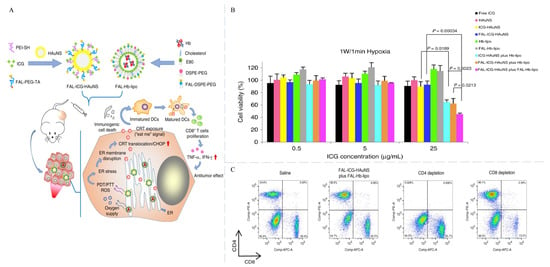
Figure 6.
(A) Schematic of oxygen-enhanced PDT-induced ICD based on ER targeting NPs. (B) CT26 cell viability after different treatments under hypoxic conditions. (C) Flow cytometry showed the percentage of CD8+ T cells and CD4+ T cells in mice spleens []. Copyright, 2019, published by the Author(s).
Liang et al. [] designed a PDT oxygen enhancement generator, which is a core gold nanocage with a shell of manganese dioxide (named: AuNC@MnO2, AM) (Figure 7A). Under laser stimulation, a high level DAMP release and DC maturation (CD83 high, CD86 high) can be triggered by AM-PDT. The immunogenic effects of AuNC@MnO2-PDT were further demonstrated in vivo to effectively inhibit the growth of primary tumors and lung metastases in 4T1-bearing mice (Figure 7B–D). Therefore, the results indicated that oxygen-enhanced PDT serves as a potent pathway for triggering ICD, which dramatically enhances PDT-mediated immune effects by promoting the recruitment of effector T cells.
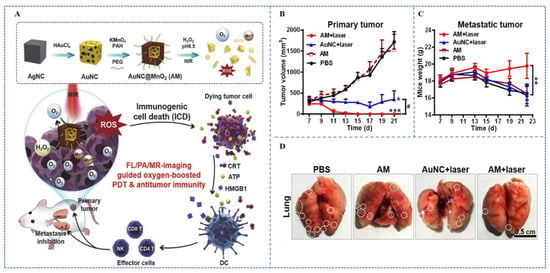
Figure 7.
(A) Schematic of the preparation and function of AuNC@MnO2 as an ICD inducer for oxygen-boosted PDT. (B) Growth curves of primary tumors in mice after different treatments of the 4T1 tumor models. (C) Growth curves of distal tumors in mice after different treatments of the 4T1 tumor models. (D) Counts of the number of metastatic lesions from resected lungs (white spots as a sign of metastatic lung nodules) []. * p < 0.05, ** p < 0.01, *** p < 0.001, # p < 0.05. Copyright, 2018, published by Elsevier.
In addition to improving oxygen delivery efficacy, developing PSs with type I mechanisms to decrease oxygen consumption of PDT is another strategy to improve immunological effect. Compared to the type II mechanism, the type I mechanism of PDT has less oxygen consumption, thereby alleviating the anoxic microenvironment of tumors during PDT []. Huang et al. developed a self-degrading conjugated polyelectrolyte (named: CP+) consisting of aggregation-induced emission (AIE) and imidazole units, which can effectively generate O2•− by a type I mechanism []. At the same time, CP+ was loaded with the immunoadjuvant cytosine-phosphate-guanine (CpG). Compared with other groups, the CP+-CpG NPs group resulted in a significant increase in the proportion of CD4+ T cells and CD8+ T cells, which indicated successful activation of the immune effects and inhibition of primary and metastatic tumors. Chen et al. designed a set of acridinium derivatives with extended D-π-A systems (named: IMA, QMA, MAMA, BMA, and BAMA) []. BAMA-PDT treatment showed significant anti-tumor efficacy in 4T1 tumor models. These results showed that the infiltration of CD8 + T cells increased 4.8 fold and the proportion of Treg cells decreased in the BAMA-PDT group compared with the PBS group in 4T1 tumors. Therefore, the type I mechanism of PDT can mitigate the decreased anti-tumor immune effects caused by hypoxia.
3.5. Immune System
In addition to the above effectors, the integrity of the patient’s immune system function is crucial for long-term inhibition of tumor growth. Korbelik et al. found that the majority of tumors with neutrophil-depleting treatment by Photofrin (Scheme 1, Compound 2)-PDT recurred at 2–3 weeks, although the immediate therapeutic effect was unaffected significantly []. This may be due to that immune deficiency commonly accompanies abnormal function of immune cells, such as T lymphocytes and B lymphocytes. Cytotoxic T lymphocytes are integral parts of the adaptive immune system which play an important role in the direct killing of tumor cells []. It was confirmed by further experiments that after depletion of CD8+ T cells, PDT treatment reduced the tumor cure rate from 100% to 50%, with a more significantly impact than CD4+ T cells depletion. In addition to specific immune cells, the activation of innate cells are also essential for immune effects []. Korbelik et al. detected that the growth of tumors in mice with severe combined immune deficiencycould not be effectively inhibited by PDT []. And, with the depletion of natural killer cells (NK cells), the anti-tumor effect of PDT in mice of immune deficiency was noticeably diminished. Therefore, the integrity of immune system plays a critical role in PDT-mediated immune effects.
4. PDT Combined with Other Therapies
Although it has been demonstrated that PDT can trigger immune effects in vivo, the efficacy is often limited by negative factors such as the tumor hypoxic microenvironment and tumor immune escape []. At present, PDT in combination with DC vaccines, immune checkpoint inhibitors, chemotherapy, and radiotherapy has been proposed to improve the anti-tumor effect [,,].
4.1. PDT Combines with DC Vaccines
Dendritic cells are powerful antigen-presenting cells that can efficiently present antigens to T and B lymphocytes []. Many studies have suggested that injecting DC vaccines into patients can induce T lymphocyte activation and IL-12 expression [,]. The ICD induced by PDT can achieve the establishment of DC vaccines []. Garg et al. produced a DC vaccine based on Hyp-PDT, which can promote the infiltration of T lymphocytes (CD8+ T cells, CD4+ T cells) and reduce the number of Treg cells in GL261 tumors []. The results indicate that Hyp-PDT-based DC vaccines can lead to the alleviation of the immunosuppressive microenvironment. Zhang et al. found that DC vaccines made from ALA-PDT can enhance the activity of effector T cells (CD8+ T cellshigh, CD4+ T cellshigh) and promote the release of cytokines (IL-12high, IFN-γhigh) in mice with PECA tumors []. The same results were also found in another study with the H22 vaccine prepared by hematoporphyrin monomethyl ether (HMME)-PDT (Scheme 1, Compound 11). Compared with the control group, the H22 vaccine group induced an increased number of CD4+CD8+CD19+ T cells and significantly inhibited tumor growth []. Therefore, the DC vaccine produced by PDT can effectively ameliorate the immune suppression microenvironment, thereby inhibiting tumor growth and enhancing survival rate.
4.2. PDT Combines with Immune Checkpoint Inhibitors
The inhibitory effects of the immune system on tumors are affected by receptors and ligands on tumors and immune cells. These receptors and their ligands are called immune checkpoints, which will be upregulated in tumor microenvironment to resist anti-tumor immunity []. Therefore, the blockade of immune checkpoint can improve the recognition of the immune system to tumor cells. In order to enhance the therapeutic efficacy of immune checkpoint blockade (ICB), the combination of immune checkpoint inhibitors-PDT has been extensively studied []. The details of the PSs and the types of immune checkpoint inhibitors are summarized in Table 3. As an example, Duan et al. loaded PS pyrolipid in the Zn-pyrophosphate (ZnP) NPs (Figure 8A), ZnP@pyro-PDT treatment sensitizes tumors to immunotherapy mediated by PD-L1 antibodies []. The combination of ZnP@pyro-PDT with PD-L1 checkpoint blockade therapy can increase the infiltration of T cells (CD8+ T cellshigh, CD4+ T cellshigh) (Figure 8D) and significantly eradicate the growth of primary tumors and prevent lung metastases in 4T1 mice models (Figure 8B,C).

Table 3.
Photodynamic therapy and immune checkpoint blockade.
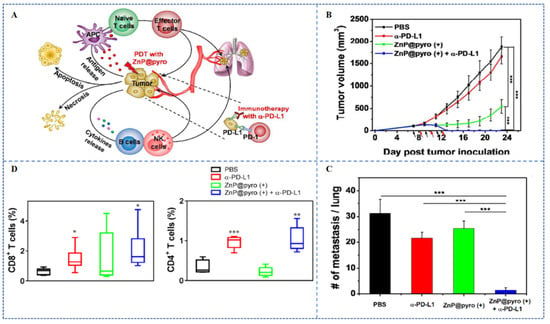
Figure 8.
(A) Schematic of the anti-tumor effect of ZnP@pyro. (B) Growth curves of tumors after different treatments in the 4T1 tumor models. (C) The numbers of tumor nodules in the lungs of mice. (D) Flow cytometry showed the percentage of CD8+ T cells and CD4+ T cells in mice metastases after different treatments, * p < 0.05, ** p < 0.01, *** p < 0.001 []. Copyright, 2016, published by American Chemical Society.
Choi et al. developed the NPs LT-NPs, which is self-assembled from the PS verteporfin (VPF) (Scheme 1, Compound 7), cathepsin B-specific cleavable peptide (FRRG) and doxorubicin (DOX) conjugates (Figure 9A) []. As a visible-light-triggered prodrug, the NPs (LT-NPs) can transform the tumor immune suppressive microenvironment into one with high immunogenicity, thereby enhancing checkpoint blocking immunotherapy. In the model of bilateral CT26 tumor-bearing mice, the combination of LT-NPs and PD-L1 checkpoint blockade therapy had shown significant advantages in inhibiting tumor growth. The combined therapy completely regressed the tumors within 100 days, and effectively alleviated the lung metastasis of tumors (Figure 9B,C). It is worth noting that ICB combined with PDT treatment is usually accompanied by multiple injections of immune checkpoint inhibitors and even multiple laser irradiation [].
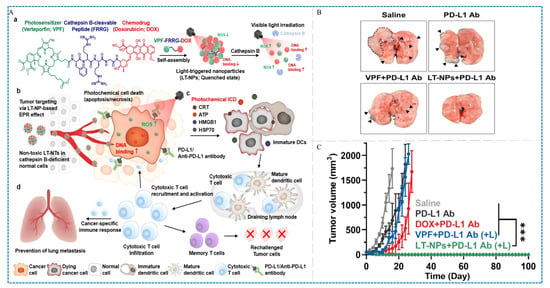
Figure 9.
(A) Schematic of the preparation, function, and anti-tumor effect of LT-NTs. (B) Growth curves of tumors after different treatments in the CT26 tumor models (arrows: metastatic tumor nodules). (C) Image of lung metastasis at day 20 after tumor inoculation, *** p < 0.001 []. Copyright, 2021, published by American Chemical Society.
4.3. PDT Combines with Chemotherapy
Chemotherapy (chem), surgery, and radiotherapy are known as the three major treatment methods for tumors. Some chemotherapy drugs, such as doxorubicin [], idarubicin [], and oxaliplatin [], have been shown to cause immunogenic cell death. Therefore, chem/PDT-induced ICD can trigger a systemic anti-tumor immune affect and reverse the immunosuppressive microenvironment of the tumors. The relevant studies on chem/PDT are summarized in Table 4. Yao et al. synthesized a self-cascading unimolecular prodrug (named AIE-pep-DOX), which is formed by coupling doxorubicin and the aggregation-induced emission PS to a caspase-3 response peptide []. Compared to the control group, in 4T1 murine models, the AIE-pep-DOX group can alleviate the tumor suppressor microenvironment by increasing the percentage of cytotoxic T cells (CD8+ T cellshigh, Tregslow), thereby inhibiting tumor growth. Hypoxia and high GSH are typical features of the tumor-suppressing microenvironment, which seriously affects the therapeutic efficacy of PDT by reducing ROS production. Wang et al. constructed a multicomponent supramolecular nanomedicine (named NPCe6/Pt) which combined β-cd modified chlorine e6, cisplatin and hydrophilic poly (oligoethylene glycol) methacrylate []. NPCe6/Pt can be used for GSH depletion, that is, triggering azo cleavage under hypoxic conditions. Therefore, NPCe6/Pt induced more DAMP release, further enhanced immunogenicity, and improved the DC maturation rate from 5.2% to 28.8% in 4T1 tumor. The percentage of CD8+ T cells and CD4+ T cells increased 1.35 fold and 1.26 fold, respectively, in the NPCe6/Pt group. In conclusion, the chem–PDT combination strategy will shed light on enhancing the immunogenicity of the tumors and improving the efficacy of PDT.

Table 4.
Photodynamic therapy and chemotherapy.
5. Conclusions and Perspectives
PDT not only kills primary tumors but also inhibits metastatic tumors by mediating both innate and specific immunity of the body. PDT-induced immune response is influenced by the localization and dosage of PSs in cells, parameters of light, concentration of oxygen in tumors, and integrity of immune function. To figure out the relationship between these influencing factors and the immune effect of PDT is very important, which assists physicians to make an optimum treatment plan to patients. Therefore, we summarized and analyzed the influencing factors of PDT-induced anti-tumor immunity in this review. Furthermore, the future direction and challenges of anti-tumor immunity induced by PDT are discussed.
Firstly, ER plays a very important role in inducing immunogenic death of tumor cells. Therefore, the development of ER-targeting PSs is an important option to enhance the anti-tumor immunity of PDT. The biosafety of new PS- or NP-packaged PSs should be further evaluated by sufficient pharmacokinetic research. Secondly, the hypoxia of tumors severely inhibits the PDT-mediated ICD effect. Therefore, the development of type I PSs with oxygen non-dependence is another strategy to improve the anti-tumor immunity of PDT. Improvement of the synthetic efficiency and quantum yield of type I PSs is an urgent problem to be solved. Finally, the combination of PDT and other immunotherapy strategies is a promising direction of improvement of outcome of PDT. Although the synergistic immune effect of combination therapies has been demonstrated by many preclinical studies, only a few combined strategies have progressed to the clinical stage. Meanwhile, the safety of the combination therapy also needs to be evaluated. Anti-tumor immunity is the most accurate and effective tumor-killing strategy. We believe that anti-tumor immunity induced by PDT will benefit more patients after comprehensive consideration of the influencing factors, such as improving the anti-tumor immunological effect through combination of PDT with PD1/PDL1, balancing the elimination efficacy to local tumors and the killing efficacy to metastatic tumors of PDT through regulating the dosage of irradiation and PSs.
Author Contributions
Conceptualization, W.C., D.C. and H.Z.; writing—original draft preparation, W.C., T.S., H.Q. and H.Z.; writing—review and editing, W.C., T.S., H.Q. and H.Z.; formal analysis, W.C., N.P., Z.W. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFA1207600), the Major Program of the National Natural Science Foundation of China (T2293750, T2293753), the Beijing Institute of Technology Research Fund Program for Young Scholars (XSQD-202023003), and the CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5–061).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucena, S.R.; Salazar, N.; Gracia-Cazana, T.; Zamarron, A.; Gonzalez, S.; Juarranz, A.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef] [PubMed]
- Togsverd-Bo, K.; Sandberg, C.; Helsing, P.; Mørk, G.; Wennberg, A.M.; Wulf, H.C.; Haedersdal, M. Cyclic photodynamic therapy delays first onset of actinic keratoses in renal transplant recipients: A 5-year randomized controlled trial with 12-month follow-up. J. Eur. Acad. Dermatol. Venereol. 2022, 36, E946–E948. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem. Photobiol. Sci. 2014, 13, 474–487. [Google Scholar] [CrossRef]
- De Santana, W.M.O.S.; Surur, A.K.; Momesso, V.M.; Lopes, P.M.; Santilli, C.V.; Fontana, C.R. Nanocarriers for photodynamic-gene therapy. Photodiagn. Photodyn. Ther. 2023, 43, 103644. [Google Scholar] [CrossRef]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; Piccionello, A.P.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; et al. New tricyclic systems as photosensitizers towards triple negative breast cancer cells. Arch. Pharmacal Res. 2022, 45, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Moserova, I.; Kralova, J. Role of ER stress response in photodynamic therapy: ROS generated in different subcellular compartments trigger diverse cell death pathways. PLoS ONE 2012, 7, e32972. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheung, Y.K.; Ng, D.K.P.; Fong, W.P. Enhancement of innate and adaptive anti-tumor immunity by serum obtained from vascular photodynamic therapy-cured BALB/c mouse. Cancer Immunol. Immunother. 2021, 70, 3217–3233. [Google Scholar] [CrossRef]
- Nkune, N.W.; Simelane, N.W.N.; Montaseri, H.; Abrahamse, H. Photodynamic Therapy-Mediated Immune Responses in Three-Dimensional Tumor Models. Int. J. Mol. Sci. 2021, 22, 12618. [Google Scholar] [CrossRef]
- Kick, G.; Messer, G.; Goetz, A.; Plewig, G.; Kind, P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kappa B DNA binding. Cancer Res. 1995, 55, 2373–2379. [Google Scholar] [PubMed]
- Volanti, C.; Matroule, J.Y.; Piette, J. Involvement of oxidative stress in NF-kappaB activation in endothelial cells treated by photodynamic therapy. Photochem. Photobiol. 2002, 75, 36–45. [Google Scholar] [CrossRef]
- de Vree, W.J.A.; Essers, M.C.; Koster, J.F.; Sluiter, W. Role of Interleukin 1 and Granulocyte Colony-Stimulating Factor in Photofrin-based Photodynamic Therapy of Rat Rhabdomyosarcoma Tumors1. Cancer Res. 1997, 57, 2555–2558. [Google Scholar] [PubMed]
- Sun, J.; Cecic, I.; Parkins, C.S.; Korbelik, M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 2002, 1, 690–695. [Google Scholar] [CrossRef]
- Kousis, P.C.; Henderson, B.W.; Maier, P.G.; Gollnick, S.O. Photodynamic Therapy Enhancement of Antitumor Immunity Is Regulated by Neutrophils. Cancer Res. 2007, 67, 10501–10510. [Google Scholar] [CrossRef] [PubMed]
- Stott, B.; Korbelik, M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol. Immunother. 2007, 56, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Cecic, I.; Serrano, K.; Gyongyossy-Issa, M.; Korbelik, M. Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett. 2005, 225, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Krysko, D.V.; Verfaillie, T.; Kaczmarek, A.; Ferreira, G.B.; Marysael, T.; Rubio, N.; Firczuk, M.; Mathieu, C.; Roebroek, A.J.; et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012, 31, 1062–1079. [Google Scholar] [CrossRef]
- Garg, A.D.; Dudek, A.M.; Ferreira, G.B.; Verfaillie, T.; Vandenabeele, P.; Krysko, D.V.; Mathieu, C.; Agostinis, P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 2013, 9, 1292–1307. [Google Scholar] [CrossRef]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef]
- Garg, A.D.; Krysko, D.V.; Golab, J.; Vandenabeele, P.; Agostinis, P. Contribution of ER Stress to Immunogenic Cancer Cell Death. In Endoplasmic Reticulum Stress in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2012; pp. 413–428. [Google Scholar]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Yan, X.; Wei, J.; Wu, Z.; Wang, Y.; Han, W. Inducing immunogenic cell death in immuno-oncological therapies. Chin. J. Cancer Res. 2022, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Panaretakis, T.; Kepp, O.; Brockmeier, U.; Tesniere, A.; Bjorklund, A.C.; Chapman, D.C.; Durchschlag, M.; Joza, N.; Pierron, G.; van Endert, P.; et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009, 28, 578–590. [Google Scholar] [CrossRef]
- Martins, I.; Kepp, O.; Schlemmer, F.; Adjemian, S.; Tailler, M.; Shen, S.; Michaud, M.; Menger, L.; Gdoura, A.; Tajeddine, N.; et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2011, 30, 1147–1158. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Manfredi, A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007, 220, 35–46. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Jalili, A.; Makowski, M.; Switaj, T.; Nowis, D.; Wilczynski, G.M.; Wilczek, E.; Chorazy-Massalska, M.; Radzikowska, A.; Maslinski, W.; Bialy, L.; et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004, 10, 4498–4508. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic Therapy–Induced Cell Surface Expression and Release of Heat Shock Proteins: Relevance for Tumor Response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Lan, G.; Culbert, A.; Song, Y.; Wu, T.; Jiang, X.; Lin, W. A Nanoscale Metal-Organic Framework to Mediate Photodynamic Therapy and Deliver CpG Oligodeoxynucleotides to Enhance Antigen Presentation and Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 1108–1112. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.K.; Castano, A.P.; Mroz, P.; Avci, P.; Hamblin, M.R. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J. Biophotonics 2014, 7, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Vummidi, B.R.; Noreen, F.; Alzeer, J.; Moelling, K.; Luedtke, N.W. Photodynamic Agents with Anti-metastatic Activities. ACS Chem. Biol. 2013, 8, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, Y.; Zou, L.; Wang, L.; Chen, H.H.; Cai, J.Y.; Xu, J.M.; Sosnovik, D.E.; Chao, W. Role of Extracellular RNA and TLR3-Trif Signaling in Myocardial Ischemia–Reperfusion Injury. J. Am. Heart Assoc. 2014, 3, e000683. [Google Scholar] [CrossRef]
- Preissner, K.T.; Fischer, S.; Deindl, E. Extracellular RNA as a Versatile DAMP and Alarm Signal That Influences Leukocyte Recruitment in Inflammation and Infection. Front. Cell Dev. Biol. 2020, 8, 619221. [Google Scholar] [CrossRef]
- Ojcius, D.M.; Noll, F.; Behnke, J.; Leiting, S.; Troidl, K.; Alves, G.T.; Müller-Redetzky, H.; Preissner, K.T.; Fischer, S. Self-extracellular RNA acts in synergy with exogenous danger signals to promote inflammation. PLoS ONE 2017, 12, e0190002. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol. Rev. 2017, 280, 126–148. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Nigro, A.; Casolaro, V.; Rumie Vittar, N.B.; Dal Col, J. Damage-Associated Molecular Patterns Modulation by microRNA: Relevance on Immunogenic Cell Death and Cancer Treatment Outcome. Cancers 2021, 13, 2566. [Google Scholar] [CrossRef]
- Rapoport, B.; Anderson, R. Realizing the Clinical Potential of Immunogenic Cell Death in Cancer Chemotherapy and Radiotherapy. Int. J. Mol. Sci. 2019, 20, 959. [Google Scholar] [CrossRef]
- Vacchelli, E.; Sistigu, A.; Yamazaki, T.; Vitale, I.; Zitvogel, L.; Kroemer, G. Autocrine signaling of type 1 interferons in successful anticancer chemotherapy. Oncoimmunology 2015, 4, e988042. [Google Scholar]
- Fucikova, J.; Moserova, I.; Urbanova, L.; Bezu, L.; Kepp, O.; Cremer, I.; Salek, C.; Strnad, P.; Kroemer, G.; Galluzzi, L.; et al. Prognostic and Predictive Value of DAMPs and DAMP-Associated Processes in Cancer. Front. Immunol. 2015, 6, 402. [Google Scholar] [CrossRef]
- Locy, H.; de Mey, S.; de Mey, W.; De Ridder, M.; Thielemans, K.; Maenhout, S.K. Immunomodulation of the Tumor Microenvironment: Turn Foe Into Friend. Front. Immunol. 2018, 9, 2909. [Google Scholar] [CrossRef] [PubMed]
- Seelige, R.; Searles, S.; Bui, J.D. Mechanisms regulating immune surveillance of cellular stress in cancer. Cell Mol. Life Sci. 2018, 75, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhou, Z.; Yang, W.; Lin, L.S.; Wang, S.; Niu, G.; Song, J.; Chen, X. Endoplasmic Reticulum Targeting to Amplify Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2020, 20, 1928–1933. [Google Scholar] [CrossRef]
- Wang, X.; Ji, J.; Zhang, H.; Fan, Z.; Zhang, L.; Shi, L.; Zhou, F.; Chen, W.R.; Wang, H.; Wang, X. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget 2015, 6, 44688–44702. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci. Rep. 2021, 11, 7205. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shim, M.K.; Yang, S.; Hwang, H.S.; Cho, H.; Kim, J.; Yun, W.S.; Moon, Y.; Kim, J.; Yoon, H.Y.; et al. Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. ACS Nano 2021, 15, 12086–12098. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 350. [Google Scholar] [CrossRef]
- Hanlon, J.G.; Adams, K.; Rainbow, A.J.; Gupta, R.S.; Singh, G. Induction of Hsp60 by Photofrin-mediated photodynamic therapy. J. Photochem. Photobiol. B Biol. 2001, 64, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Aung, T.; Li, S.; Fatuzzo, N.; Liang, X.; Lin, W. Nanoscale Metal-Organic Framework Mediates Radical Therapy to Enhance Cancer Immunotherapy. Chem 2019, 5, 1892–1913. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, N.; Sun, H.; Fu, X.; Zhai, S.; Cui, J. Self-adjuvanting photosensitizer nanoparticles for combination photodynamic immunotherapy. Biomater. Sci. 2021, 9, 6940–6949. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Xu, H.Z.; Xu, Y.H.; Yu, T.T.; Tang, J.M.; Li, K.; Wang, C.; Peng, X.C.; Li, Q.R.; Sang, X.Y.; et al. Efficient Delivery of Chlorin e6 by Polyglycerol-Coated Iron Oxide Nanoparticles with Conjugated Doxorubicin for Enhanced Photodynamic Therapy of Melanoma. Mol. Pharm. 2021, 18, 3601–3615. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chung, C.K.; Gu, Z.; Schomann, T.; Dong, X.; Veld, R.V.H.i.t.; Camps, M.G.M.; ten Dijke, P.; Ossendorp, F.A.; Cruz, L.J. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment. Mol. Biomed. 2022, 3, 26. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Z.; Chen, G.; Li, T.; Peng, Y.; Shuai, X. A pH-sensitive nanomedicine incorporating catalase gene and photosensitizer augments photodynamic therapy and activates antitumor immunity. Nano Today 2022, 43, 101390. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Liang, R.; Luo, Z.; He, H.; Wu, Z.; Tian, H.; Zheng, M.; Ma, Y.; Cai, L. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano 2018, 12, 8633–8645. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef]
- Song, H.; Cai, Z.; Li, J.; Xiao, H.; Qi, R.; Zheng, M. Light triggered release of a triple action porphyrin-cisplatin conjugate evokes stronger immunogenic cell death for chemotherapy, photodynamic therapy and cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 329. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Hu, J.; Li, Q.R.; Peng, X.C.; Xu, H.Z.; Han, N.; Li, L.G.; Yang, X.X.; Xu, X.; Yang, Z.Y.; et al. Chlorin e6-induced photodynamic effect facilitates immunogenic cell death of lung cancer as a result of oxidative endoplasmic reticulum stress and DNA damage. Int. Immunopharmacol. 2023, 115, 109661. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, H.; Luo, Z.; Zhou, H.; Liang, R.; Pan, H.; Ma, Y.; Cai, L. In Situ Photocatalyzed Oxygen Generation with Photosynthetic Bacteria to Enable Robust Immunogenic Photodynamic Therapy in Triple-Negative Breast Cancer. Adv. Funct. Mater. 2020, 30, 1910176. [Google Scholar] [CrossRef]
- Liang, R.; Liu, L.; He, H.; Chen, Z.; Han, Z.; Luo, Z.; Wu, Z.; Zheng, M.; Ma, Y.; Cai, L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Sun, Y.; Wan, C.; Zhang, Z.; Dai, X.; Lin, Z.; He, Q.; Yang, Z.; Huang, P.; et al. Co-delivery of Bee Venom Melittin and a Photosensitizer with an Organic-Inorganic Hybrid Nanocarrier for Photodynamic Therapy and Immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, F.; Deng, H.; Lin, L.; Wang, S.; Kang, F.; Yu, G.; Lau, J.; Tian, R.; Zhang, M.; et al. Smart Nanovesicle-Mediated Immunogenic Cell Death through Tumor Microenvironment Modulation for Effective Photodynamic Immunotherapy. ACS Nano 2020, 14, 620–631. [Google Scholar] [CrossRef]
- Nash, G.T.; Luo, T.; Lan, G.; Ni, K.; Kaufmann, M.; Lin, W. Nanoscale Metal–Organic Layer Isolates Phthalocyanines for Efficient Mitochondria-Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 2194–2199. [Google Scholar] [CrossRef]
- Wang, H.; Jing, G.; Niu, J.; Yang, L.; Li, Y.; Gao, Y.; Wang, H.; Xu, X.; Qian, Y.; Wang, S. A mitochondria-anchored supramolecular photosensitizer as a pyroptosis inducer for potent photodynamic therapy and enhanced antitumor immunity. J. Nanobiotechnol. 2022, 20, 513. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, C.; Wang, H.; Peng, T.; Zhang, L.; Wang, Y.; Han, W.; Shi, C. Mitochondria-Targeting Immunogenic Cell Death Inducer Improves the Adoptive T-Cell Therapy Against Solid Tumor. Front. Oncol. 2019, 9, 1196. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv. Mater. 2019, 31, e1904914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Ma, H.; Sun, Y.; Cao, J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 2022, 12, 4629–4655. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. DAMPs and PDT-mediated photo-oxidative stress: Exploring the unknown. Photochem. Photobiol. Sci. 2011, 10, 670–680. [Google Scholar] [CrossRef]
- Brodin, N.P.; Guha, C.; Tome, W.A. Photodynamic Therapy and Its Role in Combined Modality Anticancer Treatment. Technol. Cancer Res. Treat. 2015, 14, 355–368. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Teiten, M.H.; Bezdetnaya, L.; Morlière, P.; Santus, R.; Guillemin, F. Endoplasmic reticulum and Golgi apparatus are the preferential sites of Foscan® localisation in cultured tumour cells. Br. J. Cancer 2003, 88, 146–152. [Google Scholar] [CrossRef]
- Kessel, D. Death Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2021, 97, 1101–1103. [Google Scholar] [CrossRef]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. The emergence of phox-ER stress induced immunogenic apoptosis. OncoImmunology 2014, 1, 786–788. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.K.K.; Nielsen, I.Ø.; Maeda, K.; Jäättelä, M. SnapShot: Lysosomal Functions. Cell 2020, 181, 748.e741. [Google Scholar] [CrossRef]
- Doix, B.; Trempolec, N.; Riant, O.; Feron, O. Low Photosensitizer Dose and Early Radiotherapy Enhance Antitumor Immune Response of Photodynamic Therapy-Based Dendritic Cell Vaccination. Front. Oncol. 2019, 9, 811. [Google Scholar] [CrossRef]
- Morais, J.A.V.; Almeida, L.R.; Rodrigues, M.C.; Azevedo, R.B.; Muehlmann, L.A. The induction of immunogenic cell death by photodynamic therapy in B16F10 cells in vitro is effected by the concentration of the photosensitizer. PhotoDiagn. Photodyn. Ther. 2021, 35, 102392. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease. Cancer Immunol. Immunother. 2015, 64, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, L.; Tang, H.; Cao, D. Recent advances in type I organic photosensitizers for efficient photodynamic therapy for overcoming tumor hypoxia. J. Mater. Chem. B 2023, 11, 4600–4618. [Google Scholar] [CrossRef]
- Huang, H.; Xie, W.; Hu, D.; He, X.; Li, R.; Zhang, X.; Wei, Y. Type I photodynamic therapy with a self-degradable conjugated polyelectrolyte in combination with CpG adjuvant for cancer immunotherapy. Chem. Eng. J. 2023, 451, 138617. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Yi, H.B.; Wang, F.; Chu, X.; Jiang, J.H. Type I Photosensitizer Targeting G-Quadruplex RNA Elicits Augmented Immunity for Cancer Ablation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300162. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Cecic, I. Contribution of myeloid and lymphoid host cells to the curative outcome of mouse sarcoma treatment by photodynamic therapy. Cancer Lett. 1999, 137, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2018, 234, 8509–8521. [Google Scholar] [CrossRef]
- Nowarski, R.; Gagliani, N.; Huber, S.; Flavell, R.A. Innate Immune Cells in Inflammation and Cancer. Cancer Immunol. Res. 2013, 1, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Sang, W.; Xie, L.; Wang, G.; Li, J.; Zhang, Z.; Li, B.; Guo, S.; Deng, C.X.; Dai, Y. Oxygen-Enriched Metal-Phenolic X-Ray Nanoprocessor for Cancer Radio-Radiodynamic Therapy in Combination with Checkpoint Blockade Immunotherapy. Adv. Sci. 2020, 8, 2003338. [Google Scholar] [CrossRef]
- Ni, K.; Lan, G.; Song, Y.; Hao, Z.; Lin, W. Biomimetic nanoscale metal–organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chem. Sci. 2020, 11, 7641–7653. [Google Scholar] [CrossRef]
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Cui, W.; Joshi, N.S.; Jiang, A.; Kaech, S.M. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 2009, 27, 2177–2187. [Google Scholar] [CrossRef]
- Saxena, M.; Bhardwaj, N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Vandenberk, L.; Koks, C.; Verschuere, T.; Boon, L.; Van Gool, S.W.; Agostinis, P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci. Transl. Med. 2016, 8, 328ra327. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Wang, X.; Shi, L.; Fan, Z.; Zhang, G.; Yang, D.; Bahavar, C.F.; Zhou, F.; Chen, W.R.; et al. Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice. Technol. Cancer Res. Treat. 2018, 17, 1533033818785275. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Ma, W.J.; Zou, Z.H.; Gao, W.P.; Xue, Z.X.; Li, Y.X. Generation of Antitumor Vaccines for H22 Tumor on Mouse Using Photodynamic Therapy. Chin. J. Lasers 2008, 34, 631–634. [Google Scholar] [CrossRef]
- Cramer, G.M.; Moon, E.K.; Cengel, K.A.; Busch, T.M. Photodynamic Therapy and Immune Checkpoint Blockade(dagger). Photochem. Photobiol. 2020, 96, 954–961. [Google Scholar] [CrossRef]
- Jin, F.; Liu, D.; Xu, X.; Ji, J.; Du, Y. Nanomaterials-Based Photodynamic Therapy with Combined Treatment Improves Antitumor Efficacy Through Boosting Immunogenic Cell Death. Int. J. Nanomed. 2021, 16, 4693–4712. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, Z.; Huang, J.; Wang, Y.; An, Y.; Xiao, H.; Peng, Y.; Pang, P.; Han, S.; Zhu, K.; et al. Dual-Sensitive PEG-Sheddable Nanodrug Hierarchically Incorporating PD-L1 Antibody and Zinc Phthalocyanine for Improved Immuno-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 12845–12856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sang, W.; Xie, L.; Li, W.; Li, B.; Li, J.; Tian, H.; Yuan, Z.; Zhao, Q.; Dai, Y. Polyphenol-Based Nanomedicine Evokes Immune Activation for Combination Cancer Treatment. Angew. Chem. Int. Ed. Engl. 2021, 60, 1967–1975. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Xu, Y.; Ren, X.; Zhou, S.; Shang, Q.; Jiang, Y.; Luan, Y. Molecular engineering of anti-PD-L1 peptide and photosensitizer for immune checkpoint blockade photodynamic-immunotherapy. Chem. Eng. J. 2020, 400, 125995. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Jiang, W.; Kumar, A.; Zhou, S.; Cao, Z.; Zhan, S.; Yang, W.; Liu, R.; Teng, Y.; et al. Nanoconjugates to enhance PDT-mediated cancer immunotherapy by targeting the indoleamine-2,3-dioxygenase pathway. J. Nanobiotechnol. 2021, 19, 182. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal–Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef]
- Sasaki, M.; Tanaka, M.; Kojima, Y.; Nishie, H.; Shimura, T.; Kubota, E.; Kataoka, H. Anti-tumor immunity enhancement by photodynamic therapy with talaporfin sodium and anti-programmed death 1 antibody. Mol. Ther. Oncolytics 2023, 28, 118–131. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Q.; Zhang, X.; Tiemuer, A.; Wang, J.; Zhang, Y.; Sun, X.; Liu, Y. Hot-band absorption assisted single-photon frequency upconversion luminescent nanophotosensitizer for 808 nm light triggered photodynamic immunotherapy of cancer. Biomater. Sci. 2023, 11, 2167–2176. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Z.; Liu, Z.; Fu, X.; Song, H.; Zhang, Q. Self-delivery photodynamic-hypoxia alleviating nanomedicine synergizes with anti-PD-L1 for cancer immunotherapy. Int. J. Pharm. 2023, 639, 122970. [Google Scholar] [CrossRef]
- Zhao, L.; Rao, X.; Zheng, R.; Huang, C.; Kong, R.; Yu, X.; Cheng, H.; Li, S. Targeting glutamine metabolism with photodynamic immunotherapy for metastatic tumor eradication. J. Control. Release 2023, 357, 460–471. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; Zhang, J.; Liu, Y.; Ning, J.; Yang, J.; Zhang, Z.; Hou, L.; Chen, X. In vivo activated T cell targeting with PD-1/PD-L1 blockade for sequential treatment mediated cancer immunotherapy. Nano Today 2022, 44, 101492. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, X.; Wang, J.; Fan, Y.; Ren, M.; Zhang, H. Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies. Cancer Lett. 2018, 438, 17–23. [Google Scholar] [CrossRef]
- Garg, A.D.; More, S.; Rufo, N.; Mece, O.; Sassano, M.L.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology 2017, 6, e1386829. [Google Scholar] [CrossRef]
- Kepp, O.; Menger, L.; Vacchelli, E.; Locher, C.; Adjemian, S.; Yamazaki, T.; Martins, I.; Sukkurwala, A.Q.; Michaud, M.; Senovilla, L.; et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013, 24, 311–318. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Y.; Bian, K.; Zhang, B.; Wang, D. A self-cascaded unimolecular prodrug for pH-responsive chemotherapy and tumor-detained photodynamic-immunotherapy of triple-negative breast cancer. Biomaterials 2023, 292, 121920. [Google Scholar] [CrossRef]
- Wang, D.; Ma, W.; Huang, Y.; Wang, W.; Li, S.; Liu, H.; Zhao, Y.; Peng, D.; Yu, C.-Y.; Wei, H. Supramolecular nanoassemblies-mediated GSH depletion boosts synergistic chemo- and photodynamic therapy for immunogenicity enhancement. Chem. Eng. J. 2023, 468, 143731. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.Y. Multi-shell structured upconversion nanocarriers that combine IDO inhibitor-induced immunotherapy with NIR-triggered photodynamic therapy for deep tumors. Biomater. Sci. 2023, 11, 4684–4699. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, T.; Zhao, X.; Du, J.; Sun, W.; Fan, J.; Peng, X. Tumor Cell-Responsive Photodynamic Immunoagent for Immunogenicity-Enhanced Orthotopic and Remote Tumor Therapy. Adv. Healthc. Mater. 2023, 12, e2202085. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Luo, W.; Liu, Y.; Chen, J.; Deng, H.; Zhou, Z.; Shen, J. Killing three birds with one stone: Multi-stage metabolic regulation mediated by clinically usable berberine liposome to overcome photodynamic immunotherapy resistance. Chem. Eng. J. 2023, 454, 140164. [Google Scholar] [CrossRef]
- Wang, B.; Tang, D.; Karges, J.; Cui, M.; Xiao, H. A NIR-II Fluorescent PolyBodipy Delivering Cationic Pt-NHC with Type II Immunogenic Cell Death for Combined Chemotherapy and Photodynamic Immunotherapy. Adv. Funct. Mater. 2023, 33, 2214824. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Xu, H.; Zou, B.; Chen, W.; Liu, Y.; Chen, J.; Zhang, R. Nanoscale metal-organic framework delivers rapamycin to induce tissue immunogenic cell death and potentiates cancer immunotherapy. Nanomedicine 2023, 50, 102678. [Google Scholar] [CrossRef]
- Wu, H.; Du, X.; Xu, J.; Kong, X.; Li, Y.; Liu, D.; Yang, X.; Ye, L.; Ji, J.; Xi, Y.; et al. Multifunctional biomimetic nanoplatform based on photodynamic therapy and DNA repair intervention for the synergistic treatment of breast cancer. Acta Biomater. 2023, 157, 551–565. [Google Scholar] [CrossRef]
- Mai, Z.; Zhong, J.; Zhang, J.; Chen, G.; Tang, Y.; Ma, W.; Li, G.; Feng, Z.; Li, F.; Liang, X.J.; et al. Carrier-Free Immunotherapeutic Nano-Booster with Dual Synergistic Effects Based on Glutaminase Inhibition Combined with Photodynamic Therapy. ACS Nano 2023, 17, 1583–1596. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).