Systemic Dendrimer-Peptide Therapies for Wet Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.2.1. Nuclear Magnetic Resonance
2.2.2. High-Performance Liquid Chromatography
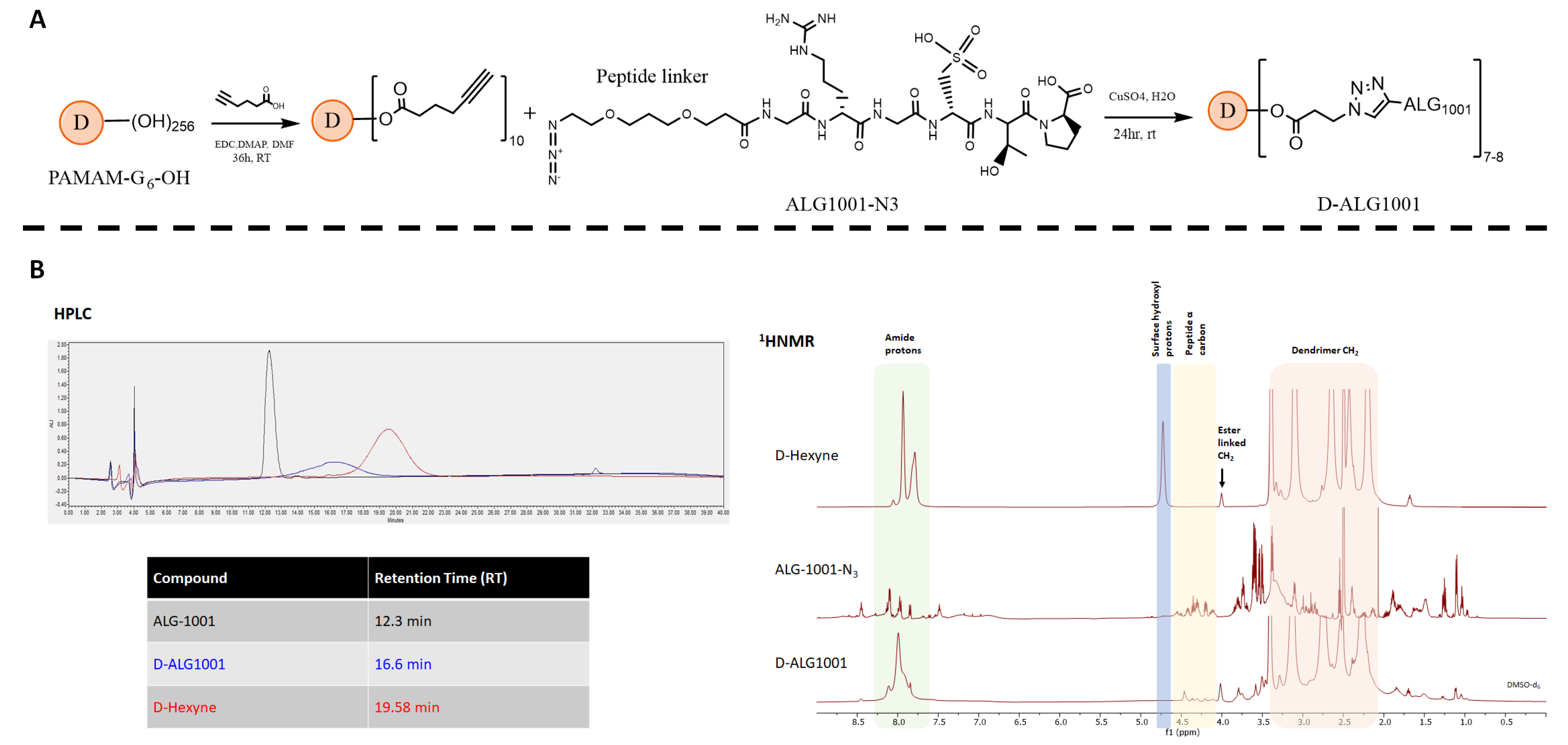
2.3. Synthesis of Dendrimer Conjugates
2.3.1. Synthesis of Dendrimer-Hexyne
2.3.2. Synthesis of BOC-GABA-D-Hexyne
2.3.3. Synthesis of GABA-D-Hexyne
2.3.4. Synthesis of Cy5-D-Hexyne
2.3.5. Synthesis of D-ALG1001 and Cy5-D-ALG1001
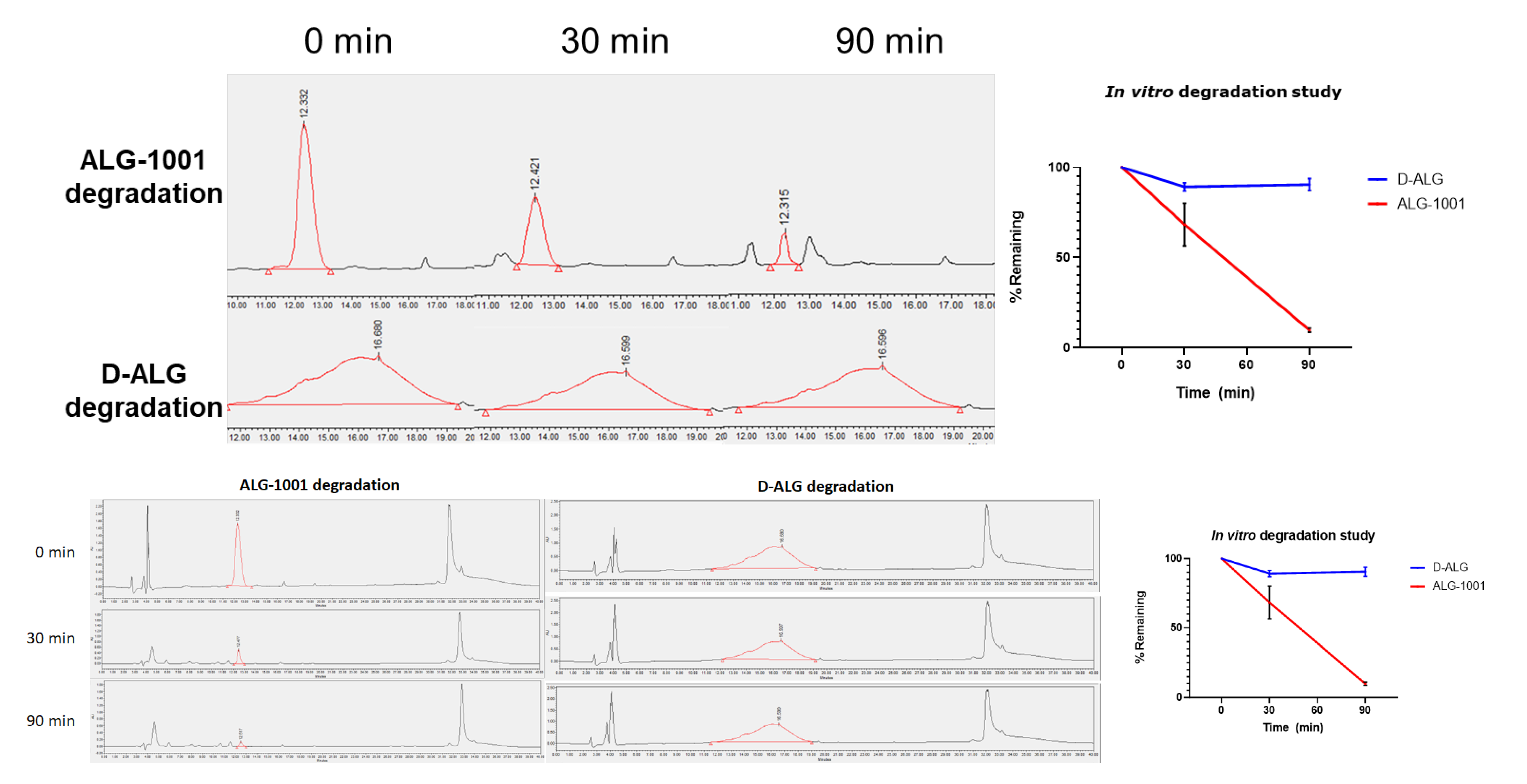
2.4. In Vitro Stability under Enzymatic Degradation
2.5. Cell Culture
2.6. In Vitro Evaluation of D-ALG Efficacy
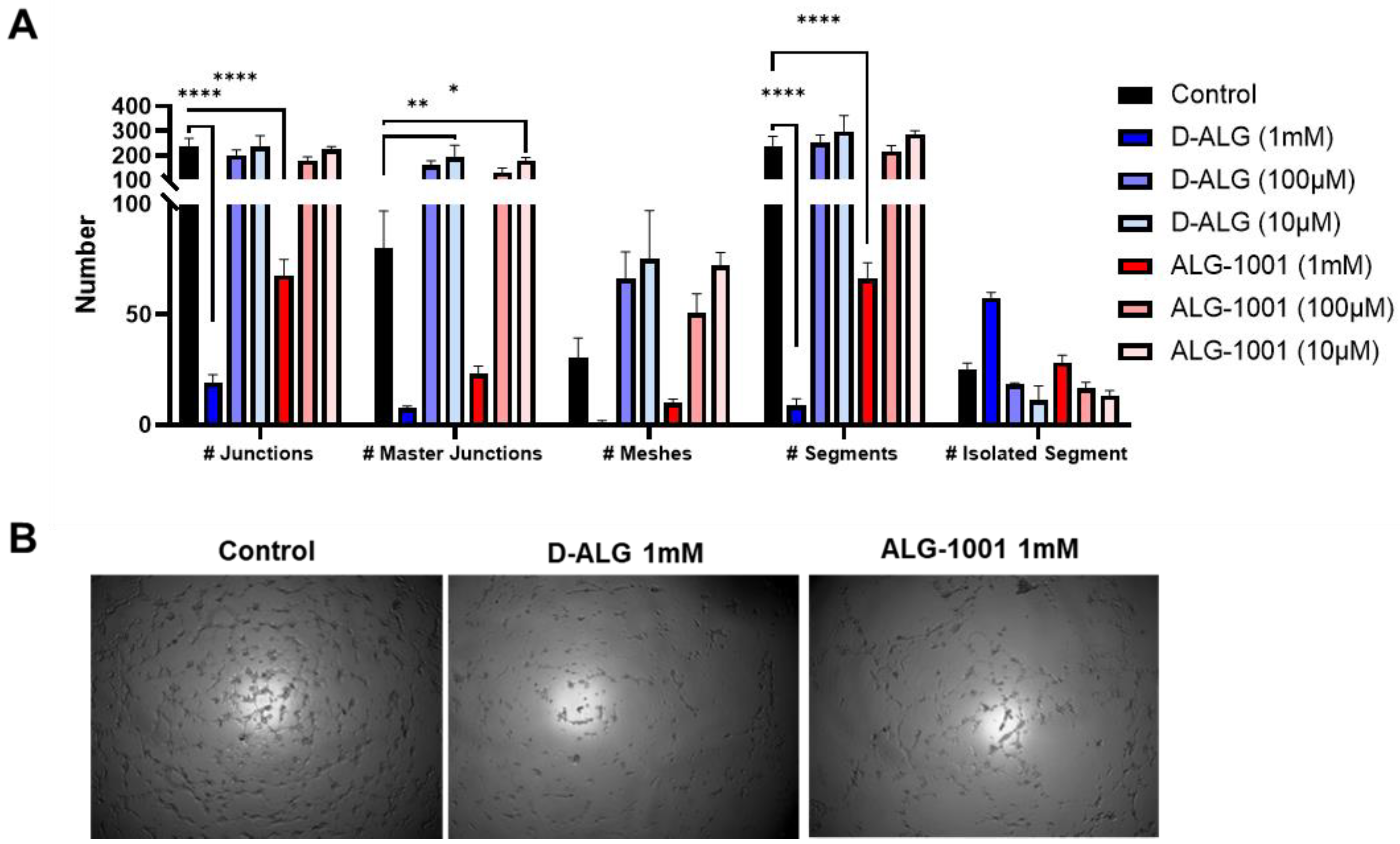
2.6.1. Vessel Formation Assay
2.6.2. Wound-Healing Assay
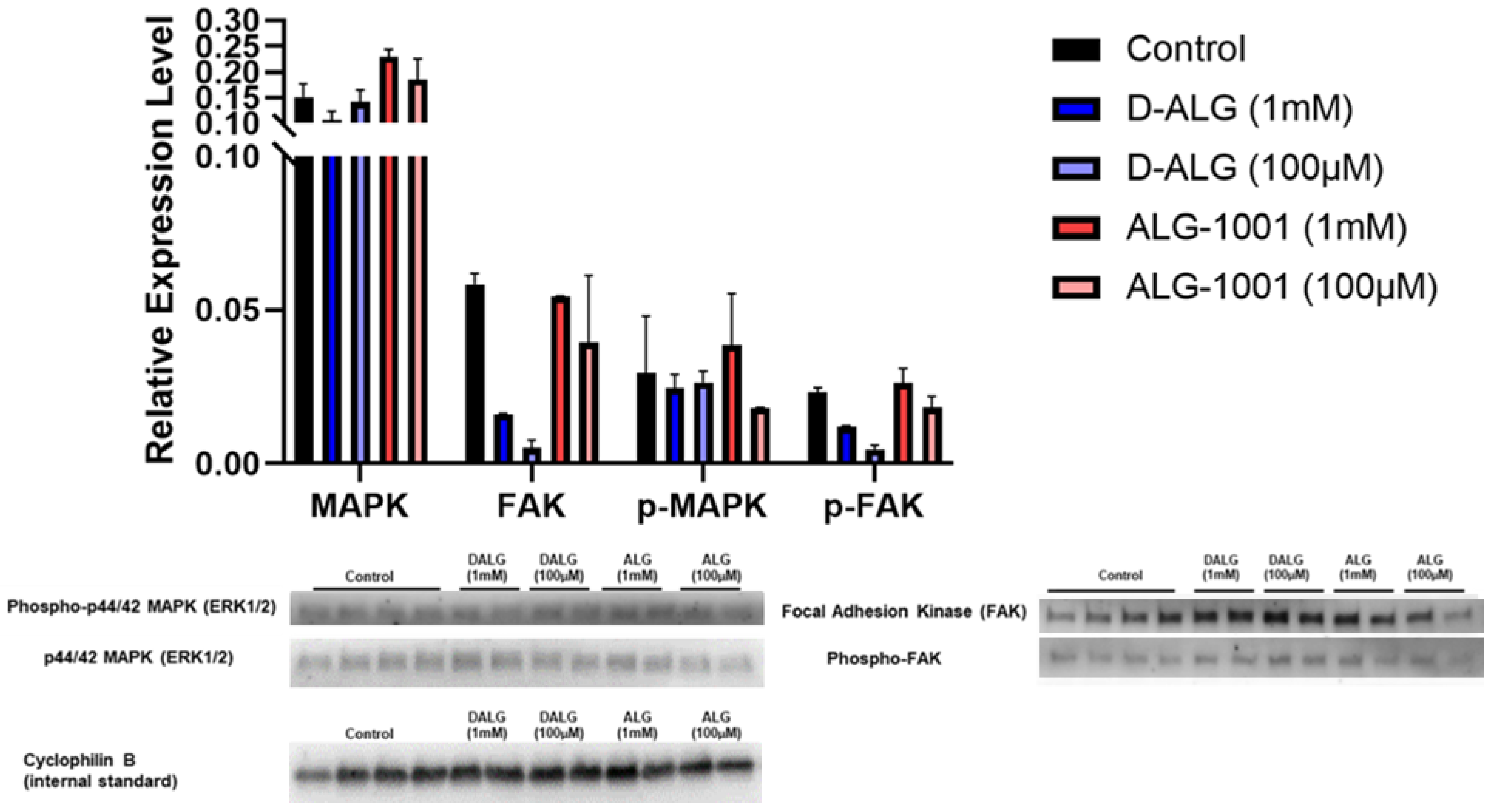
2.6.3. VEGF Activation for Western Blotting and PCR
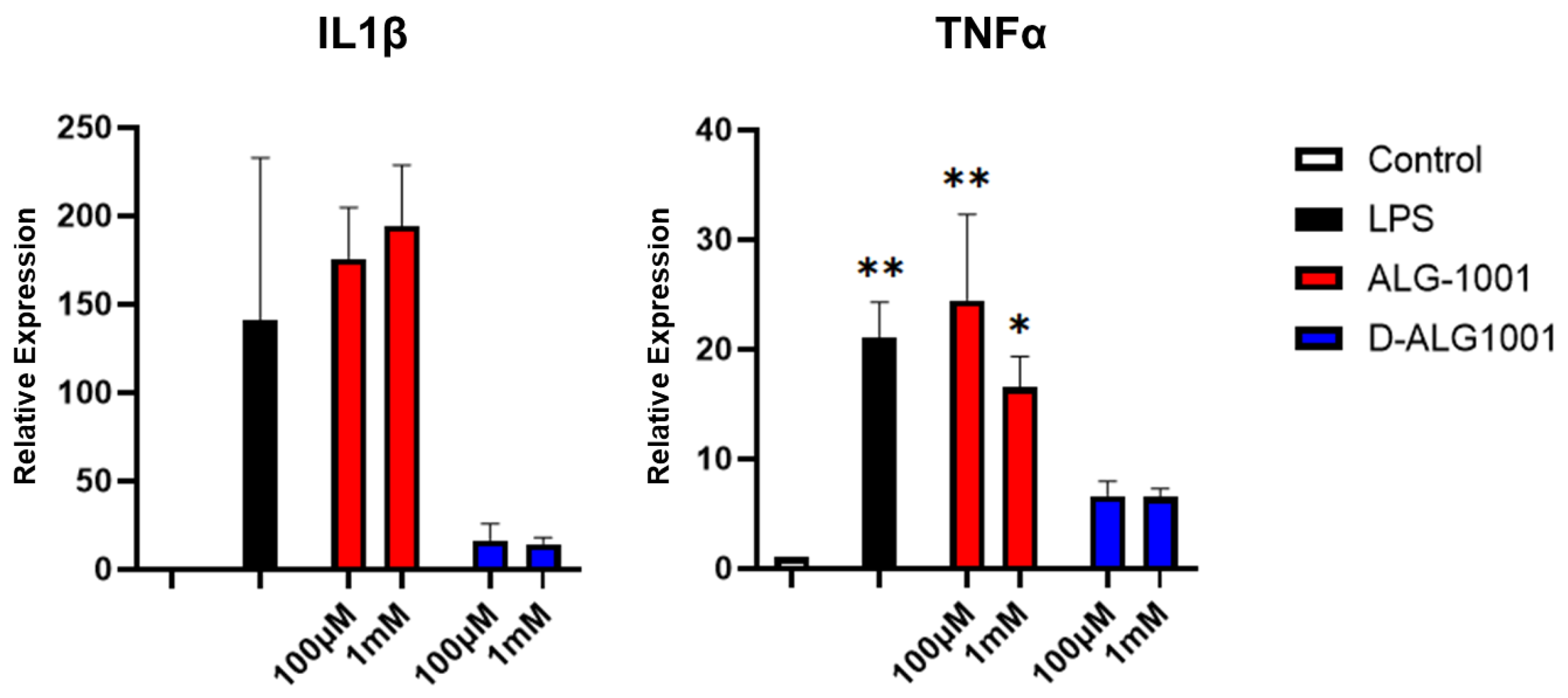
2.6.4. In Vitro Inflammation Model
2.7. In Vivo Evaluation of D-ALG at Attenuating Choroidal Neovascularization
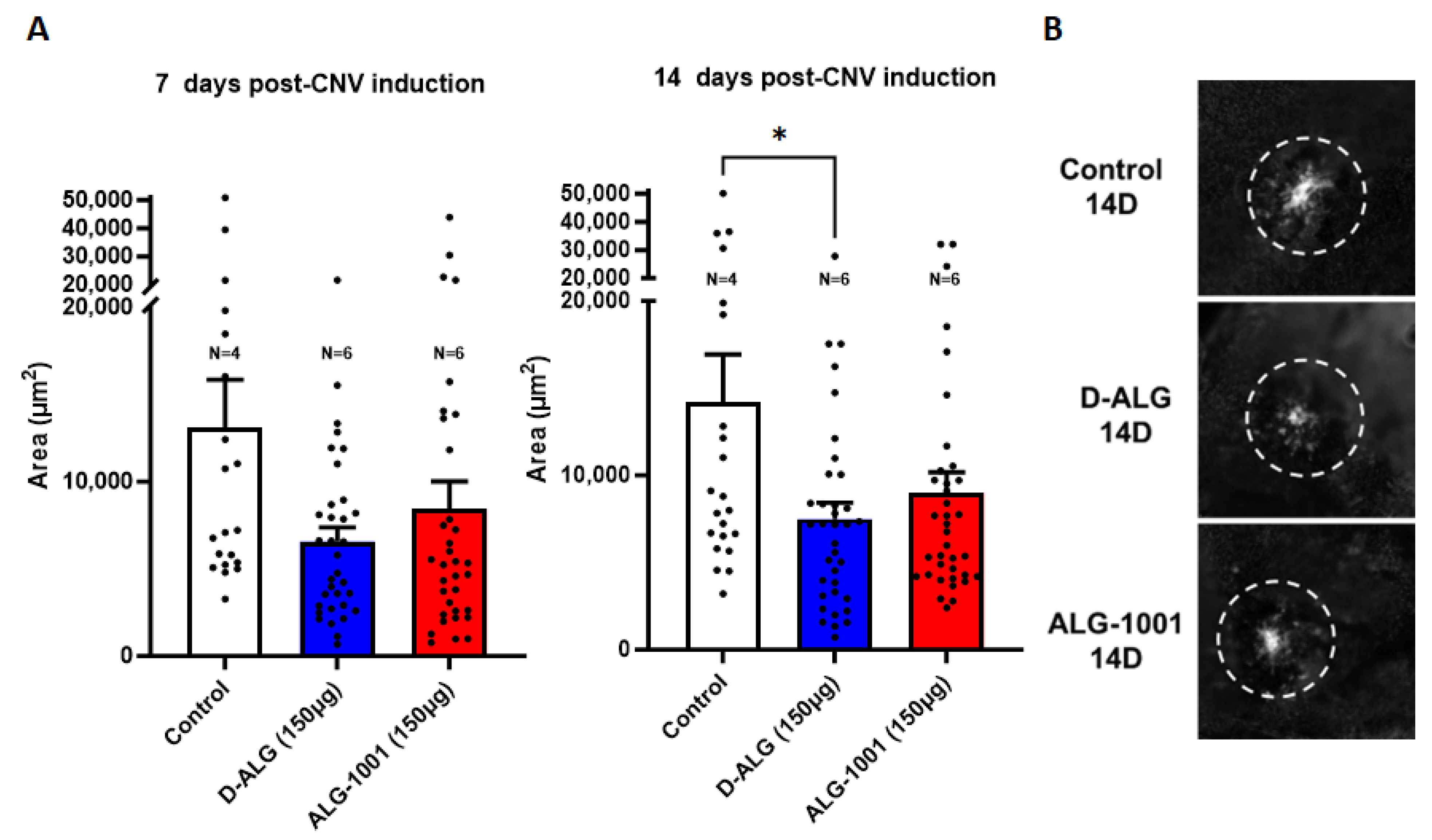
2.7.1. In Vivo Laser CNV Rat Models
2.7.2. Tissue Preparation for Western Blotting and qPCR
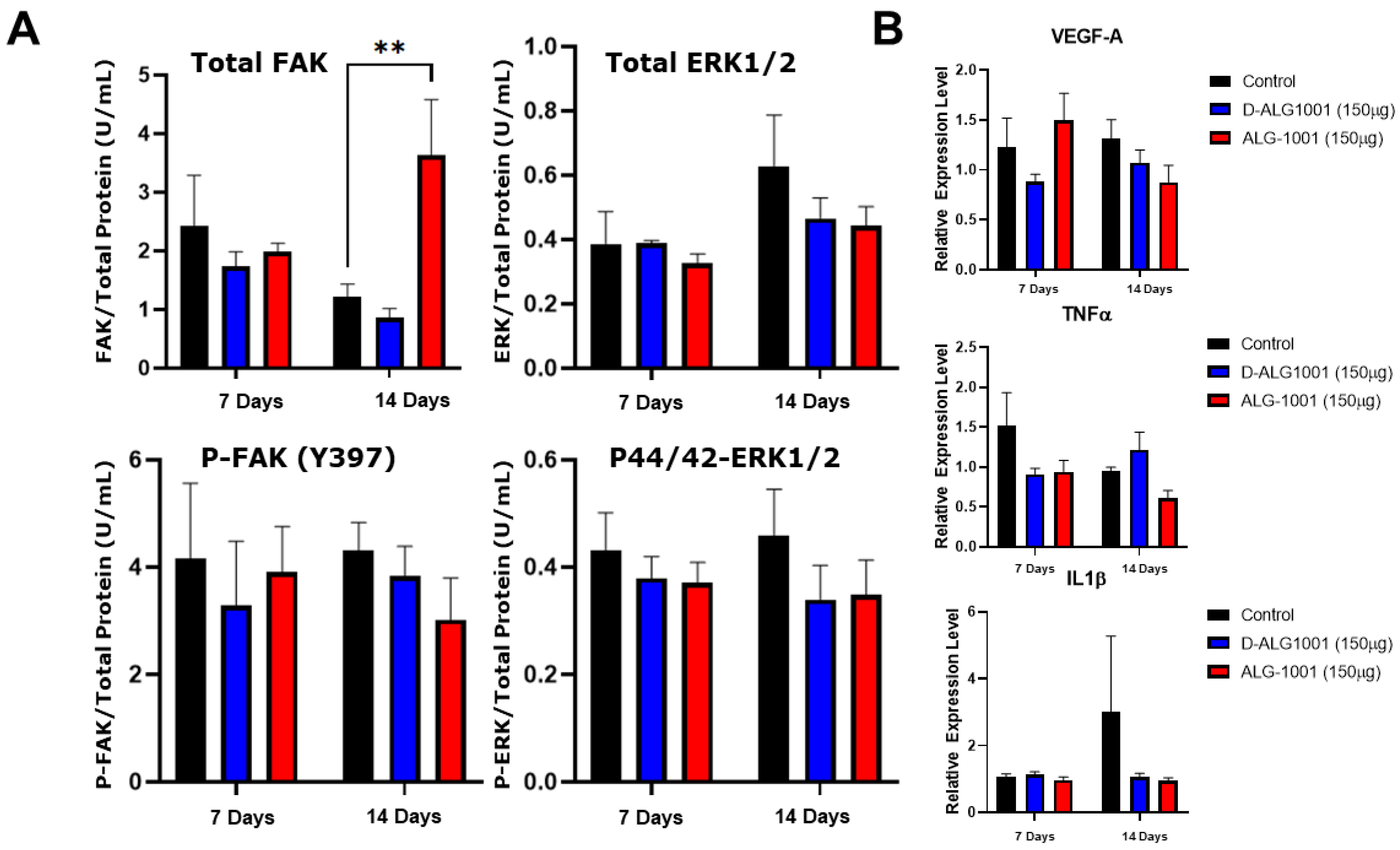
2.7.3. Pathway Activation Analysis with Western Blot and ELISA
2.7.4. qPCR Analysis
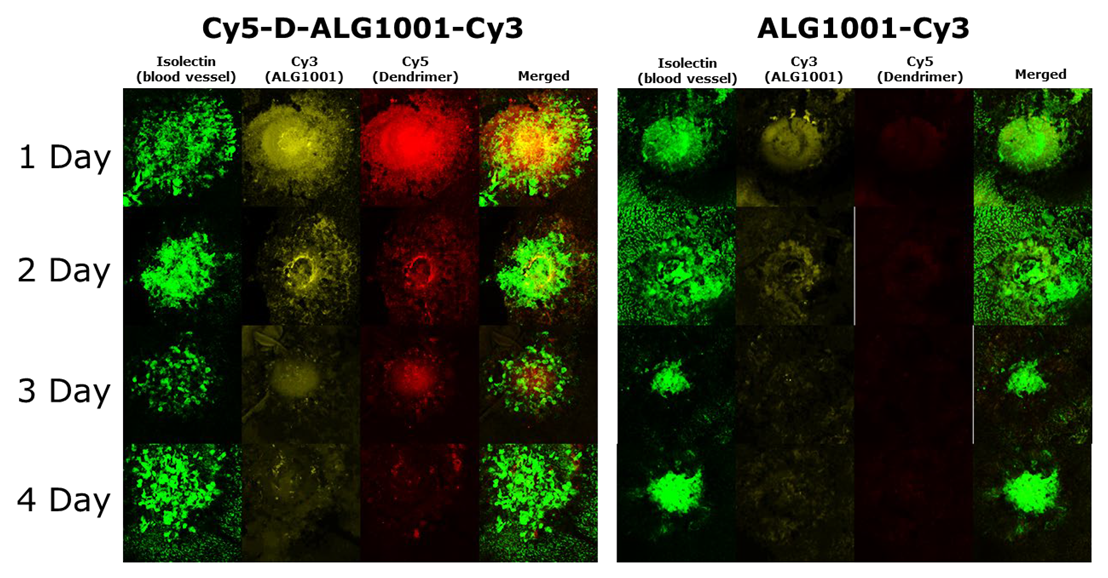
2.7.5. Biodistribution
2.7.6. Imaging
2.7.7. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of D-ALG1001 Intermediates and Conjugates
3.2. In Vitro Stability under Enzymatic Degradation
3.3. In Vitro Vessel Formation Assay
3.4. Wound-Healing Assay
3.5. Western Blot on Endothelial Cell Activation
3.6. Attenuation of Inflammatory Response in Murine Microglia
3.7. Biodistribution of Systemically Administered ALG-1001 and D-ALG in Laser CNV Model
3.8. Systemically Administered D-ALG Attenuates FAK and ERK Activation
3.9. In Vivo Attenuation of CNV Formation
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hobbs, S.D.; Pierce, K. Wet Age-related Macular Degeneration (Wet AMD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular macular degeneration: A review of etiology, risk factors, and recent advances in research and therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar]
- Chang, L.K.; Sarraf, D. Tears of the retinal pigment epithelium: An old problem in a new era. Retina 2007, 27, 523–534. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef]
- Witmer, A.N.; Vrensen, G.F.J.M.; Van Noorden, C.J.F.; Schlingemann, R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Miller, J.W.; Le Couter, J.; Strauss, E.C.; Ferrara, N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 2013, 120, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Tobe, T.; Okamoto, N.; Vinores, M.A.; Derevjanik, N.L.; Vinores, S.A.; Zack, D.J.; Campochiaro, P.A. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Investig. Ophthalmol. Vis. Sci. 1998, 39, 180–188. [Google Scholar]
- Schlingemann, R.O. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.L.; Schwartz, S.G.; Flynn, H.W.; Scott, I.U. Anti-VEGF Treatment Strategies for Wet AMD. J. Ophthalmol. 2012, 2012, 786870. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Gordon, G.M. Systemic Safety of Prolonged Monthly Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2016, 134, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Matsui, K.; Ikuno, Y.; Lai, T.Y.Y.; Gemmy Cheung, C.M. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog. Retin. Eye Res. 2018, 63, 92–106. [Google Scholar] [CrossRef]
- Treatments for Wet AMD (Advanced Neovascular AMD) | National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/age-related-macular-degeneration/wet-amd-type-late-age-related-macular-degeneration-read-about-treatments-wet-amd-anti-vegf (accessed on 6 May 2022).
- Age-Related Macular Degeneration (AMD) | Johns Hopkins Medicine. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/agerelated-macular-degeneration-amd (accessed on 6 May 2022).
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, M.; Uchida, A.; Kurihara, T.; Kamoshita, M.; Minami, S.; Shinoda, H.; Tsubota, K.; Ozawa, Y. Non-responsiveness to intravitreal aflibercept treatment in neovascular age-related macular degeneration: Implications of serous pigment epithelial detachment. Sci. Rep. 2016, 6, 29619. [Google Scholar] [CrossRef] [PubMed]
- Gau, D.; Vignaud, L.; Francoeur, P.; Koes, D.; Guillonneau, X.; Roy, P. Inhibition of ocular neovascularization by novel anti-angiogenic compound. Exp. Eye Res. 2021, 213, 108861. [Google Scholar] [CrossRef]
- Vogt, D.; Deiters, V.; Herold, T.R.; Guenther, S.R.; Kortuem, K.U.; Priglinger, S.G.; Wolf, A.; Schumann, R.G. Optimal Patient Adherence and Long-Term Treatment Outcomes of Neovascular Age-Related Macular Degeneration in Real-Life. Curr. Eye Res. 2022, 47, 889–896. [Google Scholar] [CrossRef]
- Van Hove, I.; Hu, T.-T.; Beets, K.; Van Bergen, T.; Etienne, I.; Stitt, A.W.; Vermassen, E.; Feyen, J.H.M. Targeting RGD-binding integrins as an integrative therapy for diabetic retinopathy and neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2021, 85, 100966. [Google Scholar] [CrossRef]
- Bhatwadekar, A.D.; Kansara, V.; Luo, Q.; Ciulla, T. Anti-integrin therapy for retinovascular diseases. Expert Opin. Investig. Drugs 2020, 29, 935–945. [Google Scholar] [CrossRef]
- Wolf, A.T.; Harris, A.; Oddone, F.; Siesky, B.; Verticchio Vercellin, A.; Ciulla, T.A. Disease progression pathways of wet AMD: Opportunities for new target discovery. Expert Opin. Ther. Targets 2022, 26, 5–12. [Google Scholar] [CrossRef]
- Hu, T.-T.; Vanhove, M.; Porcu, M.; Van Hove, I.; Van Bergen, T.; Jonckx, B.; Barbeaux, P.; Vermassen, E.; Feyen, J.H.M. The potent small molecule integrin antagonist THR-687 is a promising next-generation therapy for retinal vascular disorders. Exp. Eye Res. 2019, 180, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, W.; Zhang, Y.; Meza, L.; Tseng, H.; Takada, Y.; Ames, J.B.; Lam, K.S. Optimization of RGD-Containing Cyclic Peptides against αvβ3 Integrin. Mol. Cancer Ther. 2016, 15, 232–240. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Lu, F.; Xu, S.; Hu, W.; Huang, J.; Gu, Q.; Sun, X. The antiangiogenic effects of integrin alpha5beta1 inhibitor (ATN-161) in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7213–7220. [Google Scholar] [CrossRef]
- Chappelow, A.V.; Kaiser, P.K. Neovascular age-related macular degeneration: Potential therapies. Drugs 2008, 68, 1029–1036. [Google Scholar] [CrossRef]
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib-a novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert Opin. Investig. Drugs 2020, 29, 547–554. [Google Scholar] [CrossRef]
- Joshi, P.; Chung, C.Y.; Aukhil, I.; Erickson, H.P. Endothelial cells adhere to the RGD domain and the fibrinogen-like terminal knob of tenascin. J. Cell Sci. 1993, 106 Pt 1, 389–400. [Google Scholar] [CrossRef]
- Fibronectin and Integrin. Available online: https://www.ks.uiuc.edu/Research/fibronectin/ (accessed on 2 April 2021).
- Maturi, R.; Jaffe, G.J.; Ehlers, J.P.; Kaiser, P.K.; Boyer, D.S.; Heier, J.S.; Kornfield, J.A.; Kuppermann, B.D.; Quiroz-Mercado, H.; Aubel, J.; et al. Safety and Efficacy of Risuteganib in Intermediate Non-exudative Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1944. [Google Scholar]
- Zhou, D.; Chwa, M.; Shao, Z.; Koo, J.M.; Park, J.Y.; Karageozian, H.L.; Karageozian, V.H.; Kenney, C.M.; Kornfield, J.A. Mechanism of Action of Risuteganib for Retinal Diseases through Protection of Retinal Pigment Epithelium (RPE) and Enhancement of Mitochondrial Functions. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4949. [Google Scholar]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug delivery to the posterior segment of the eye: Biopharmaceutic and pharmacokinetic considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Zarei, M.; Roohipoor, R.; Mahmoudzadeh, R.; Yaseri, M.; Riazi-Esfahani, H. Epidemiology of intravitreal injections in iran: Indications and referral patterns in a tertiary hospital. Clin. Ophthalmol. 2020, 14, 1201–1206. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Nagai, H.; Hirano, Y.; Yoshida, M.; Ogura, Y. Incidence of the Complications After Intravitreal Injections. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5120. [Google Scholar]
- Miller, A.; Wilneff, M.A.; Yazji, A.; Petrinec, E.; Carbone, M.; Miller, C.; McCrossin, C.; Donkor, R.; Miller, D.G. Analysis of urgent follow up visits and complications after intravitreal injections: A retrospective cohort study. Int. J. Retina Vitreous 2022, 8, 8. [Google Scholar] [CrossRef]
- Jonas, J.B.; Spandau, U.H.; Schlichtenbrede, F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye 2008, 22, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.P.; Bhutto, I.A.; Wu, T.; Ho, K.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release 2021, 335, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Arteaga Cabeza, O.; Zhang, Z.; Smith Khoury, E.; Sheldon, R.A.; Sharma, A.; Zhang, F.; Slusher, B.S.; Kannan, R.M.; Kannan, S.; Ferriero, D.M. Neuroprotective effects of a dendrimer-based glutamate carboxypeptidase inhibitor on superoxide dismutase transgenic mice after neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 2021, 148, 105201. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, K.B.; White, D.T.; Kambhamptati, S.P.; Lee, G.Y.; Fu, T.-M.; Sahoo, A.; Saxena, M.T.; Betzig, E.; Kannan, R.M.; Mumm, J.S. Dendrimer-targeted immunosuppression of microglia reactivity super-accelerates photoreceptor regeneration in the zebrafish retina. bioRxiv 2020. [Google Scholar] [CrossRef]
- DeRidder, L.; Sharma, A.; Liaw, K.; Sharma, R.; John, J.; Kannan, S.; Kannan, R.M. Dendrimer-tesaglitazar conjugate induces a phenotype shift of microglia and enhances β-amyloid phagocytosis. Nanoscale 2021, 13, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Reddy, R.; Sharma, A.; Li, J.; Chang, M.; Sharma, R.; Salazar, S.; Kannan, S.; Kannan, R.M. Targeted systemic dendrimer delivery of CSF-1R inhibitor to tumor-associated macrophages improves outcomes in orthotopic glioblastoma. Bioeng. Transl. Med. 2021, 6, e10205. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Pitha, I.; Parikh, K.S. A new era in posterior segment ocular drug delivery: Translation of systemic, cell-targeted, dendrimer-based therapies. Adv. Drug Deliv. Rev. 2023, 200, 115005. [Google Scholar] [CrossRef]
- Zhang, F.; Trent Magruder, J.; Lin, Y.-A.; Crawford, T.C.; Grimm, J.C.; Sciortino, C.M.; Wilson, M.A.; Blue, M.E.; Kannan, S.; Johnston, M.V.; et al. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J. Control. Release 2017, 249, 173–182. [Google Scholar] [CrossRef]
- Zhang, F.; Nance, E.; Alnasser, Y.; Kannan, R.; Kannan, S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J. Neuroinflamm. 2016, 13, 65. [Google Scholar] [CrossRef]
- Nance, E.; Zhang, F.; Mishra, M.K.; Zhang, Z.; Kambhampati, S.P.; Kannan, R.M.; Kannan, S. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 2016, 101, 96–107. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Faraday, N.; Aita, J.S.; Kumar, S.; Mehta, I.; Choi, H.A.; Cleland, J.L.; Robinson, K.; McCullough, L.D.; Ng, D.K.; et al. Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. Sci. Transl. Med. 2022, 14, eabo2652. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-J.; Bu, J.; Mickel, P.; Han, Y.; Rawding, P.A.; Wang, J.; Kang, H.; Hong, H.; Král, P.; Hong, S. Dendrimer-Peptide Conjugates for Effective Blockade of the Interactions between SARS-CoV-2 Spike Protein and Human ACE2 Receptor. Biomacromolecules 2023, 24, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.S.; Mallick, S.; Kwon, Y.-E.; Kim, Y.-J.; Kim, G.H.; Choi, J.S. PAMAM Dendrimer Conjugated with Cell-penetrating Peptide-derived Oligopeptides for Enhanced Cell Uptake and Gene Delivery. Bull. Korean Chem. Soc. 2015, 36, 2477–2483. [Google Scholar] [CrossRef]
- Chi, L.A.; Asgharpour, S.; Correa-Basurto, J.; Bandala, C.R.; Martínez-Archundia, M. Unveiling the G4-PAMAM capacity to bind and protect Ang-(1-7) bioactive peptide by molecular dynamics simulations. J. Comput. Aided Mol. Des. 2022, 36, 653–675. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, W.; Wu, T.; Kannan, S.; Kannan, R.M. Dendrimer-siRNA Conjugates for Targeted Intracellular Delivery in Glioblastoma Animal Models. ACS Appl. Mater. Interfaces 2022, 14, 46290–46303. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, S.C.; Ji, W.H.; Jin, S.-H.; Kim, J.H. Synthesis of diblock codendrimer by double click chemistry. Bull. Korean Chem. Soc. 2012, 33, 4103–4108. [Google Scholar] [CrossRef]
- Arseneault, M.; Wafer, C.; Morin, J.-F. Recent advances in click chemistry applied to dendrimer synthesis. Molecules 2015, 20, 9263–9294. [Google Scholar] [CrossRef] [PubMed]
- Samarasimhareddy, M.; Shamir, M.; Shalev, D.E.; Hurevich, M.; Friedler, A. A rapid and efficient building block approach for click cyclization of peptoids. Front. Chem. 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Fuaad, A.A.H.; Azmi, F.; Skwarczynski, M.; Toth, I. Peptide conjugation via CuAAC “click” chemistry. Molecules 2013, 18, 13148–13174. [Google Scholar] [CrossRef]
- Li, H.; Aneja, R.; Chaiken, I. Click chemistry in peptide-based drug design. Molecules 2013, 18, 9797–9817. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, K.R.; Sharma, A.; Tallon, C.; Lovell, L.; Thomas, A.G.; Zhu, X.; Wiseman, R.; Wu, Y.; Kambhampati, S.P.; Liaw, K.; et al. Dendrimer-2PMPA selectively blocks upregulated microglial GCPII activity and improves cognition in a mouse model of multiple sclerosis. Nanotheranostics 2022, 6, 126–142. [Google Scholar] [CrossRef]
- Tang, W.; Becker, M.L. “Click” reactions: A versatile toolbox for the synthesis of peptide-conjugates. Chem. Soc. Rev. 2014, 43, 7013–7039. [Google Scholar] [CrossRef]
- Freimann, K.; Arukuusk, P.; Kurrikoff, K.; Pärnaste, L.; Raid, R.; Piirsoo, A.; Pooga, M.; Langel, Ü. Formulation of Stable and Homogeneous Cell-Penetrating Peptide NF55 Nanoparticles for Efficient Gene Delivery In Vivo. Mol. Ther. Nucleic Acids 2018, 10, 28–35. [Google Scholar] [CrossRef]
- Liaw, K.; Zhang, F.; Mangraviti, A.; Kannan, S.; Tyler, B.; Kannan, R.M. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng. Transl. Med. 2020, 5, e10160. [Google Scholar] [CrossRef]
- McGregor, D.P. Discovering and improving novel peptide therapeutics. Curr. Opin. Pharmacol. 2008, 8, 616–619. [Google Scholar] [CrossRef]
- Weinstock, M.T.; Francis, J.N.; Redman, J.S.; Kay, M.S. Protease-resistant peptide design-empowering nature’s fragile warriors against HIV. Biopolymers 2012, 98, 431–442. [Google Scholar] [CrossRef]
- Lei, R.; Hou, J.; Chen, Q.; Yuan, W.; Cheng, B.; Sun, Y.; Jin, Y.; Ge, L.; Ben-Sasson, S.A.; Chen, J.; et al. Self-Assembling Myristoylated Human α-Defensin 5 as a Next-Generation Nanobiotics Potentiates Therapeutic Efficacy in Bacterial Infection. ACS Nano 2018, 12, 5284–5296. [Google Scholar] [CrossRef]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014, 91, e51312. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Yarrow, J.C.; Perlman, Z.E.; Westwood, N.J.; Mitchison, T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Abbi, S.; Ueda, H.; Zheng, C.; Cooper, L.A.; Zhao, J.; Christopher, R.; Guan, J.-L. Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol. Biol. Cell 2002, 13, 3178–3191. [Google Scholar] [CrossRef]
- Underwood, P.A.; Bean, P.A.; Gamble, J.R. Rate of endothelial expansion is controlled by cell:cell adhesion. Int. J. Biochem. Cell Biol. 2002, 34, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.-L.A.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Fabian-Jessing, B.K.; Jakobsen, T.S.; Jensen, E.G.; Alsing, S.; Hansen, S.; Aagaard, L.; Askou, A.L.; Bek, T.; Corydon, T.J. Animal models of choroidal neovascularization: A systematic review. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11. [Google Scholar] [CrossRef]
- Shah, R.S.; Soetikno, B.T.; Lajko, M.; Fawzi, A.A. A Mouse Model for Laser-induced Choroidal Neovascularization. J. Vis. Exp. 2015, 106, e53502. [Google Scholar] [CrossRef]
- Umeda, N.; Kachi, S.; Akiyama, H.; Zahn, G.; Vossmeyer, D.; Stragies, R.; Campochiaro, P.A. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol. Pharmacol. 2006, 69, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.J.; Bessho, K.; Cheng, L.; Bartsch, D.-U.; Jones, T.R.; Bergeron-Lynn, G.; Freeman, W.R. Inhibition of choroidal neovascularization in rats by the urokinase-derived peptide A6. Investig. Ophthalmol. Vis. Sci. 2004, 45, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, Y.; Iesato, Y.; Imai, A.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Yamauchi, A.; Igarashi, K.; Tanaka, M.; et al. Pathophysiological function of endogenous calcitonin gene-related peptide in ocular vascular diseases. Am. J. Pathol. 2015, 185, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Liu, C.; Kannan, R.M. Systemic Dendrimer-Peptide Therapies for Wet Age-Related Macular Degeneration. Pharmaceutics 2023, 15, 2428. https://doi.org/10.3390/pharmaceutics15102428
Wu T, Liu C, Kannan RM. Systemic Dendrimer-Peptide Therapies for Wet Age-Related Macular Degeneration. Pharmaceutics. 2023; 15(10):2428. https://doi.org/10.3390/pharmaceutics15102428
Chicago/Turabian StyleWu, Tony, Chang Liu, and Rangaramanujam M. Kannan. 2023. "Systemic Dendrimer-Peptide Therapies for Wet Age-Related Macular Degeneration" Pharmaceutics 15, no. 10: 2428. https://doi.org/10.3390/pharmaceutics15102428
APA StyleWu, T., Liu, C., & Kannan, R. M. (2023). Systemic Dendrimer-Peptide Therapies for Wet Age-Related Macular Degeneration. Pharmaceutics, 15(10), 2428. https://doi.org/10.3390/pharmaceutics15102428






