Characterization and Bio-Evaluation of the Synergistic Effect of Simvastatin and Folic Acid as Wound Dressings on the Healing Process
Abstract
:1. Introduction
2. Materials and Methods
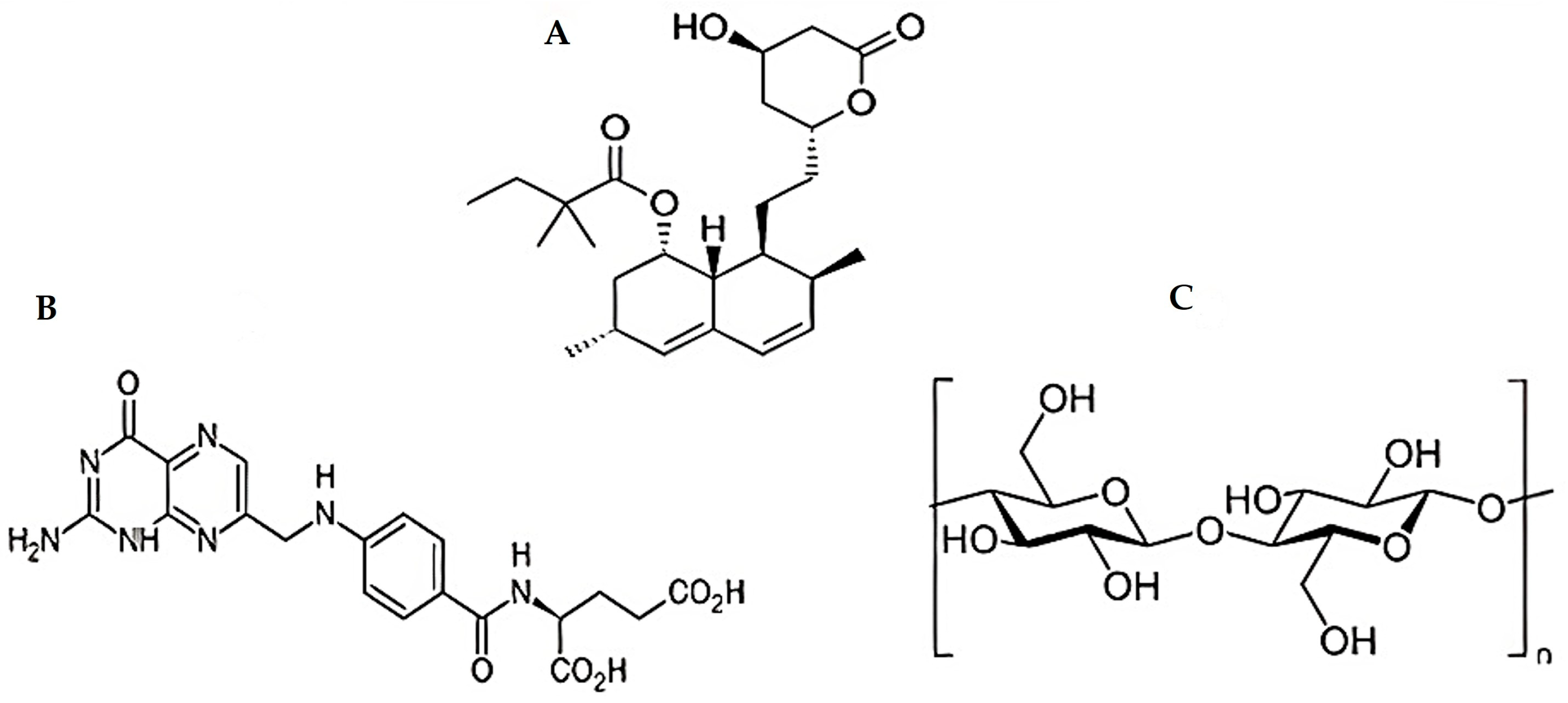
2.1. Materials
2.2. Animals and Ethical Approval
2.3. Methods
2.3.1. Preparation of SMV-FA Polymeric Films
2.3.2. Experimental Design
2.3.3. Characterization of Prepared SMV-FAPFs
Films Expansion Profile (Expansion%)
Tensile Strength
In Vitro Release Study
Thermal Analysis and Infrared Spectroscopy
X-ray Diffraction Studies
Microscopic Examination
Weight Variation
Folding Endurance
Film Thickness
Drug Content
2.3.4. Efficient Formula Selection
2.3.5. In Vivo Study
Animal Experiments
2.3.6. Histopathological Evaluation
2.3.7. CoTI and JAK3 RNA Isolation and Real-Time PCR (RT-PCR) Investigation for Skin Biopsies
3. Results and Discussion
3.1. Fabrication of SMV-FA Polymeric Film
3.2. DSC Studies
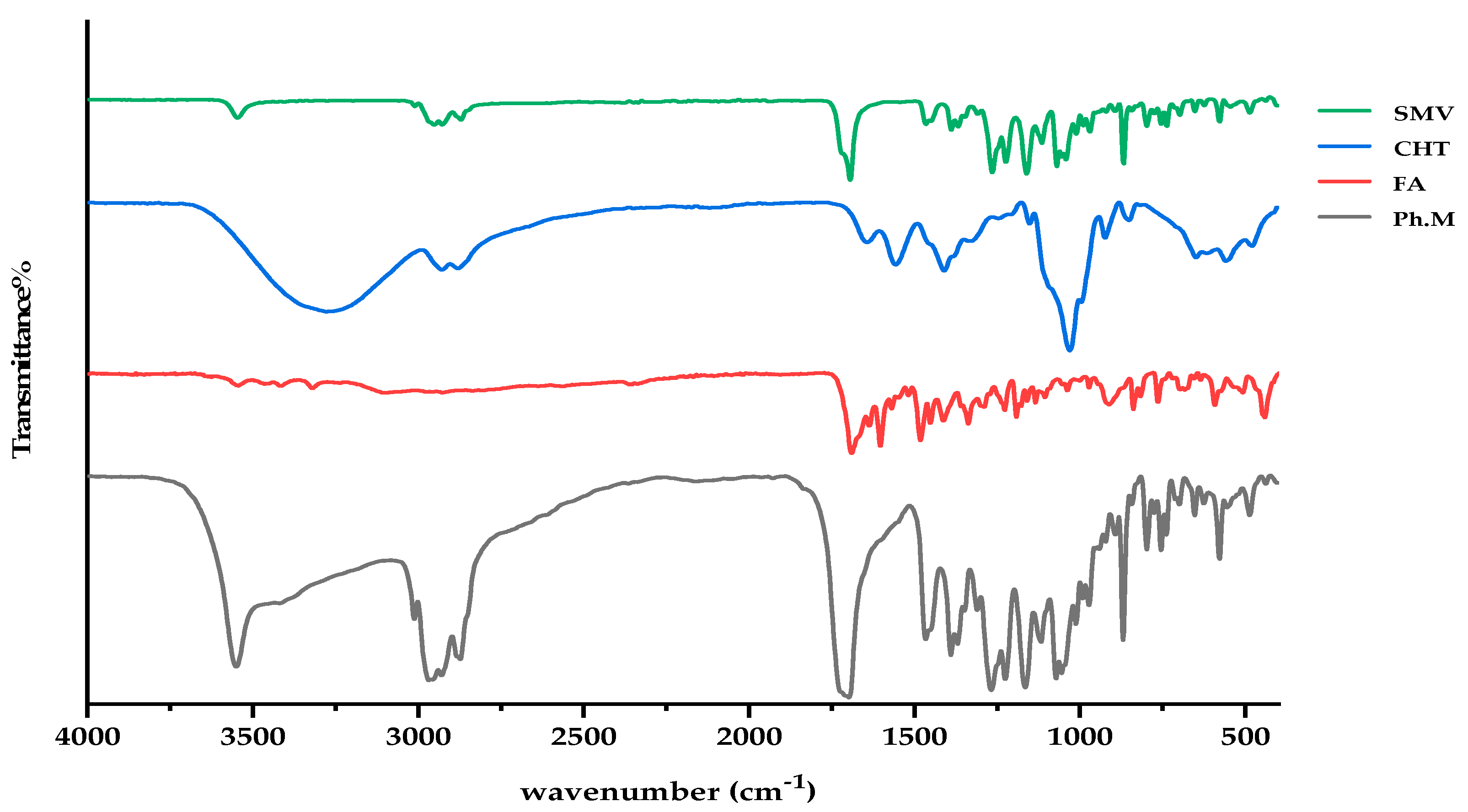
3.3. FTIR Investigations
3.4. X-ray Diffraction (XRD)
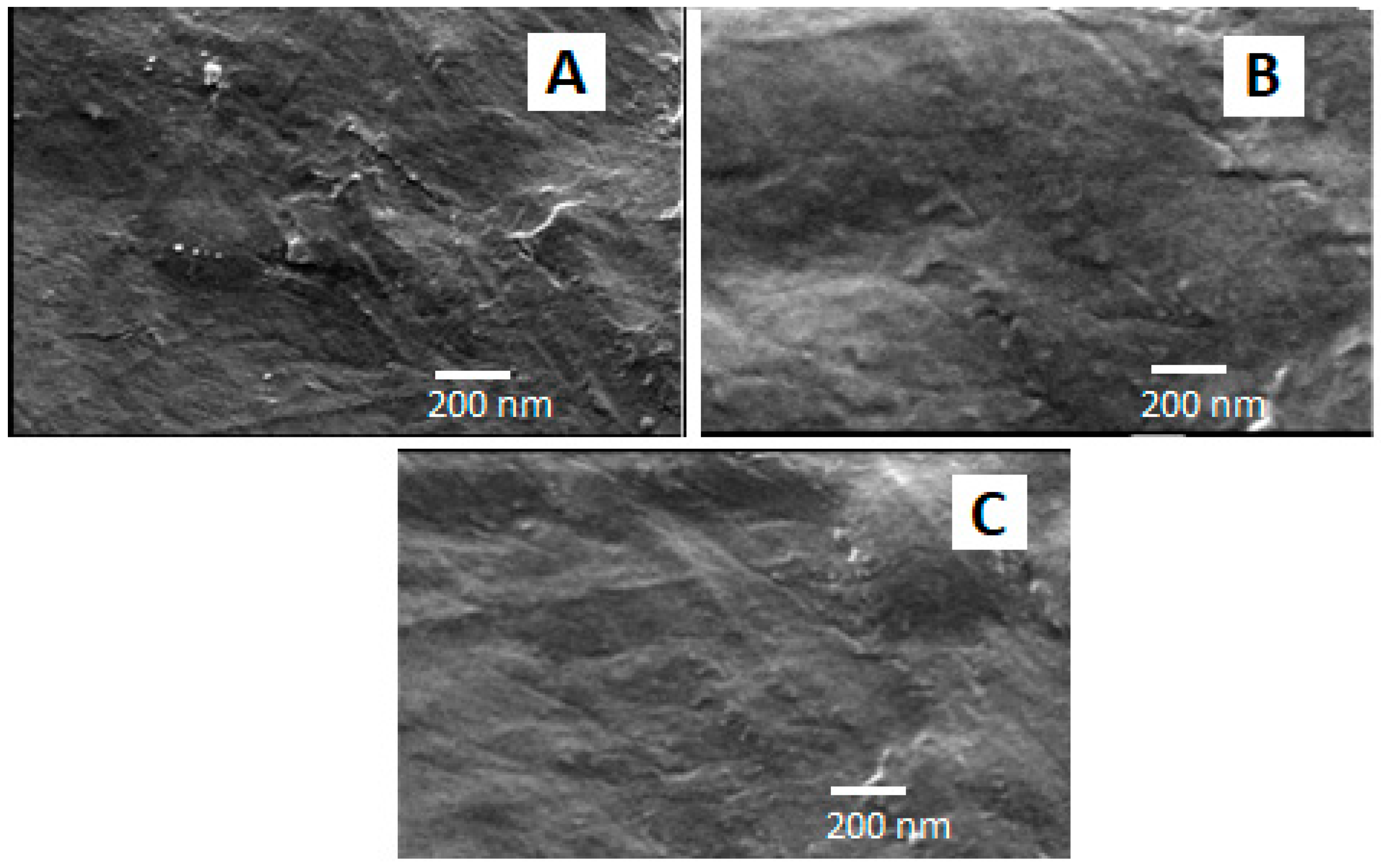
3.5. Scanning Electron Microscope
3.6. Response Surface Methodology and Optimization of Formulation Factors
3.6.1. Tensile Strength
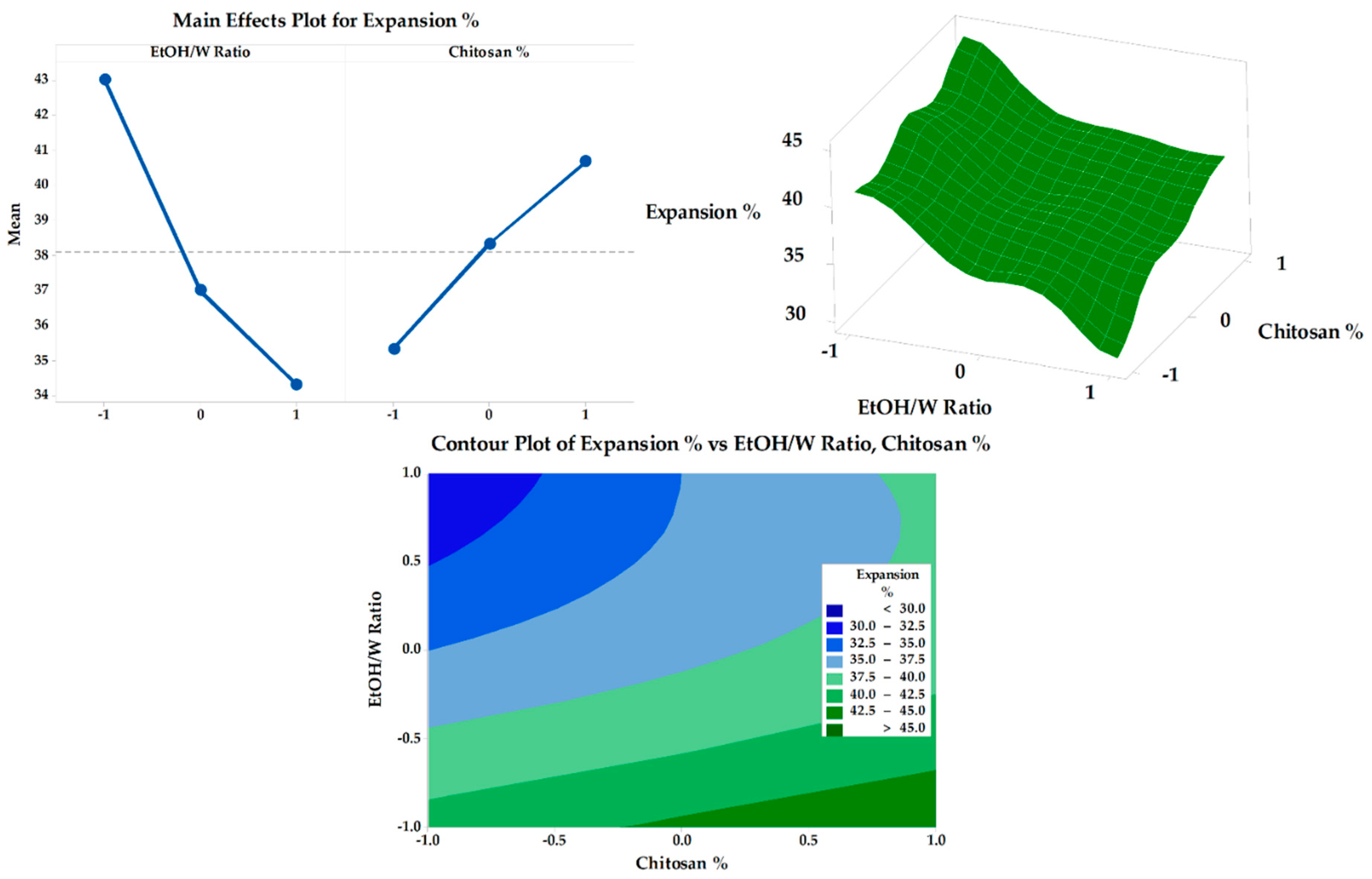
3.6.2. Expansion Percentage
3.6.3. In Vitro SMV-FAPFs Release
3.7. Physical and Mechanical Characterization of the Prepared Films
3.8. In Vivo Study
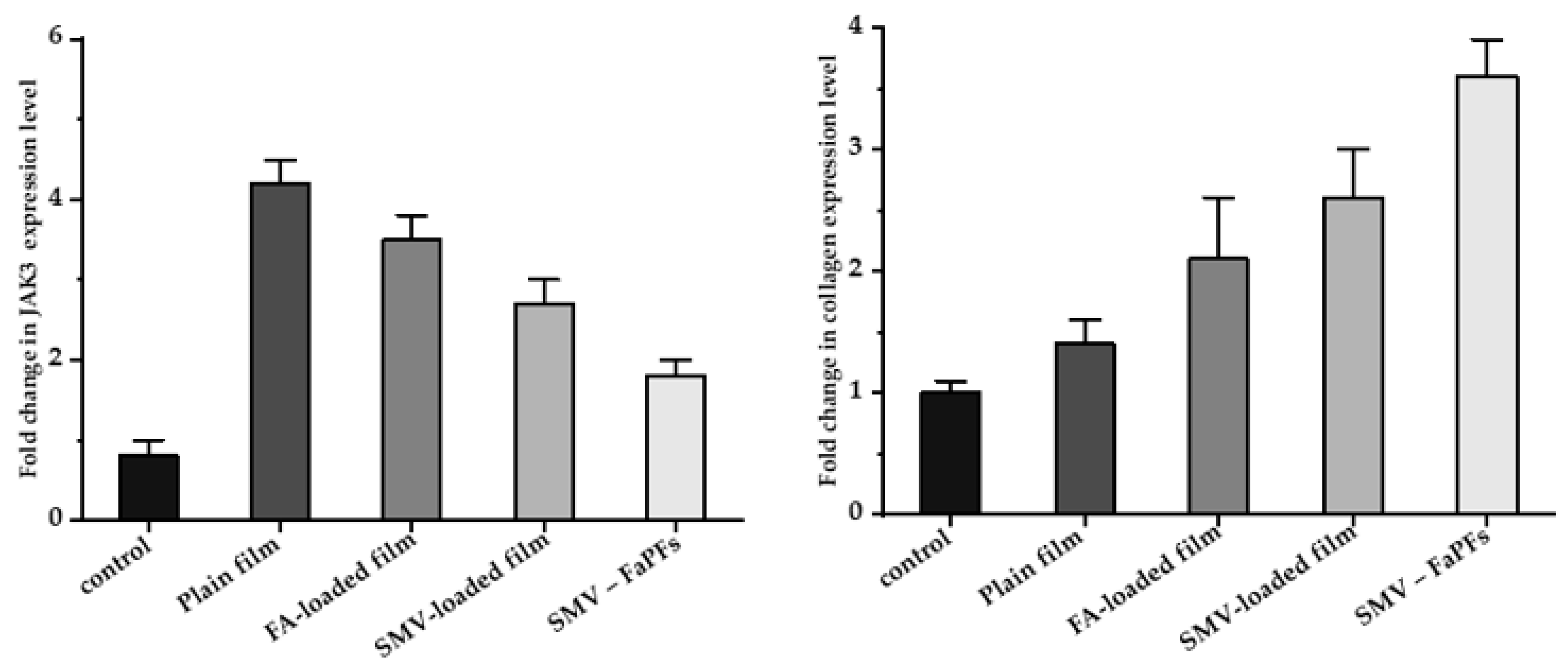
3.9. RNA Isolation and Real-Time PCR (RT-PCR) Analysis of CoTI and JAK3 in Skin Biopsies
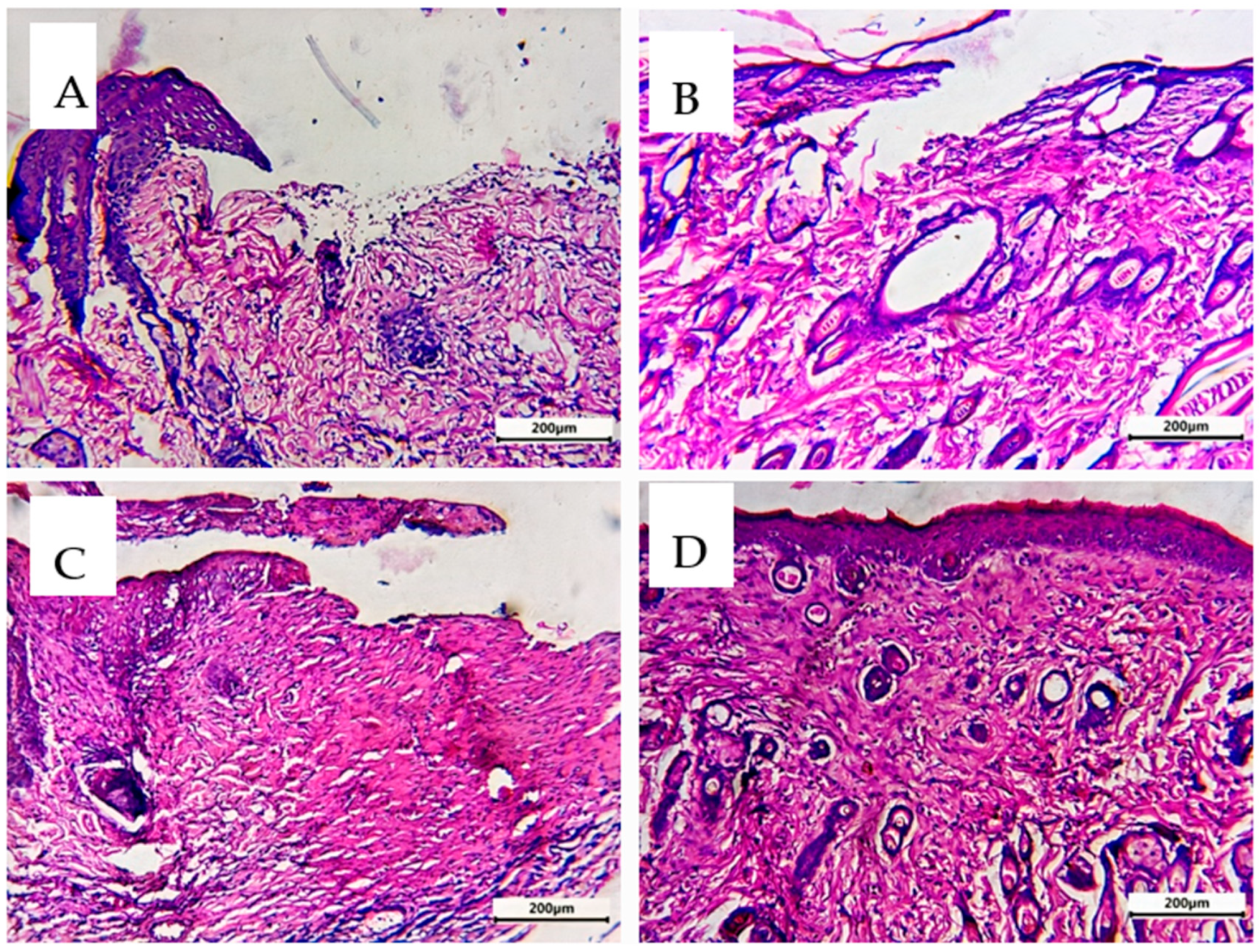
3.10. Comparative Histological Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikareepaisan, P.; Ruktanonchai, U.; Supaphol, P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr. Polym. 2011, 83, 1457–1469. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Ficai, D.; Ficai, A.; Andronescu, E. Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings. Antioxidants 2023, 12, 34. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- El-Ferjani, R.; Ahmad, M.; Dhiyaaldeen, S.M.; Harun, F.W.; Ibrahim, M.Y.; Adam, H.; Al-Obaidi, M.M.J.; Batran, R.A. In Vivo assessment of antioxidant and wound healing improvement of a new schiff base derived Co (ii) complex in rats. Sci. Rep. 2016, 6, 38748. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Khan, N.R.; Razaque, G.; Shah, S.U.; Shahid, M.G.; Albarqi, H.A.; Alqahtani, A.A.; Alasiri, A.; Basit, H.M. Microwave-treated physically cross-linked sodium alginate and sodium carboxymethyl cellulose blend polymer film for open incision wound healing in diabetic animals—A Novel perspective for skin tissue regeneration application. Pharmaceutics 2023, 15, 418. [Google Scholar]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- El-Say, K.M.; Ahmed, T.A.; Badr-Eldin, S.M.; Fahmy, U.; Aldawsari, H.; Ahmed, O.A. Enhanced permeation parameters of optimized nanostructured simvastatin transdermal films: Ex vivo and In Vivo evaluation. Pharm. Dev. Techol. 2015, 20, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Mendes, M.; Cabral, C.; Mare, R.; Paolino, D.; Vitorino, C. Hybrid nanostructured films for topical administration of simvastatin as coadjuvant treatment of melanoma. J. Pharm. Sci. 2019, 108, 3396–3407. [Google Scholar] [CrossRef]
- Alizadeh, J.; Zeki, A.A.; Mirzaei, N.; Tewary, S.; Rezaei Moghadam, A.; Glogowska, A.; Nagakannan, P.; Eftekharpour, E.; Wiechec, E.; Gordon, J.W.; et al. Mevalonate Cascade Inhibition by Simvastatin Induces the Intrinsic Apoptosis Pathway via Depletion of Isoprenoids in Tumor Cells. Sci. Rep. 2017, 7, 44841. [Google Scholar] [CrossRef]
- López-Álvarez, M.; López-Puente, V.; Rodríguez-Valencia, C.; Angelomé, P.C.; Liz-Marzán, L.M.; Serra, J.; Pastoriza-Santos, I.; González, P. Osteogenic effects of simvastatin-loaded mesoporous titania thin films. Biomed. Mat. 2018, 13, 025017. [Google Scholar] [CrossRef]
- Rohilla, A.; Rohilla, S.; Kumar, A.; Khan, M.U.; Deep, A. Pleiotropic effects of statins: A boulevard to cardioprotection. Arab. J. Chem. 2016, 9, S21–S27. [Google Scholar] [CrossRef]
- Duman, N.; Duman, R.; Tosun, M.; Akıcı, M.; Göksel, E.; Gökçe, B.; Alagöz, O. Topical folinic acid enhances wound healing in rat model. Adv. Med. Sci. 2018, 63, 347–352. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M. Electrically triggered drug delivery from novel electrospun poly (lactic acid)/graphene oxide/quercetin fibrous scaffolds for wound dressing applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin–chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef] [PubMed]
- El-Rasoul, A.; Ahmed, M.M. Chitosan polymer as a coat of calcium alginate microcapsules loaded by non-steroidal antiinflammatory drug. Bull. Pharm. Sci. Assiut 2010, 33, 179–186. [Google Scholar] [CrossRef]
- Elsayed, M. Controlled release alginate-chitosan microspheres of tolmetin sodium prepared by internal gelation technique and characterized by response surface modeling. Braz. J. Pharm. Sci. 2021, 56, e18414. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Bio. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanontechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Saddik, M.S.; Elsayed, M.M.; Abdel-Rheem, A.A.; El-Mokhtar, M.A.; Mosa, E.S.; Al-Hakkani, M.F.; Al-Shelkamy, S.A.; Khames, A.; Daha, M.A.; Abdel-Aleem, J.A. A Novel C@ Fe@ Cu Nanocomposite Loaded with Doxorubicin Tailored for the Treatment of Hepatocellular Carcinoma. Pharmaceutics 2022, 14, 1845. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, H.M.; Abdel-Aleem, J.A.; Ahmed, M.M. Development and optimization of itopride hydrochloride fast disintegrating tablets using factorial design and response surface methodology. Int. J. Pharm. Sci. Res. 2015, 6, 1661. [Google Scholar]
- El-Shenawy, A.A.; Elsayed, M.M.; Atwa, G.M.; Abourehab, M.A.; Mohamed, M.S.; Ghoneim, M.M.; Mahmoud, R.A.; Sabry, S.A.; Anwar, W.; El-Sherbiny, M. Anti-Tumor Activity of Orally Administered Gefitinib-Loaded Nanosized Cubosomes against Colon Cancer. Pharmaceutics 2023, 15, 680. [Google Scholar] [CrossRef]
- Ahmed, M.M. Effect of different formulation variables on release characteristics of gastro-floating microspheres of ethyl cellulose/carbopol 934P encapsulating sorafenib. Int. J. Pharm. Pharm. Sci. 2019, 11, 64–70. [Google Scholar] [CrossRef]
- Matthews, K.H.; Stevens, H.N.E.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M. Lyophilised wafers as a drug delivery system for wound healing containing methylcellulose as a viscosity modifier. Int. J. Pharm. 2005, 289, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Pierscionek, B.; Alany, R.G. Novel in situ gelling ocular films for the opioid growth factor-receptor antagonist-naltrexone hydrochloride: Fabrication, mechanical properties, mucoadhesion, tolerability and stability studies. Int. J. Pharm. 2014, 477, 631–642. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Rel. 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Aboelez, M.O.; Mohamed, M.S.; Mahmoud, R.A.; El-Shenawy, A.A.; Mahmoud, E.A.; Al-Karmalawy, A.A.; Santali, E.Y.; Alshehri, S.; Elsadek, M.E.M. Tailoring of rosuvastatin calcium and atenolol bilayer tablets for the management of hyperlipidemia associated with hypertension: A preclinical study. Pharmaceutics 2022, 14, 1629. [Google Scholar] [CrossRef]
- Gopal, A.; Kant, V.; Gopalakrishnan, A.; Tandan, S.K.; Kumar, D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014, 731, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Shailajan, S.; Menon, S.; Pednekar, S.; Singh, A. Wound healing efficacy of Jatyadi Taila: In Vivo evaluation in rat using excision wound model. J. Ethnopharmacol. 2011, 138, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Verpoorte, R.; Suresh, B. Evaluation of In Vivo wound healing activity of Hypericum patulum (Family: Hypericaceae) leaf extract on different wound model in rats. J. Ethnopharmacol. 2000, 70, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Gautam, M.; Goel, S.; Purohit, V.; Sharma, H.; Goel, R. Evaluation of In Vivo wound healing activity of Bacopa monniera on different wound model in rats. BioMed Res. Int. 2013, 2013, 972028. [Google Scholar] [CrossRef]
- Che Hamzah, N.; Mohammed, A.; Sirajudeen, K.; Asari, M.; Hamzah, Z.; Shaik, I. Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats. Asian Pacif. J. Trop. Biomed. 2019, 9, 140–149. [Google Scholar]
- Adamskaya, N.; Dungel, P.; Mittermayr, R.; Hartinger, J.; Feichtinger, G.; Wassermann, K.; Redl, H.; van Griensven, M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury 2011, 42, 917–921. [Google Scholar] [CrossRef]
- Ahanger, A.A.; Leo, M.D.; Gopal, A.; Kant, V.; Tandan, S.K.; Kumar, D. Pro-healing effects of bilirubin in open excision wound model in rats. Int. Wound J. 2016, 13, 398–402. [Google Scholar] [CrossRef]
- Gürses, İ.; Özeren, M.; Serin, M.; Yücel, N.; Erkal, H.Ş. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol. Res. Pract. 2014, 210, 863–871. [Google Scholar] [CrossRef]
- Kiss, N.; Hamar, P. Histopathological evaluation of contrast-induced acute kidney injury rodent models. BioMed Res. Int. 2016, 2016, 3763250. [Google Scholar] [CrossRef] [PubMed]
- Serin, M.; Gülbaş, H.; Gürses, İ.; Erkal, H.Ş.; Yücel, N. The histopathological evaluation of the effectiveness of melatonin as a protectant against acute lung injury induced by radiation therapy in a rat model. Int. J. Radiat. Biol. 2007, 83, 187–193. [Google Scholar] [CrossRef]
- Ghanbarabadi, M.; Iranshahi, M.; Amoueian, S.; Mehri, S.; Motamedshariaty, V.S.; Mohajeri, S.A. Neuroprotective and memory enhancing effects of auraptene in a rat model of vascular dementia: Experimental study and histopathological evaluation. Neurosci. Lett. 2016, 623, 13–21. [Google Scholar] [CrossRef]
- Whittaker, P.; Hines, F.A.; Robl, M.G.; Dunkel, V.C. Histopathological evaluation of liver, pancreas, spleen, and heart from iron-overloaded sprague-dawley rats* 1, 2. Toxicol. Pathol. 1996, 24, 558–563. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.; Glasson, S.; Carlson, C. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the rat. Osteoarthitis Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Heinemeier, K.; Olesen, J.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.; Schjerling, P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiolog. 2007, 582, 1303–1316. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, J.; Du, Y.; Hu, B.; Zhou, Z.; Lou, J. Dynamic changes in the gene expression profile during rat oral carcinogenesis induced by 4-nitroquinoline 1-oxide. Mol. Med. Rep. 2016, 13, 2561–2569. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; de Moraes, M.A.; Nogueira, G.M.; Rodas, A.C.; Higa, O.Z.; Beppu, M.M. Glycerin and ethanol as additives on silk fibroin films: Insoluble and malleable films. J. Appl. Polym. Sci. 2013, 128, 115–122. [Google Scholar] [CrossRef]
- Felton, L.A.; O’Donnell, P.B.; McGinity, J.W. Mechanical properties of polymeric films prepared from aqueous dispersions. In Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; CRC Press: Boca Raton, FL, USA, 2008; pp. 125–148. [Google Scholar]
- Briggs, F.; Browne, D.; Asuri, P. Role of Polymer Concentration and Crosslinking Density on Release Rates of Small Molecule Drugs. Int. J. Mol. Sci. 2022, 23, 4118. [Google Scholar] [CrossRef]
- Abdelkader, H.; Wertheim, D.; Pierscionek, B.; Alany, R.G. Curcumin In Situ Gelling Polymeric Insert with Enhanced Ocular Performance. Pharmaceutics 2020, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Buabeid, M.; Arafa, E.-S.A.; Yaseen, H.S.; Umar, M.I.; Murtaza, G. Anti-inflammatory effect of simvastatin by impeding TNF-α and interleukin-1ß pathways: Antiangiogenic activity of simvastatin and simvastatin-loaded silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L. Current concepts in soft connective tissue wound healing. Br. J. Surg. 1983, 70, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Haukipuro, K.; Melkko, J.; Risteli, L.; Kairaluoma, M.; Risteli, J. Synthesis of type I collagen in healing wounds in humans. Ann. Surg. 1991, 213, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Taga, T.; Kishimoto, T. Signaling mechanisms through cytokine receptors that share signal transducing receptor components. Curr. Opin. Immunol. 1995, 7, 17–23. [Google Scholar] [CrossRef]
- Alves de Medeiros, A.K.; Speeckaert, R.; Desmet, E.; Van Gele, M.; De Schepper, S.; Lambert, J. JAK3 as an Emerging Target for Topical Treatment of Inflammatory Skin Diseases. PLoS ONE 2016, 11, e0164080. [Google Scholar] [CrossRef] [PubMed]





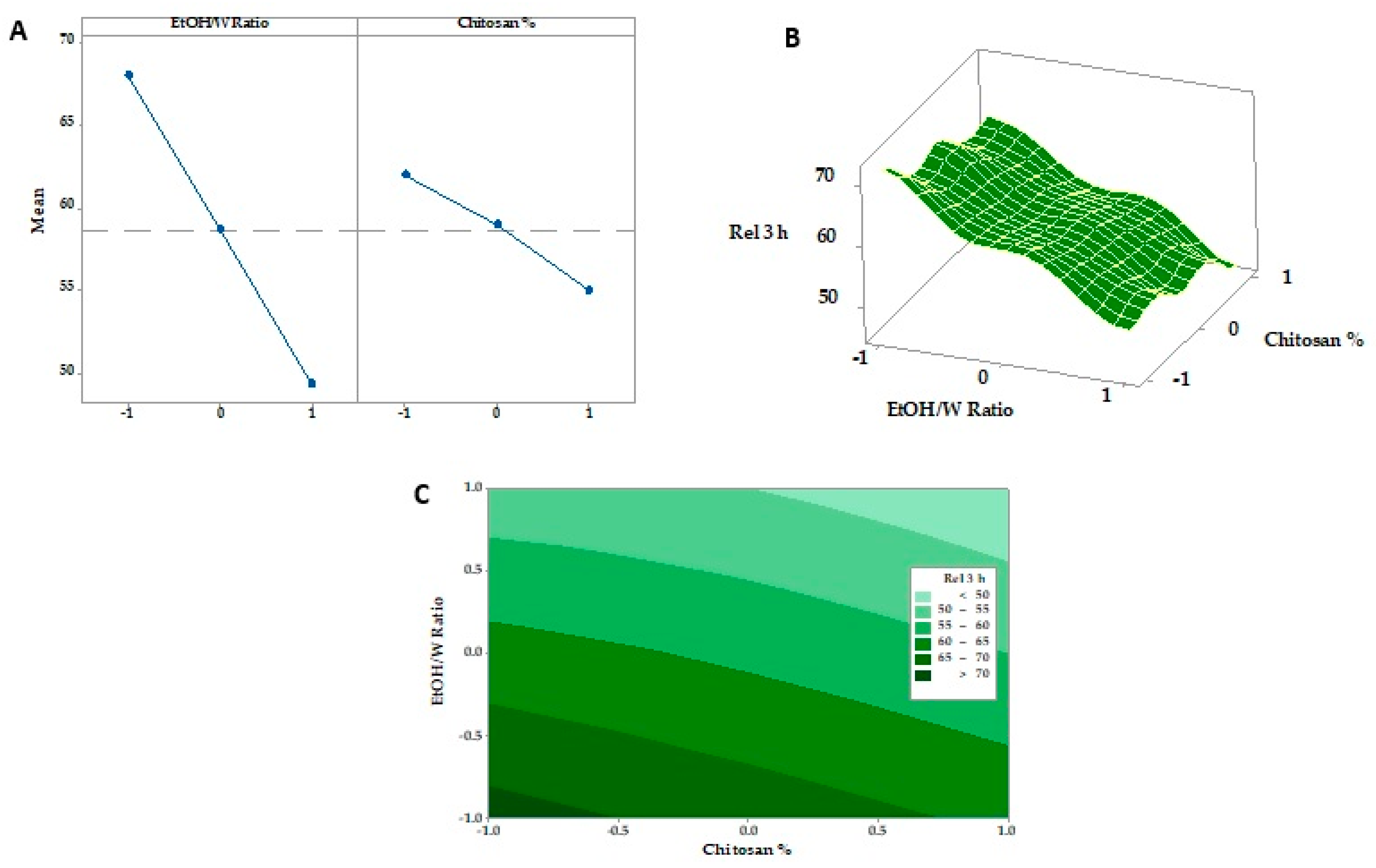

| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
|---|---|---|---|---|---|---|---|---|---|
| SMV (mg) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| EtOH/W ratio (% v/v) | 10 | 10 | 10 | 20 | 20 | 20 | 30 | 30 | 30 |
| Chitosan (% w/v) | 0.5 | 1 | 1.5 | 0.5 | 1 | 1.5 | 0.5 | 1 | 1.5 |
| Folic acid (mg) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Glycerol (% v/v) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Water to (mL) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| F. Code | TS (MPa) | Expansion % | Cumulative % Released at 3 h |
|---|---|---|---|
| F1 | 16.43 ± 0.92 | 41.32 ± 1.32 | 72.87 ± 2.32 |
| F2 | 18.34 ± 0.87 | 43.65 ± 1.45 | 68. 24 ± 1.98 |
| F3 | 19.84 ± 0.83 | 45.54 ± 1.67 | 64.54 ± 2.54 |
| F4 | 14.65 ± 1.12 | 35.55 ± 1.98 | 62.76 ± 2.11 |
| F5 | 15.55 ± 1.62 | 37.12 ± 1.55 | 59.43 ± 3.01 |
| F6 | 16.97 ± 0.98 | 39.87 ± 1.39 | 55.33 ± 3.23 |
| F7 | 11.21 ± 0.57 | 30.88 ± 1.24 | 52.98 ± 2.89 |
| F8 | 12.34 ± 0.54 | 35.66 ± 1.66 | 50.43 ± 1.76 |
| F9 | 14.39 ± 0.77 | 38.54 ± 1.77 | 46.44 ± 2.89 |
| Formulation Code | F1 | F2 | F3 |
|---|---|---|---|
| Thickness (μm) | 165.45 ± 5.87 | 181.66 ± 4.39 | 187.98 ± 5.28 |
| Weight 1 cm2 (mg) | 7.7 ± 0.3 | 8.5 ± 0.2 | 9.3 ± 0.3 |
| SMV content (w/w) % | 9.65 ± 0.32 | 9.43 ± 0.35 | 9.24 ± 0.28 |
| Surface pH | 6.9 ± 0.19 | 7.2 ± 0.15 | 7.3 ± 0.14 |
| Moisture uptake % | 4.65 ± 0.43 | 4.52 ± 0.32 | 4.9 ± 0.54 |
| Tensile strength (Mpa) | 16.43 ± 0.92 | 18.34 ± 0.87 | 19.84 ± 0.83 |
| Folding endurance | 335 ± 4 | 367 ± 6 | 400 ± 7 |
| Expansion % | 41.32 ± 1.32 | 43.65 ± 1.45 | 45.54 ± 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, M.A.; Alotaibi, B.S.; Elsayed, M.M.A.; Alosaimi, M.E.; Hussein, A.K.; Abduljabbar, M.H.; Lee, K.-T.; Abdelkader, H.; El-Mokhtar, M.A.; Hassan, A.H.E.; et al. Characterization and Bio-Evaluation of the Synergistic Effect of Simvastatin and Folic Acid as Wound Dressings on the Healing Process. Pharmaceutics 2023, 15, 2423. https://doi.org/10.3390/pharmaceutics15102423
Hashem MA, Alotaibi BS, Elsayed MMA, Alosaimi ME, Hussein AK, Abduljabbar MH, Lee K-T, Abdelkader H, El-Mokhtar MA, Hassan AHE, et al. Characterization and Bio-Evaluation of the Synergistic Effect of Simvastatin and Folic Acid as Wound Dressings on the Healing Process. Pharmaceutics. 2023; 15(10):2423. https://doi.org/10.3390/pharmaceutics15102423
Chicago/Turabian StyleHashem, Mahmoud A., Badriyah S. Alotaibi, Mahmoud M. A. Elsayed, Manal E. Alosaimi, Amal K. Hussein, Maram H. Abduljabbar, Kyung-Tae Lee, Hamdy Abdelkader, Mohamed A. El-Mokhtar, Ahmed H.E. Hassan, and et al. 2023. "Characterization and Bio-Evaluation of the Synergistic Effect of Simvastatin and Folic Acid as Wound Dressings on the Healing Process" Pharmaceutics 15, no. 10: 2423. https://doi.org/10.3390/pharmaceutics15102423







