Nanoparticle-Mediated Strategies for Enhanced Drug Penetration and Retention in the Airway Mucosa
Abstract
:1. Introduction
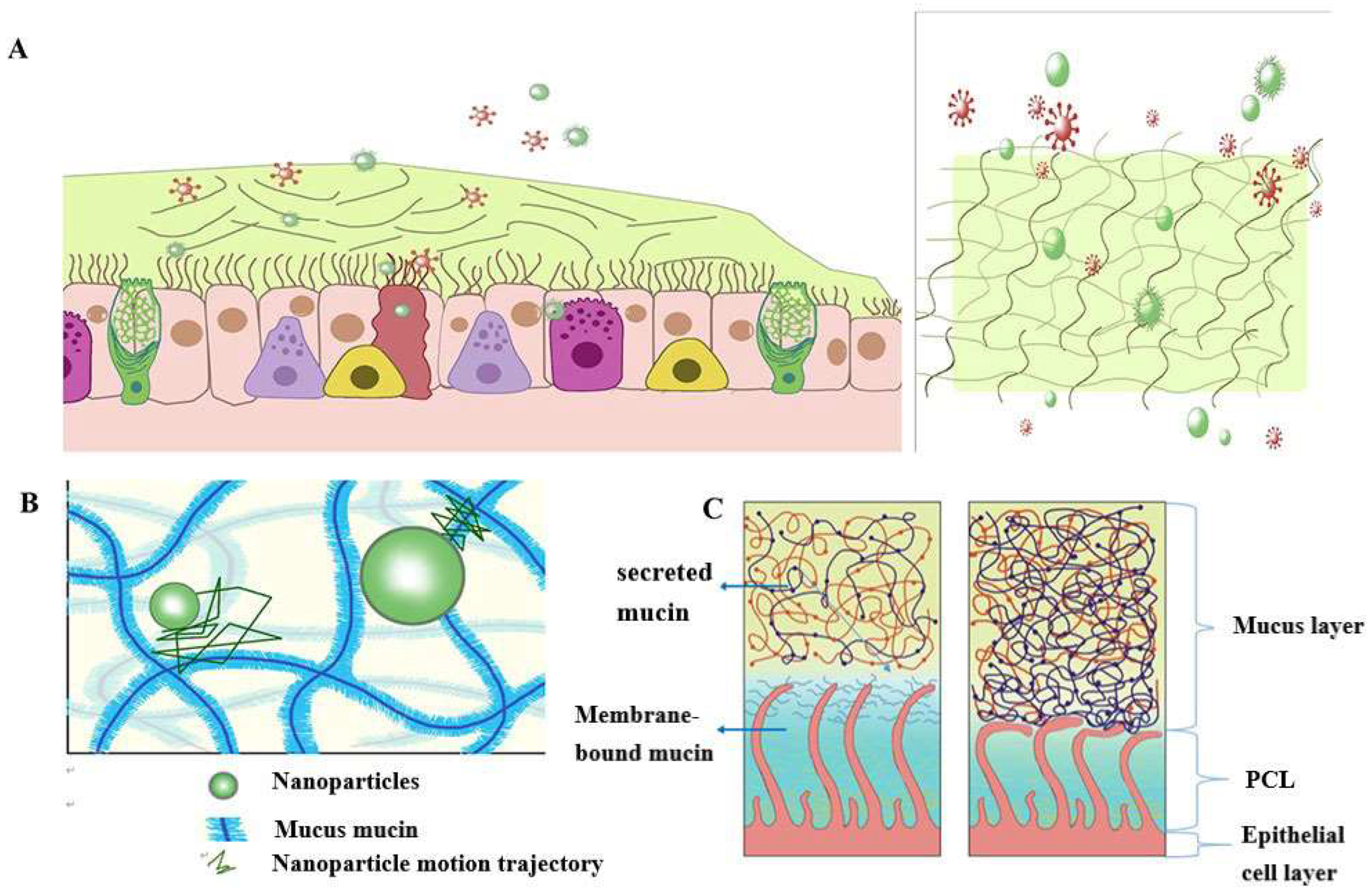
2. Pathophysiology of Airway Mucus
3. Nanoparticle-Mediated Effective Enhancement of Drug Retention and Penetration in the Airway Mucosa
3.1. Mucosal Permeation System
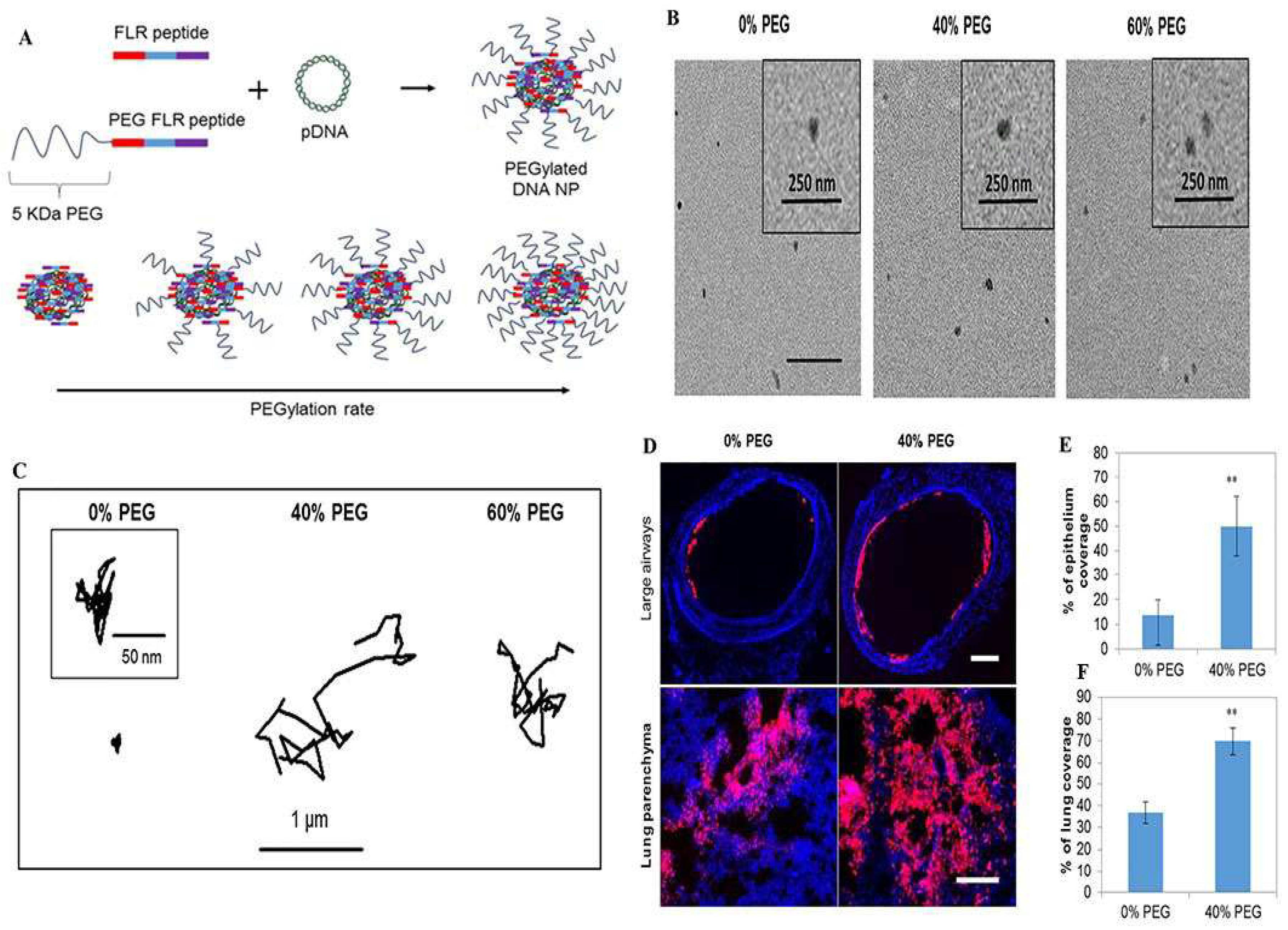
3.1.1. Nanoparticles Based on Hydrophilic Polymer Modifications
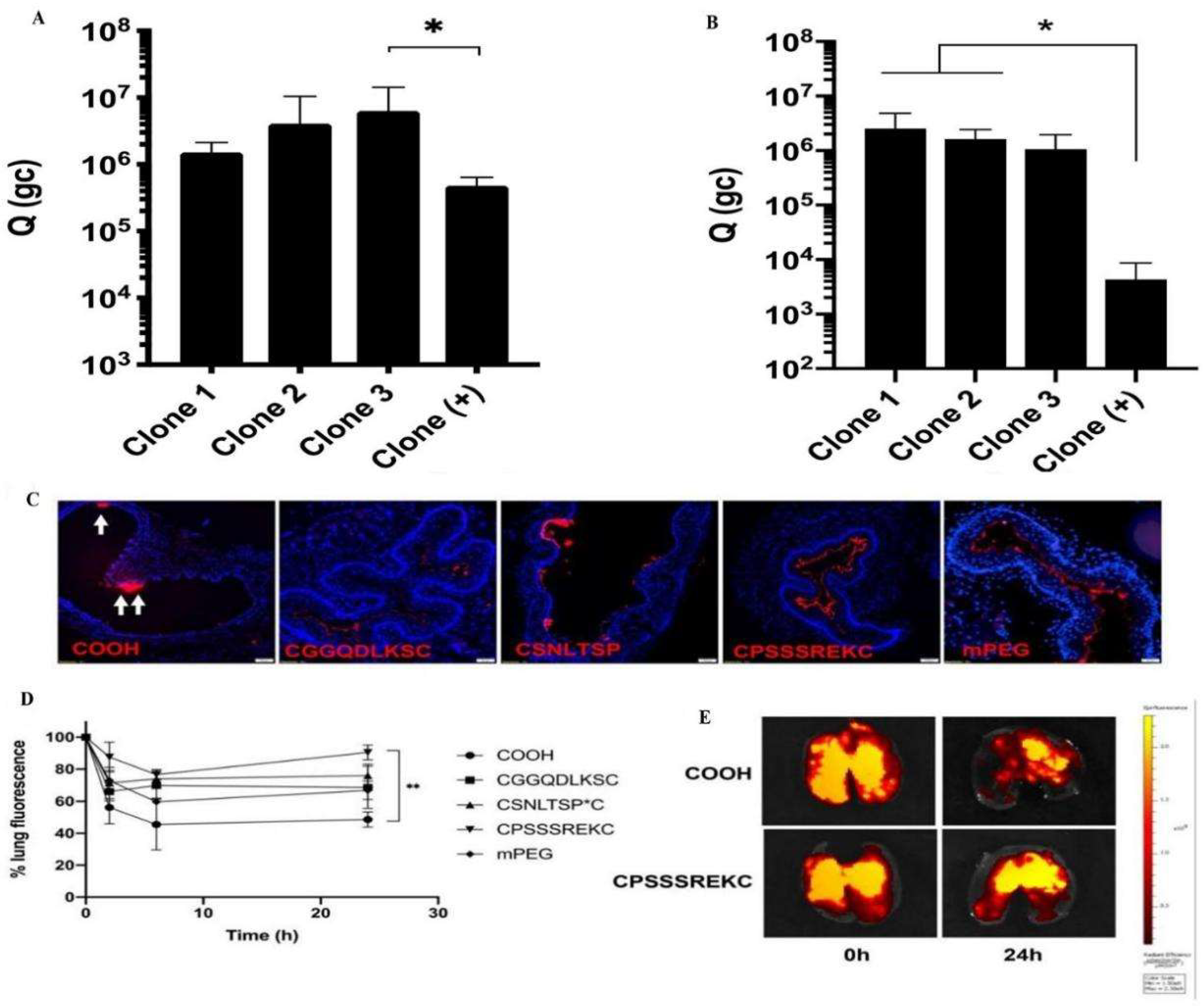
3.1.2. Nanoparticles Based on Cell-Penetrating Peptide Modifications
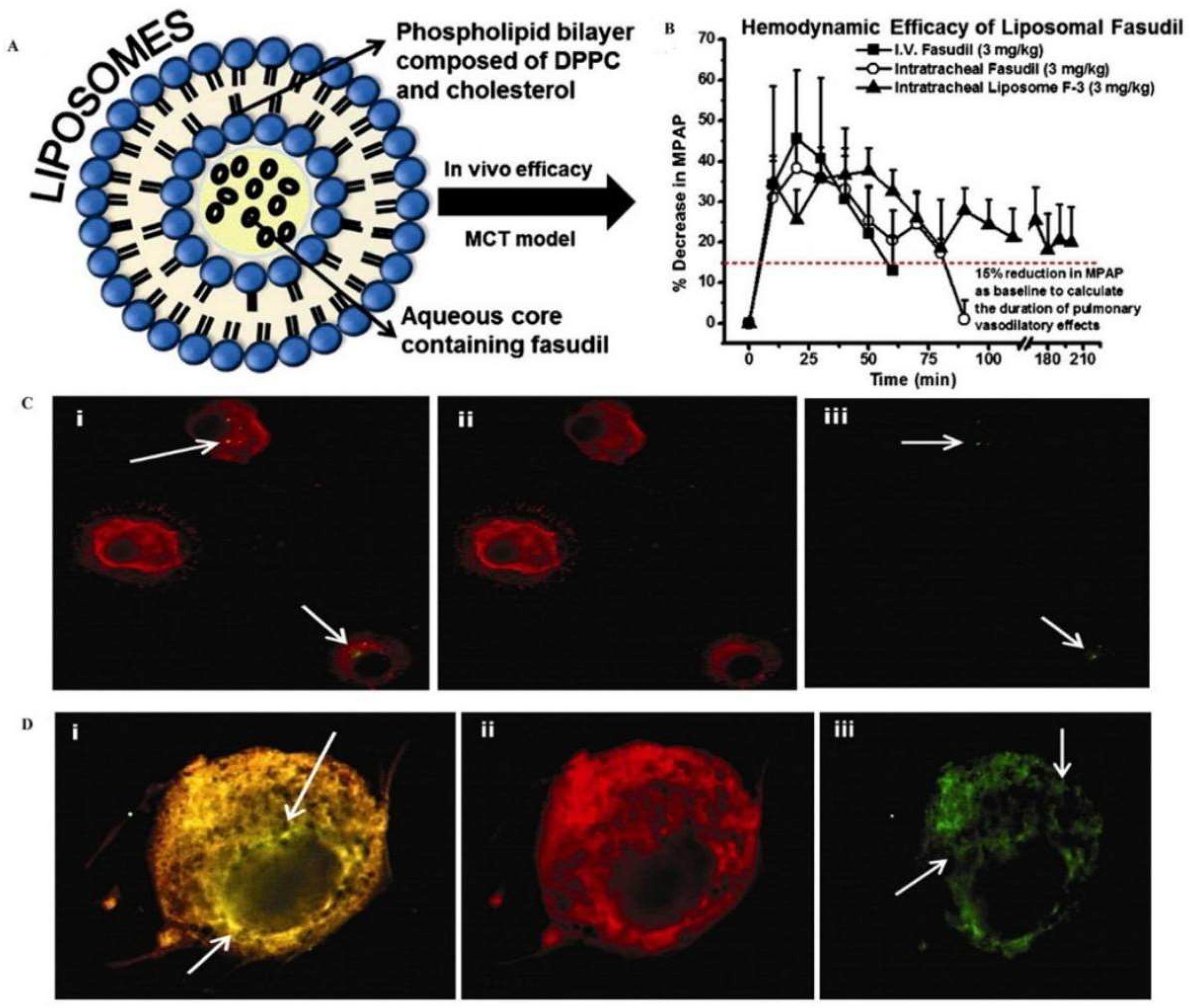
3.1.3. Lipid-Based Nanoparticles
3.2. Mucosal Adhesion System
3.2.1. Nanoparticles Based on Chitosan Modification
Nanoparticles Modified with Thiolated Chitosan
Nanoparticles Modified with Quaternized Chitosan
3.2.2. Hyaluronic Acid-Based Modified Nanoparticles
3.3. Mucolytic Agent Modified Nanoparticles
3.3.1. Nanoparticles Based on Thiol Modification Containing Free Sulfhydryl Groups
3.3.2. Protein Hydrolase-Based Modified Nanoparticles
3.4. Clinical Study of Drug Delivery Targeting Mucus
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Osman, G.; Rodriguez, J.; Chan, S.Y.; Chisholm, J.; Duncan, G.; Kim, N.; Tatler, A.L.; Shakesheff, K.M.; Hanes, J.; Suk, J.S.; et al. PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J. Control. Release 2018, 285, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.G.; Flanagan, D.R.; Donovan, M.D. The limiting role of mucus in drug absorption: Drug permeation through mucus solution. Int. J. Pharm. 1995, 126, 179–187. [Google Scholar] [CrossRef]
- Sears, P.R.; Davis, C.W.; Chua, M.; Sheehan, J.K. Mucociliary interactions and mucus dynamics in ciliated human bronchial epithelial cell cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L181–L186. [Google Scholar] [CrossRef] [PubMed]
- Button, B.; Cai, L.H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Vladar, E.K.; Janssen, W.J.; Evans, C.M. Mucociliary Defense: Emerging Cellular, Molecular, and Animal Models. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), S210–S215. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell. Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, H. Inhalation therapy for bronchial asthma: Strategies and targets. Curr. Opin. Pulm. Med. 2003, 9 (Suppl. S1), S3–S7. [Google Scholar] [CrossRef]
- Duncan, G.A.; Jung, J.; Hanes, J.; Suk, J.S. The Mucus Barrier to Inhaled Gene Therapy. Mol. Ther. 2016, 24, 2043–2053. [Google Scholar] [CrossRef]
- Cingolani, E.; Alqahtani, S.; Sadler, R.C.; Prime, D.; Stolnik, S.; Bosquillon, C. In vitro investigation on the impact of airway mucus on drug dissolution and absorption at the air-epithelium interface in the lungs. Eur. J. Pharm. Biopharm. 2019, 141, 210–220. [Google Scholar] [CrossRef]
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013, 65, 1816–1827. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shukla, D.; Mishra, B.; Singh, S. Lipid—An emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. 2009, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, X.; Dou, Y.; Xu, J.; Zhang, J.; Fan, T.; Du, J.; Liu, K.; Deng, Y.; Zhao, L.; et al. Exendin-4 Loaded Nanoparticles with a Lipid Shell and Aqueous Core Containing Micelles for Enhanced Intestinal Absorption. J. Biomed. Nanotechnol. 2015, 11, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, Z.R.; Gong, T.; Chen, G.Q.; Sun, X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials 2012, 33, 1583–1588. [Google Scholar] [CrossRef]
- Cortesi, R.; Campioni, M.; Ravani, L.; Drechsler, M.; Pinotti, M.; Esposito, E. Cationic lipid nanosystems as carriers for nucleic acids. New Biotechnol. 2014, 31, 44–54. [Google Scholar] [CrossRef]
- das Neves, J.; Bahia, M.F.; Amiji, M.M.; Sarmento, B. Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opin. Drug Deliv. 2011, 8, 1085–1104. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control. Release 2014, 192, 131–140. [Google Scholar] [CrossRef]
- Bures, P.; Huang, Y.; Oral, E.; Peppas, N.A. Surface modifications and molecular imprinting of polymers in medical and pharmaceutical applications. J. Control. Release 2001, 72, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that “Slip” through the Human Mucus Barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Monaco, V.; Stefanini, C. Assessing the Tidal Volume through Wearables: A Scoping Review. Sensors 2021, 21, 4124. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.F.; Méndez, G.A.; Lagos, R.A.; Vargas, M.S. Mucociliary clearance system in lung defense. Rev. Med. Chil. 2014, 142, 606–615. [Google Scholar] [CrossRef]
- Meziu, E.; Koch, M.; Fleddermann, J.; Schwarzkopf, K.; Schneider, M.; Kraegeloh, A. Visualization of the structure of native human pulmonary mucus. Int. J. Pharm. 2021, 597, 120238. [Google Scholar] [CrossRef] [PubMed]
- Button, B.; Okada, S.F.; Frederick, C.B.; Thelin, W.R.; Boucher, R.C. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci. Signal. 2013, 6, ra46. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, M.; Stein, P.C.; Flaten, G.E.; di Cagno, M.P. Impact of Mucin on Drug Diffusion: Development of a Straightforward in Vitro Method for the Determination of Drug Diffusivity in the Presence of Mucin. Pharmaceutics 2020, 12, 168. [Google Scholar] [CrossRef]
- Song, D.; Cahn, D.; Duncan, G.A. Mucin Biopolymers and Their Barrier Function at Airway Surfaces. Langmuir 2020, 36, 12773–12783. [Google Scholar] [CrossRef]
- Wan, F.; Herzberg, M.; Huang, Z.; Hassenkam, T.; Nielsen, H.M. A free-floating mucin layer to investigate the effect of the local microenvironment in lungs on mucin-nanoparticle interactions. Acta Biomater. 2020, 104, 115–123. [Google Scholar] [CrossRef]
- Kirkham, S.; Sheehan, J.K.; Knight, D.; Richardson, P.S.; Thornton, D.J. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 2002, 361, 537–546. [Google Scholar] [CrossRef]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef]
- Wu, T.; Liao, W.; Wang, W.; Zhou, J.; Tan, W.; Xiang, W.; Zhang, J.; Guo, L.; Chen, T.; Ma, D.; et al. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr. Polym. 2018, 197, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sands, M.; Kron, M.A.; Brown, R.B. Pentamidine: A review. Rev. Infect. Dis. 1985, 7, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Yanagawa, Y.; Tachikawa, T.; Deki, Y.; Uchiyama, H.; Shirakawa, Y.; Tozuka, Y. Development of porous particles using dextran as an excipient for enhanced deep lung delivery of rifampicin. Int. J. Pharm. 2019, 555, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Rubin, E.J.; Bifani, P.; Mathys, V.; Lim, V.; Au, M.; Jang, J.C.; Nam, J.; Dick, T.; Walker, J.R.; et al. para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis. J. Biol. Chem. 2013, 288, 28951. [Google Scholar] [CrossRef]
- Gopal, P.; Gruber, G.; Dartois, V.; Dick, T. Pharmacological and Molecular Mechanisms Behind the Sterilizing Activity of Pyrazinamide. Trends Pharmacol. Sci. 2019, 40, 930–940. [Google Scholar] [CrossRef]
- Murgia, X.; Pawelzyk, P.; Schaefer, U.F.; Wagner, C.; Willenbacher, N.; Lehr, C.M. Size-Limited Penetration of Nanoparticles into Porcine Respiratory Mucus after Aerosol Deposition. Biomacromolecules 2016, 17, 1536–1542. [Google Scholar] [CrossRef]
- Webster, M.J.; Tarran, R. Slippery When Wet: Airway Surface Liquid Homeostasis and Mucus Hydration. Curr. Top Membr. 2018, 81, 293–335. [Google Scholar] [CrossRef]
- Kaufman, H.; Arfken, G. Mathematical Methods for Physicists. Can. Math Bull. 1967, 10, 624. [Google Scholar] [CrossRef]
- Butnarasu, C.; Caron, G.; Pacheco, D.P.; Petrini, P.; Visentin, S. Cystic Fibrosis Mucus Model to Design More Efficient Drug Therapies. Mol. Pharm. 2022, 19, 520–531. [Google Scholar] [CrossRef]
- Dawson, M.; Wirtz, D.; Hanes, J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J. Biol. Chem. 2003, 278, 50393–50401. [Google Scholar] [CrossRef]
- Suk, J.S.; Lai, S.K.; Wang, Y.Y.; Ensign, L.M.; Zeitlin, P.L.; Boyle, M.P.; Hanes, J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.P.; Butnarasu, C.S.; Briatico Vangosa, F.; Pastorino, L.; Visai, L.; Visentin, S.; Petrini, P. Disassembling the complexity of mucus barriers to develop a fast screening tool for early drug discovery. J. Mater. Chem. B 2019, 7, 4940–4952. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2021, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, J.; Yang, Y.; Zhu, C.; Su, Q.; Guo, S.; Sun, J.; Gan, Y.; Shi, X.; Gao, H. Rotation-Facilitated Rapid Transport of Nanorods in Mucosal Tissues. Nano Lett. 2016, 16, 7176–7182. [Google Scholar] [CrossRef] [PubMed]
- Kolte, A.; Patil, S.; Lesimple, P.; Hanrahan, J.W.; Misra, A. PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int. J. Pharm. 2017, 524, 382–396. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Zhong, Q.; Bielski, E.R.; da Rocha, S.R.P. Nanoparticles of pH-Responsive, PEG-Doxorubicin Conjugates: Interaction with an in Vitro Model of Lung Adenocarcinoma and Their Direct Formulation in Propellant-Based Portable Inhalers. Mol. Pharmaceut. 2017, 14, 3866–3878. [Google Scholar] [CrossRef]
- Laffleur, F.; Hintzen, F.; Shahnaz, G.; Rahmat, D.; Leithner, K.; Bernkop-Schnurch, A. Development and in vitro evaluation of slippery nanoparticles for enhanced diffusion through native mucus. Nanomedicine 2014, 9, 387–396. [Google Scholar] [CrossRef]
- Leal, J.; Dong, T.; Taylor, A.; Siegrist, E.; Gao, F.; Smyth, H.D.C.; Ghosh, D. Mucus-penetrating phage-displayed peptides for improved transport across a mucus-like model. Int. J. Pharm. 2018, 553, 57–64. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; McMurtry, I.F.; Oka, M.; Nozik-Grayck, E.; Komatsu, M.; Ahsan, F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J. Control. Release 2013, 167, 189–199. [Google Scholar] [CrossRef]
- Karamanidou, T.; Karidi, K.; Bourganis, V.; Kontonikola, K.; Kammona, O.; Kiparissides, C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 2015, 97 Pt A, 223–229. [Google Scholar] [CrossRef]
- Conte, G.; Costabile, G.; Baldassi, D.; Rondelli, V.; Bassi, R.; Colombo, D.; Linardos, G.; Fiscarelli, E.V.; Sorrentino, R.; Miro, A.; et al. Hybrid Lipid/Polymer Nanoparticles to Tackle the Cystic Fibrosis Mucus Barrier in siRNA Delivery to the Lungs: Does PEGylation Make the Difference? ACS Appl. Mater. Interfaces 2022, 14, 7565–7578. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, J.W.; Cha, H.R.; Jung, S.Y.; Lee, J.E.; Jung, S.S.; Kim, J.O.; Kim, S.Y.; Lee, C.S.; Park, H.S. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int. J. Nanomed. 2012, 7, 1329–1343. [Google Scholar] [CrossRef]
- Barreto, E.; Serra, M.F.; Dos Santos, R.V.; Dos Santos, C.E.; Hickmann, J.; Cotias, A.C.; Pão, C.R.; Trindade, S.G.; Schimidt, V.; Giacomelli, C.; et al. Local Administration of Gold Nanoparticles Prevents Pivotal Pathological Changes in Murine Models of Atopic Asthma. J. Biomed. Nanotechnol. 2015, 11, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Dunnhaupt, S.; Barthelmes, J.; Hombach, J.; Sakloetsakun, D.; Arkhipova, V.; Bernkop-Schnurch, A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int. J. Pharm. 2011, 408, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Agnihotri, V.V.; Patil, K.Y.; Pardeshi, S.R.; Surana, S.J. Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Int. J. Biol. Macromol. 2020, 165 Pt A, 445–459. [Google Scholar] [CrossRef]
- du Plessis, L.H.; Kotze, A.F.; Junginger, H.E. Nasal and rectal delivery of insulin with chitosan and N-trimethyl chitosan chloride. Drug Deliv. 2010, 17, 399–407. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.; Liu, T.; Cun, D.; Fang, L.; Yang, M. Inhaled hyaluronic acid microparticles extended pulmonary retention and suppressed systemic exposure of a short-acting bronchodilator. Carbohydr. Polym. 2017, 172, 197–204. [Google Scholar] [CrossRef]
- Liu, T.T.; Han, M.H.; Tian, F.; Cun, D.M.; Rantanen, J.; Yang, M.S. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: In vitro and in vivo evaluation. Carbohyd. Polym. 2018, 181, 1143–1152. [Google Scholar] [CrossRef]
- Lababidi, N.; Montefusco-Pereira, C.V.; de Souza Carvalho-Wodarz, C.; Lehr, C.M.; Schneider, M. Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur. J. Pharm. Biopharm. 2020, 157, 200–210. [Google Scholar] [CrossRef]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Xu, E.Y.; Moehwald, M.; Chen, L.; Zhang, X.; Mao, S. Inhalable PLGA microspheres: Tunable lung retention and systemic exposure via polyethylene glycol modification. Acta Biomater. 2021, 123, 325–334. [Google Scholar] [CrossRef]
- Craparo, E.F.; Porsio, B.; Sardo, C.; Giammona, G.; Cavallaro, G. Pegylated Polyaspartamide-Polylactide-Based Nanoparticles Penetrating Cystic Fibrosis Artificial Mucus. Biomacromolecules 2016, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.P.; Azevedo, N.F.; Pereira, M.O. Emergent bacteria in cystic fibrosis: In vitro biofilm formation and resilience under variable oxygen conditions. Biomed. Res. Int. 2014, 2014, 678301. [Google Scholar] [CrossRef]
- Costerton, J.W. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 2001, 9, 50–52. [Google Scholar] [CrossRef]
- Staudinger, B.J.; Muller, J.F.; Halldorsson, S.; Boles, B.; Angermeyer, A.; Nguyen, D.; Rosen, H.; Baldursson, O.; Gottfreethsson, M.; Guethmundsson, G.H.; et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2014, 189, 812–824. [Google Scholar] [CrossRef]
- Bahamondez-Canas, T.F.; Zhang, H.; Tewes, F.; Leal, J.; Smyth, H.D.C. PEGylation of Tobramycin Improves Mucus Penetration and Antimicrobial Activity against Pseudomonas aeruginosa Biofilms in Vitro. Mol. Pharm. 2018, 15, 1643–1652. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. Acs. Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Peng, X.; Liu, X.; Arasappan, D.; Wylie, D.C.; Schwartz, S.H.; Fullmer, J.J.; McWilliams, B.C.; Smyth, H.D.C.; Ghosh, D. Peptides as surface coatings of nanoparticles that penetrate human cystic fibrosis sputum and uniformly distribute in vivo following pulmonary delivery. J. Control. Release 2020, 322, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Zanin, M.; Baviskar, P.; Webster, R.; Webby, R. The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016, 19, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, A.; Gurer, E.S.; Yapar, E.A.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, F.; Perera, G.; Hauptstein, S.; Muller, C.; Laffleur, F.; Bernkop-Schnurch, A. In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS) for leuprorelin. Int. J. Pharm. 2014, 472, 20–26. [Google Scholar] [CrossRef]
- Friedl, H.; Dunnhaupt, S.; Hintzen, F.; Waldner, C.; Parikh, S.; Pearson, J.P.; Wilcox, M.D.; Bernkop-Schnurch, A. Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 2013, 102, 4406–4413. [Google Scholar] [CrossRef]
- Kandil, R.; Merkel, O.M. Pulmonary delivery of siRNA as a novel treatment for lung diseases. Ther. Deliv. 2019, 10, 203–206. [Google Scholar] [CrossRef]
- Sanchez, A.D.; Paunovska, K.; Cristian, A.; Dahlman, J.E. Treating Cystic Fibrosis with mRNA and CRISPR. Hum. Gene Ther. 2020, 31, 940–955. [Google Scholar] [CrossRef]
- Kim, N.; Duncan, G.A.; Hanes, J.; Suk, J.S. Barriers to inhaled gene therapy of obstructive lung diseases: A review. J. Control. Release 2016, 240, 465–488. [Google Scholar] [CrossRef]
- Esim, O.; Savaser, A.; Ozkan, C.K.; Tas, C.; Ozkan, Y. Investigation of the mucoadhesivity, swelling, and drug release mechanisms of indomethacin buccal tablets: Effect of formulation variables. Drug Dev. Ind. Pharm. 2020, 46, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Park, T.E.; Reesor, E.; Cherukula, K.; Hasan, A.; Firdous, J.; Singh, B.; Kang, S.K.; Choi, Y.J.; Park, I.K.; et al. Mucoadhesive Chitosan Derivatives as Novel Drug Carriers. Curr. Pharm. Des. 2015, 21, 4285–4309. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, L.; Vertuccio, L.; Barra, G.; Naddeo, C.; Sorrentino, A.; Lavorgna, M.; Raimondo, M.; Calabrese, E. Eco-friendly polymer nanocomposites designed for self-healing applications. Polymer 2021, 223, 123718. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Shim, S.; Yoo, H.S. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Mar. Drugs 2020, 18, 605. [Google Scholar] [CrossRef]
- Shitrit, Y.; Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 2017, 517, 247–255. [Google Scholar] [CrossRef]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Kast, C.E. Chemically modified chitosans as enzyme inhibitors. Adv. Drug Deliv. Rev. 2001, 52, 127–137. [Google Scholar] [CrossRef]
- Mahmood, A.; Lanthaler, M.; Laffleur, F.; Huck, C.W.; Bernkop-Schnurch, A. Thiolated chitosan micelles: Highly mucoadhesive drug carriers. Carbohydr. Polym. 2017, 167, 250–258. [Google Scholar] [CrossRef]
- Chopra, S.; Mahdi, S.; Kaur, J.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J. Pharm. Pharmacol. 2006, 58, 1021–1032. [Google Scholar] [CrossRef]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hao, S.; Sun, B.; Zhao, D.; Yan, X.; Jin, Z.; Zhao, K. Quaternized Chitosan Nanoparticles in Vaccine Applications. Curr. Med. Chem. 2020, 27, 4932–4944. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. In vitro evaluation of N-(2-hydroxy) propyl-3-trimethyl ammonium chitosan for oral insulin delivery. Carbohyd. Polym. 2011, 84, 103–109. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Mu, B.; Fan, L.; Wang, A. Facile preparation of magnetic 2-hydroxypropyltrimethyl ammonium chloride chitosan/Fe3O4/halloysite nanotubes microspheres for the controlled release of ofloxacin. Carbohydr. Polym. 2014, 102, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Zambito, Y.; Di Colo, G.; Caramella, C. Buccal penetration enhancement properties of N-trimethyl chitosan: Influence of quaternization degree on absorption of a high molecular weight molecule. Int. J. Pharm. 2005, 297, 146–155. [Google Scholar] [CrossRef]
- Thanou, M.M.; Kotze, A.F.; Scharringhausen, T.; Luessen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Hamman, J.H.; Stander, M.; Kotze, A.F. Effect of the degree of quaternisation of N-trimethyl chitosan chloride on absorption enhancement: In vivo evaluation in rat nasal epithelia. Int. J. Pharm. 2002, 232, 235–242. [Google Scholar] [CrossRef]
- Jiao, Y.; Pang, X.; Zhai, G.X. Advances in Hyaluronic Acid-Based Drug Delivery Systems. Curr. Drug Targets 2016, 17, 720–730. [Google Scholar] [CrossRef]
- Griesser, J.; Hetenyi, G.; Bernkop-Schnurch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef]
- Marques, L.; Vale, N. Salbutamol in the Management of Asthma: A Review. Int. J. Mol. Sci. 2022, 23, 14207. [Google Scholar] [CrossRef]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. Combination of urea-crosslinked hyaluronic acid and sodium ascorbyl phosphate for the treatment of inflammatory lung diseases: An in vitro study. Eur. J. Pharm. Sci. 2018, 120, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.J.; Whateley, T.L.; Thomas, M.; Eccleston, G.M. Controlled drug delivery to the lung: Influence of hyaluronic acid solution conformation on its adsorption to hydrophobic drug particles. Int. J. Pharm. 2007, 330, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sheffner, A.L. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann. N. Y. Acad. Sci. 1963, 106, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Ruiz, A.; Cerda, M.; Martinez-Losa, M.; Cortijo, J.; Santangelo, F.; Serrano-Mollar, A.; Llombart-Bosch, A.; Morcillo, E.J. Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur. Respir. J. 2003, 22, 900–905. [Google Scholar] [CrossRef]
- Sipos, T.; Merkel, J.R. An effect of calcium ions on the activity, heat stability, and structure of trypsin. Biochemistry 1970, 9, 2766–2775. [Google Scholar] [CrossRef]
- Rosenecker, J.; Naundorf, S.; Rudolph, C. Airway Surface Liquid Contains Endogenous Dnase Activity Which Can Be Activated by Exogenous Magnesium. Eur. J. Med. Res. 2009, 14, 304–308. [Google Scholar] [CrossRef]
- Sanders, N.N.; De Smedt, S.C.; Van Rompaey, E.; Simoens, P.; De Baets, F.; Demeester, J. Cystic fibrosis sputum: A barrier to the transport of nanospheres. Am. J. Respir. Crit. Care Med. 2000, 162, 1905–1911. [Google Scholar] [CrossRef]
- Alton, E.W.; Stern, M.; Farley, R.; Jaffe, A.; Chadwick, S.L.; Phillips, J.; Davies, J.; Smith, S.N.; Browning, J.; Davies, M.G.; et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: A double-blind placebo-controlled trial. Lancet 1999, 353, 947–954. [Google Scholar] [CrossRef]
- Noone, P.G.; Hohneker, K.W.; Zhou, Z.; Johnson, L.G.; Foy, C.; Gipson, C.; Jones, K.; Noah, T.L.; Leigh, M.W.; Schwartzbach, C.; et al. Safety and biological efficacy of a lipid-CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis. Mol. Ther. 2000, 1, 105–114. [Google Scholar] [CrossRef]
- He, C.; Zhao, D. Chitosan-N-Arginine Nanoparticles, and Preparation Method and Application Thereof. CN115252640A, 1 November 2022. [Google Scholar]
- Hanes, J.; Ensign, L. Mucus-Penetrating Budesonide Nanosuspension Enema for Local Treatment of Inflammatory Bowel Disease. US2020306202A1, 1 October 2020. [Google Scholar]
- Suk Jung, S.; Hanes, J.S. Mucus Penetrating Gene Carriers. US2017246320A1, 31 August 2017. [Google Scholar]
- Ensign, L.; Cone, R. Nanoparticle Formulations with Enhanced Mucosal Penetration. US2016317459A1, 3 November 2016. [Google Scholar]
- Li, N.; Li, X. Preparation Method of Powder Inhalation for Slow-Release Delivery of COPD (Chronic Obstructive Pulmonary Disease) Treatment Medicine in Targeted Small Airway. CN115227682A, 25 October 2022. [Google Scholar]
- Yu, L.; Liu, L. Acid-Sensitive Group Modified Viscous Inert Nano-Particles as Well as Preparation Method and Application of Acid-Sensitive Group Modified Viscous Inert Nano-Particles. CN115192734A, 18 October 2022. [Google Scholar]
- Niedermeyer, W. Use of Nanoparticles for Treating Respiratory Infections Associated with Cystic Fibrosis. AU2020315585A1, 3 March 2022. [Google Scholar]
- Tagalakis, A.; Hart, S. Use of Alginate Oligomers to Enhance the Translocation of Micro/Nanoparticles Across Mucus Layers. US2021030891A1, 4 February 2021. [Google Scholar]
- Liu, W.; Rao, R. Polyphosphoester Polymer, Preparation Method Thereof, Modified Porous Silicon Nanoparticles and Preparation Method and Application of Modified Porous Silicon Nanoparticles. CN109762170A, 17 May 2019. [Google Scholar]


| Nanoparticle Type | Formulation Details | Results | Reference |
|---|---|---|---|
|
|
| [45] |
|
| [46] | |
|
| [47] | |
|
|
| [48] |
|
| [1] | |
|
|
| [49] |
|
| [50] | |
|
| [51] | |
|
|
| [52] |
|
| [53] | |
|
|
| [54] |
|
| [55] | |
|
| [56] | |
|
|
| [57] |
|
| [58] | |
|
|
| [59] |
| Patent Name | Inventors | Public Information | Priority Date | Reference |
|---|---|---|---|---|
| Chitosan-N-arginine nanoparticles, and preparation method and application thereof | HE CHAOLIANG ZHAO DAN | CN115252640 (A) 2022–11–01 CN115252640 (B) 2023–08–29 | 2022–06–23 | [110] |
| MUCUS-PENETRATING BUDESONIDE NANOSUSPENSION ENEMA FOR LOCAL TREATMENT OF INFLAMMATORY BOWEL DISEASE | HANES JUSTIN [US] ENSIGN LAURA M [US] | US2020306202 (A1) 2020–10–01 | 2017–07–14 | [111] |
| MUCUS PENETRATING GENE CARRIERS | SUK JUNG SOO [US] HANES JUSTIN SCOT [US] | US2017246320 (A1) 2017–08–31 US10729786 (B2) 2020–08–04 | 2011–02–08 | [112] |
| NANOPARTICLE FORMULATIONS WITH ENHANCED MUCOSAL PENETRATION | ENSIGN LAURA [US] CONE RICHARD [US] | US2016317459 (A1) 2016–11–03 US9629813 (B2) 2017–04–25 | 2012–01–19 | [113] |
| Preparation method of powder inhalation for slow-release delivery of COPD (chronic obstructive pulmonary disease) treatment medicine in targeted small airway | LI NAN LI XU | CN115227682 (A) 2022–10–25 | 2022–07–25 | [114] |
| Acid-sensitive group modified viscous inert nanoparticles as well as preparation method and application of acid-sensitive group modified viscous inert nanoparticles | YU LINLING LIU LING | CN115192734 (A) 2022–10–18 | 2022–02–22 | [115] |
| Use of nanoparticles for treating respiratory infections associated with cystic fibrosis | NIEDERMEYER WILLIAM | AU2020315585 (A1) 2022–03–03 | 2019–07–12 | [116] |
| USE OF ALGINATE OLIGOMERS TO ENHANCE THE TRANSLOCATION OF MICRO/NANOPARTICLES ACROSS MUCUS LAYERS | TAGALAKIS ARISTIDES [GB] HART STEPHEN [GB] | US2021030891 (A1) 2021–02–04 | 2018–03–19 | [117] |
| Polyphosphoester polymer, preparation method thereof, modified porous silicon nanoparticles and preparation method and application of modified porous silicon nanoparticles | LIU WEI RAO RONG | CN109762170 (A) 2019–05–17 CN109762170 (B) 2020–03–31 | 2019–01–23 | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Sha, X. Nanoparticle-Mediated Strategies for Enhanced Drug Penetration and Retention in the Airway Mucosa. Pharmaceutics 2023, 15, 2457. https://doi.org/10.3390/pharmaceutics15102457
Yan X, Sha X. Nanoparticle-Mediated Strategies for Enhanced Drug Penetration and Retention in the Airway Mucosa. Pharmaceutics. 2023; 15(10):2457. https://doi.org/10.3390/pharmaceutics15102457
Chicago/Turabian StyleYan, Xin, and Xianyi Sha. 2023. "Nanoparticle-Mediated Strategies for Enhanced Drug Penetration and Retention in the Airway Mucosa" Pharmaceutics 15, no. 10: 2457. https://doi.org/10.3390/pharmaceutics15102457





