Small Molecule Cocktails Promote Fibroblast-to-Leydig-like Cell Conversion for Hypogonadism Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of HFFs
2.2. Isolation and Culture of MEFs
2.3. Isolation and Culture of PLCs
2.4. Generation of LLCs
2.5. Immunofluorescence Assays
2.6. RNA-Seq
2.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.8. Western Blot
2.9. Testosterone Measurement
2.10. LM-CS Analyses
2.11. Animals()
2.12. Cell Tracking
2.13. Castration and Cell Transplantation
2.14. Cut-Tag Analyses
2.15. Statistical Analyses
3. Results
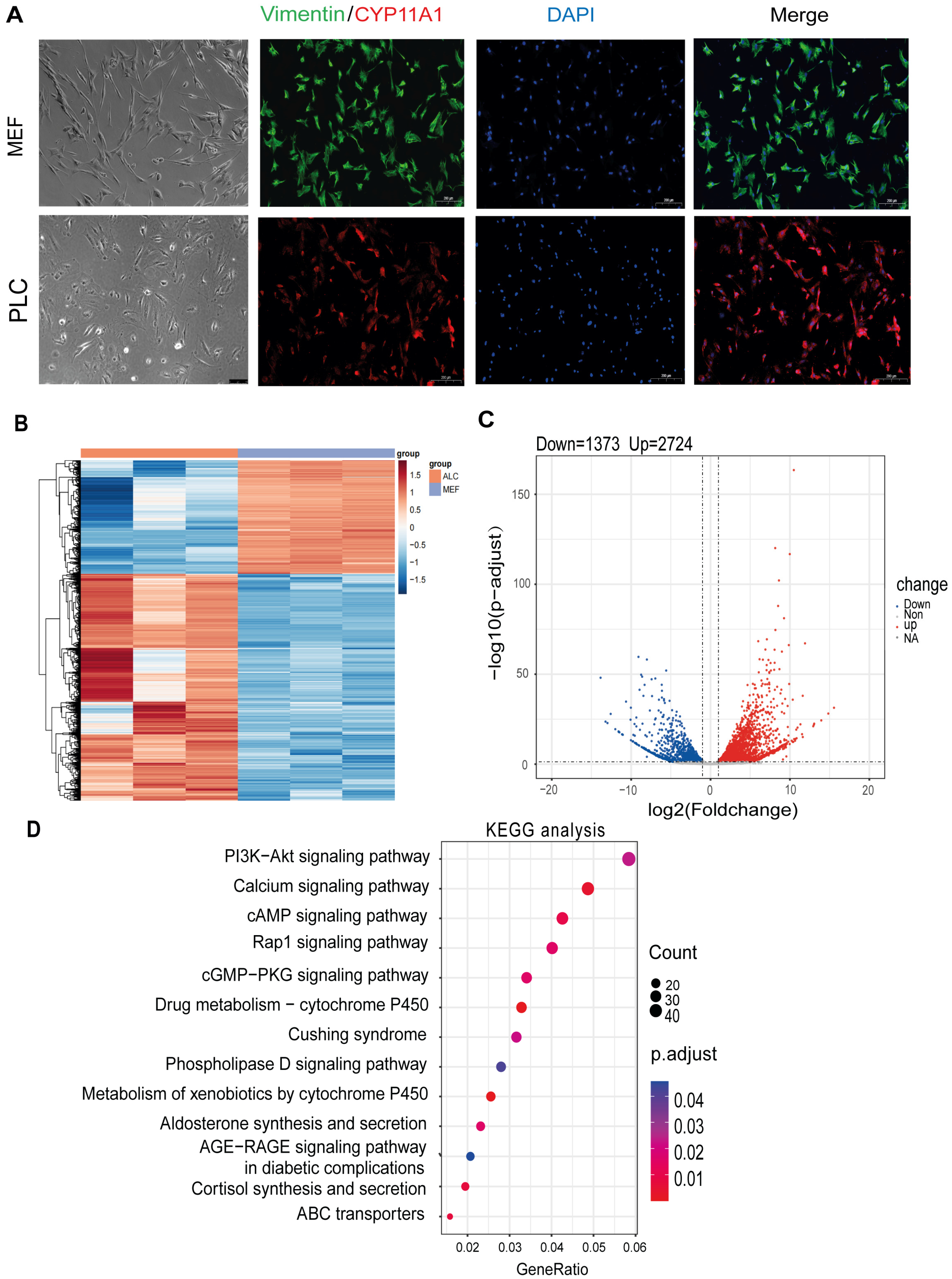
3.1. Transcriptome Analysis Reveals Distinct Signaling Pathways between Fibroblasts and LCs
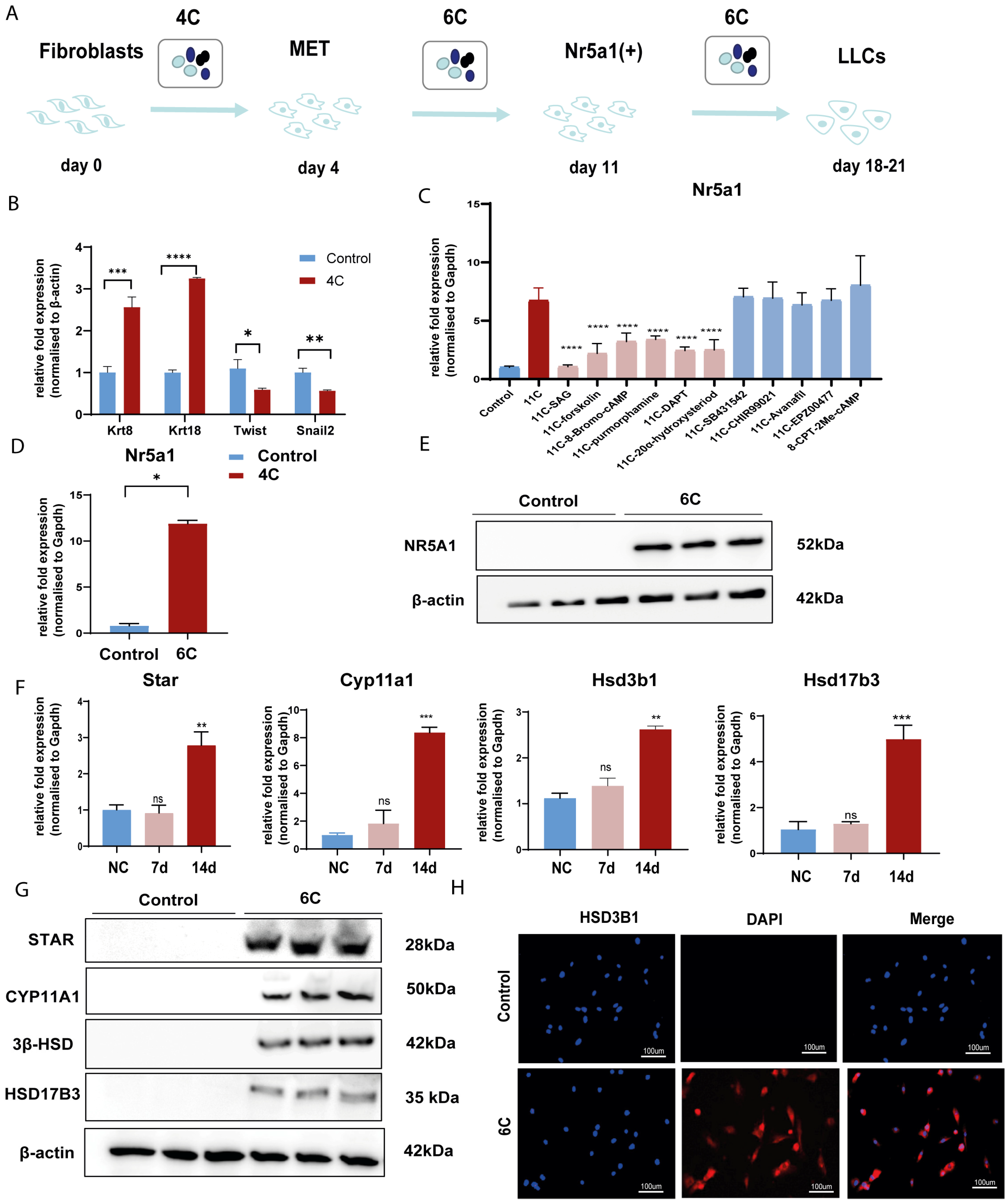
3.2. Screen out the Combination of Small Molecule Compounds That Activate NR5A1 Expression in Fibroblasts
3.3. Convert Fibroblast into LLCs by Small Molecular Compounds In Vitro
3.4. Verify the Therapeutic Effect of LLCs Transplantation on Testosterone Castration In Vivo
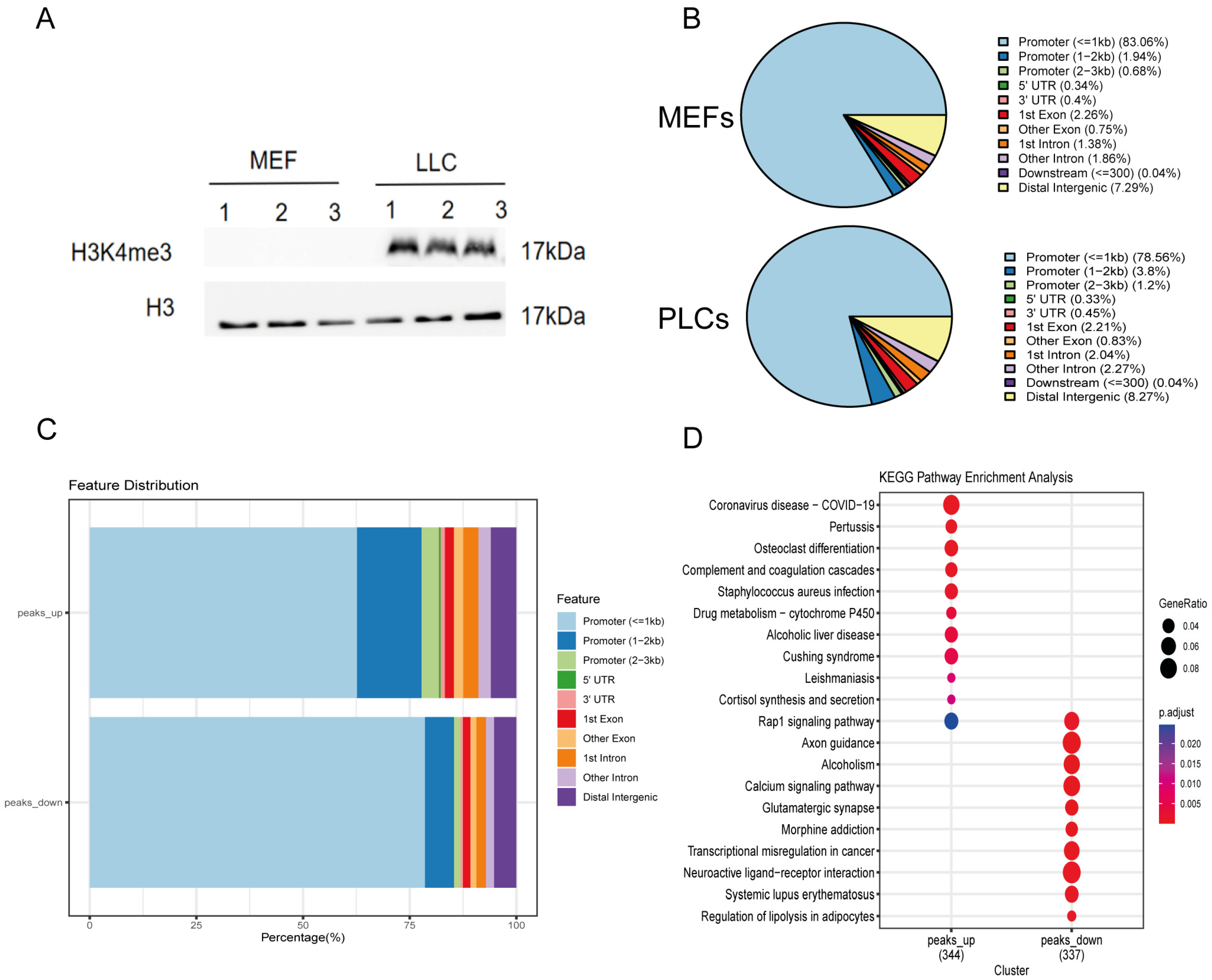
3.5. Fibroblast Altered the Epigenetic Modification of H3K4me3 by Small Molecule Cocktails
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeap, B.B.; Wu, F.C.W. Clinical practice update on testosterone therapy for male hypogonadism: Contrasting perspectives to optimize care. Clin. Endocrinol. 2019, 90, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Shen, X.B.; Yu, N.; Shang, X.J.; Gu, Y.Q.; Zuo, L.D.; Xiong, C.L.; Ye, Z.; Zhou, Y.Z. Prevalence of late-onset hypogonadism among middle-aged and elderly males in China: Results from a national survey. Asian J. Androl. 2021, 23, 170–177. [Google Scholar]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstrom, W.J.G.; Palmert, M.R.; Corona, G.; Dohle, G.R.; et al. Paediatric and adult-onset male hypogonadism. Nat. Rev. Dis. Primers 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Chioma, L.; Cappa, M. Hypogonadism in male infants and adolescents: New androgen formulations. Horm. Res. Paediatr. 2021, 1–9. [Google Scholar] [CrossRef]
- Ide, V.; Vanderschueren, D.; Antonio, L. Treatment of Men with Central Hypogonadism: Alternatives for Testosterone Replacement Therapy. Int. J. Mol. Sci. 2020, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Papadopoulos, V. Advances in stem cell research for the treatment of primary hypogonadism. Nat. Rev. Urol. 2021, 18, 487–507. [Google Scholar] [CrossRef]
- Vogiatzi, M.; Tursi, J.P.; Jaffe, J.S.; Hobson, S.; Rogol, A.D. Testosterone Use in Adolescent Males: Current Practice and Unmet Needs. J. Endocr. Soc. 2021, 5, bvaa161. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D. Pubertal androgen therapy in boys. Pediatr. Endocrinol. Rev. 2005, 2, 383–390. [Google Scholar] [PubMed]
- Sun, J.; Xi, Y.B.; Zhang, Z.D.; Shen, P.; Li, H.Y.; Yin, M.Z.; Li, W.Y.; Shi, C.R. Leydig cell transplantation restores androgen production in surgically castrated prepubertal rats. Asian J. Androl. 2009, 11, 405–409. [Google Scholar] [CrossRef]
- Huang, H.; Zhong, L.; Zhou, J.; Hou, Y.; Zhang, Z.; Xing, X.; Sun, J. Leydig-like cells derived from reprogrammed human foreskin fibroblasts by CRISPR/dCas9 increase the level of serum testosterone in castrated male rats. J. Cell. Mol. Med. 2020, 24, 3971–3981. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zou, X.; Zhong, L.; Hou, Y.; Zhou, J.; Zhang, Z.; Xing, X.; Sun, J. CRISPR/dCas9-mediated activation of multiple endogenous target genes directly converts human foreskin fibroblasts into Leydig-like cells. J. Cell. Mol. Med. 2019, 23, 6072–6084. [Google Scholar] [CrossRef]
- Hou, Y.P.; Zhang, Z.Y.; Xing, X.Y.; Zhou, J.; Sun, J. Direct conversion of human fibroblasts into functional Leydig-like cells by SF-1, GATA4 and NGFI-B. Am. J. Transl. Res. 2018, 10, 175–183. [Google Scholar]
- Gray, C.S. Quality assurance in a rehabilitation facility: A decentralized approach. QRB Qual. Rev. Bull. 1988, 14, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wang, G.; Wang, J.; Zhang, Z.; Fu, Y.; Cheng, L.; Meng, G.; Lyu, Y.; Zhu, J.; Li, Y.; et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 2022, 605, 325–331. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Zhang, W.; Yang, M.; Ding, Y.; Xu, S.; Gu, J.; Li, Y.; Yin, J.; Gao, Y. Antioxidant Small Molecule Compound Chrysin Promotes the Self-Renewal of Hematopoietic Stem Cells. Front. Pharmacol. 2020, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, M.; Afzal, E.; Ebrahimi, M. Manipulation of Hematopoietic Stem Cell Fate by Small Molecule Compounds. Stem Cells Dev. 2018, 27, 1175–1190. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, Y.; Zhang, Z.; Xing, X.; Zou, X.; Zhong, L.; Huang, H.; Zhang, Z.; Sun, J. Conversion of human fibroblasts into functional Leydig-like cells by small molecules and a single factor. Biochem. Biophys. Res. Commun. 2019, 516, 1–7. [Google Scholar] [CrossRef]
- Fabbri-Scallet, H.; de Sousa, L.M.; Maciel-Guerra, A.T.; Guerra-Junior, G.; de Mello, M.P. Mutation update for the NR5A1 gene involved in DSD and infertility. Hum. Mutat. 2020, 41, 58–68. [Google Scholar] [CrossRef]
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal-epithelial transition in development and reprogramming. Nat. Cell Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Z.; Wu, X.; Chen, H.; Xu, W.; Xiang, Q.; Zhang, Q.; Chen, J.; Ge, R.S.; Su, Z.; et al. Direct Reprogramming of Mouse Fibroblasts toward Leydig-like Cells by Defined Factors. Stem Cell Rep. 2017, 8, 39–53. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Chen, Y.; Xi, H.; Zhao, S.; Ma, L.; Xu, Z.; Han, Z.; Zhao, J.; Ge, R.; et al. Differentiation of human induced pluripotent stem cells into Leydig-like cells with molecular compounds. Cell Death Dis. 2019, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Chen, Y.; Wang, L.; Xu, Z.; Ahmed, J.; Ge, R.; Chu, M.; Guo, X. Differentiation of human umbilical cord mesenchymal stem cells into Leydig-like cells with defined molecular compounds. Hum. Cell 2020, 33, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Ji, W.; Wang, L.; Chen, X.; Zhao, S.; Xu, Z.; Ge, R.; Guo, X. Differentiation of human adipose derived stem cells into Leydig-like cells with molecular compounds. J. Cell. Mol. Med. 2019, 23, 5956–5969. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Zhang, T.; Li, Q.; Mei, J.; Liang, J.; Li, Z.; Li, H.; Xiang, Q.; Zhang, Q.; et al. Conversion of Fibroblast into Functional Leydig-like Cell Using Defined Small Molecules. Stem Cell Rep. 2020, 15, 408–423. [Google Scholar] [CrossRef]
- Kawai, Y.; Noguchi, J.; Akiyama, K.; Takeno, Y.; Fujiwara, Y.; Kajita, S.; Tsuji, T.; Kikuchi, K.; Kaneko, H.; Kunieda, T. A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction 2011, 141, 217–225. [Google Scholar] [CrossRef]
- Manna, P.R.; Slominski, A.T.; King, S.R.; Stetson, C.L.; Stocco, D.M. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: Involvement of cAMP/PKA signaling. Endocrinology 2014, 155, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, N.J.; Baburski, A.Z.; Bjelic, M.M.; Sokanovic, S.J.; Mihajlovic, A.I.; Drljaca, D.M.; Janjic, M.M.; Kostic, T.S.; Andric, S.A. In vivo blockade of alpha1-adrenergic receptors mitigates stress-disturbed cAMP and cGMP signaling in Leydig cells. Mol. Hum. Reprod. 2014, 20, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, H.; Abujarour, R.; Zhu, S.; Young Joo, J.; Lin, T.; Hao, E.; Scholer, H.R.; Hayek, A.; Ding, S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells 2009, 27, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Liu, Y.; Lu, F.; Iwabuchi, K.A.; Shen, L.; Inoue, A.; Zhang, Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 2014, 159, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Chenais, N.; Le Cam, A.; Guillet, B.; Lareyre, J.J.; Labbe, C. TGFbeta inhibition and mesenchymal to epithelial transition initiation by Xenopus egg extract: First steps towards early reprogramming in fish somatic cell. Sci. Rep. 2023, 13, 9967. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.G. Cell biology of Leydig cells in the testis. Int. Rev. Cytol. 2004, 233, 181–241. [Google Scholar] [PubMed]
- Avallet, O.; Vigier, M.; Perrard-Sapori, M.H.; Saez, J.M. Transforming growth factor beta inhibits Leydig cell functions. Biochem. Biophys. Res. Commun. 1987, 146, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Song, T.; Sun, M.; Wang, T.; Tan, J.; Zhang, J. Dhh signaling pathway regulates reconstruction of seminiferous tubule-like structure. Reprod. Biol. 2022, 22, 100684. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ikeda, Y.; Parker, K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994, 77, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chen, J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2006, 2, 29–30. [Google Scholar] [CrossRef]
- Adhikari, R.; Chen, C.; Kim, W.K. Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken. Genes 2020, 11, 1360. [Google Scholar] [CrossRef]
- Dwyer, J.R.; Sever, N.; Carlson, M.; Nelson, S.F.; Beachy, P.A.; Parhami, F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 2007, 282, 8959–8968. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Cohen-Tannoudji, J.; Counis, R.; Garner, C.W.; Huhtaniemi, I.; Kraemer, F.B.; Stocco, D.M. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: Its role in the regulation of the steroidogenic acute regulatory protein. J. Biol. Chem. 2013, 288, 8505–8518. [Google Scholar] [CrossRef] [PubMed]


| Full Name | Functions | Source | Working Concentration | Molecular Weight | Structure |
|---|---|---|---|---|---|
| CHIR99021 | GSK3 inhibition | Sigma (Kawasaki-shi, Kanagawa, Japan, Cat. No: SML1046) | 10 μM | 465.34 | 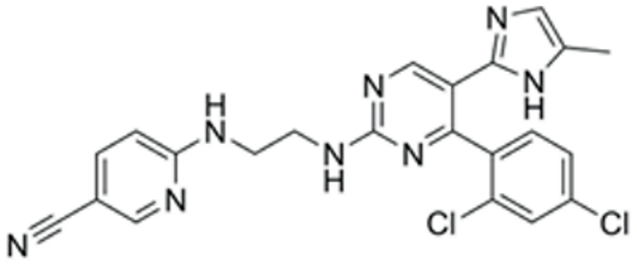 |
| EPZ004777 | DOT1L inhibition | MCE (Monmouth Junction, NJ, USA, Cat. No: HY-15227) | 10 μM | 539.67 |  |
| SB431542 | TGFβ inhibition | Selleck (Houston, TX, USA, Cat. No: S1067) | 2 μM | 384.4 | 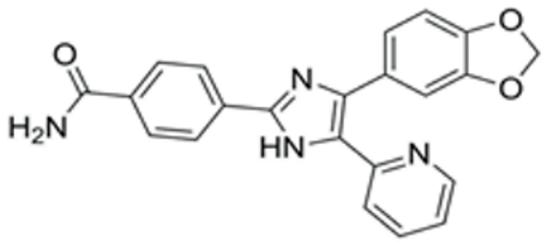 |
| SAG | Desert Hedgehog activation | Selleck (Cat. No: S7779) | 0.2 μM | 490.06 | 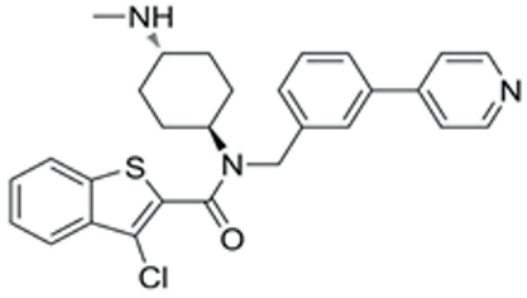 |
| Forskolin | cAMP/PKA activation | Sigma (Cat. No: F6886) | 10 μM | 410.5 |  |
| 8-Bromo-cAMP | cAMP/PKA activation | Sigma (Cat. No: B7880) | 1 μM | 430.08 |  |
| Purmorphamin | Desert Hedgehog activation | Selleck (Cat. No: S3042) | 1 μM | 520.6 |  |
| DAPT | Notch inhibition | Sigma (Cat. No: D5942) | 1 μM | 432.46 |  |
| 20α-hydroxy-cholesterol | Hedgehog activation | MCE (Cat. No: HY-123 16) | 2 μM | 402.65 | 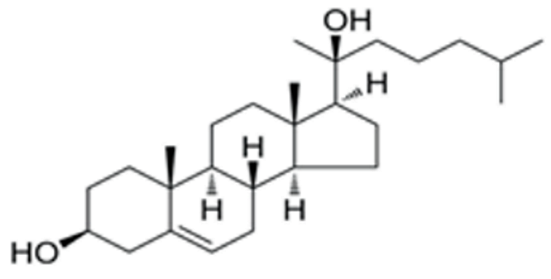 |
| 8-CPT-2Me-cAMP | Rap1 activation | MCE (Cat. No: HY-1075 43) | 2 μM | 507.82 |  |
| Avanafil | cGMP/PKG activation | MCE (Cat. No: HY-18252) | 2 μM | 483.95 | 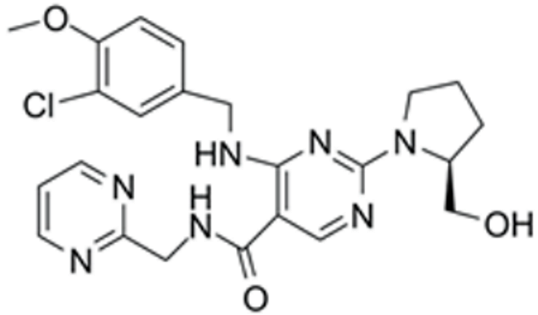 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, F.; Bai, K.; Hou, Y.; Zou, X.; Sun, J. Small Molecule Cocktails Promote Fibroblast-to-Leydig-like Cell Conversion for Hypogonadism Therapy. Pharmaceutics 2023, 15, 2456. https://doi.org/10.3390/pharmaceutics15102456
Yuan F, Bai K, Hou Y, Zou X, Sun J. Small Molecule Cocktails Promote Fibroblast-to-Leydig-like Cell Conversion for Hypogonadism Therapy. Pharmaceutics. 2023; 15(10):2456. https://doi.org/10.3390/pharmaceutics15102456
Chicago/Turabian StyleYuan, Fei, Kaiping Bai, Yanping Hou, Xiangyu Zou, and Jie Sun. 2023. "Small Molecule Cocktails Promote Fibroblast-to-Leydig-like Cell Conversion for Hypogonadism Therapy" Pharmaceutics 15, no. 10: 2456. https://doi.org/10.3390/pharmaceutics15102456






