Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
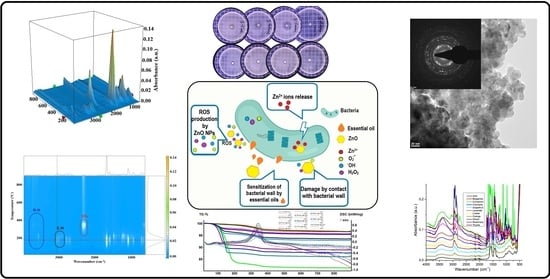
3.1. X-ray Diffraction Analysis (XRD)
3.2. Scanning and Transmission Electron Microscopy
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Transmission Electron Microscopy (TEM)
3.3. Spectroscopic Studies
3.3.1. UV-Vis and Fluorescence (PL) Spectroscopy
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Thermal Analysis (TG-DSC)
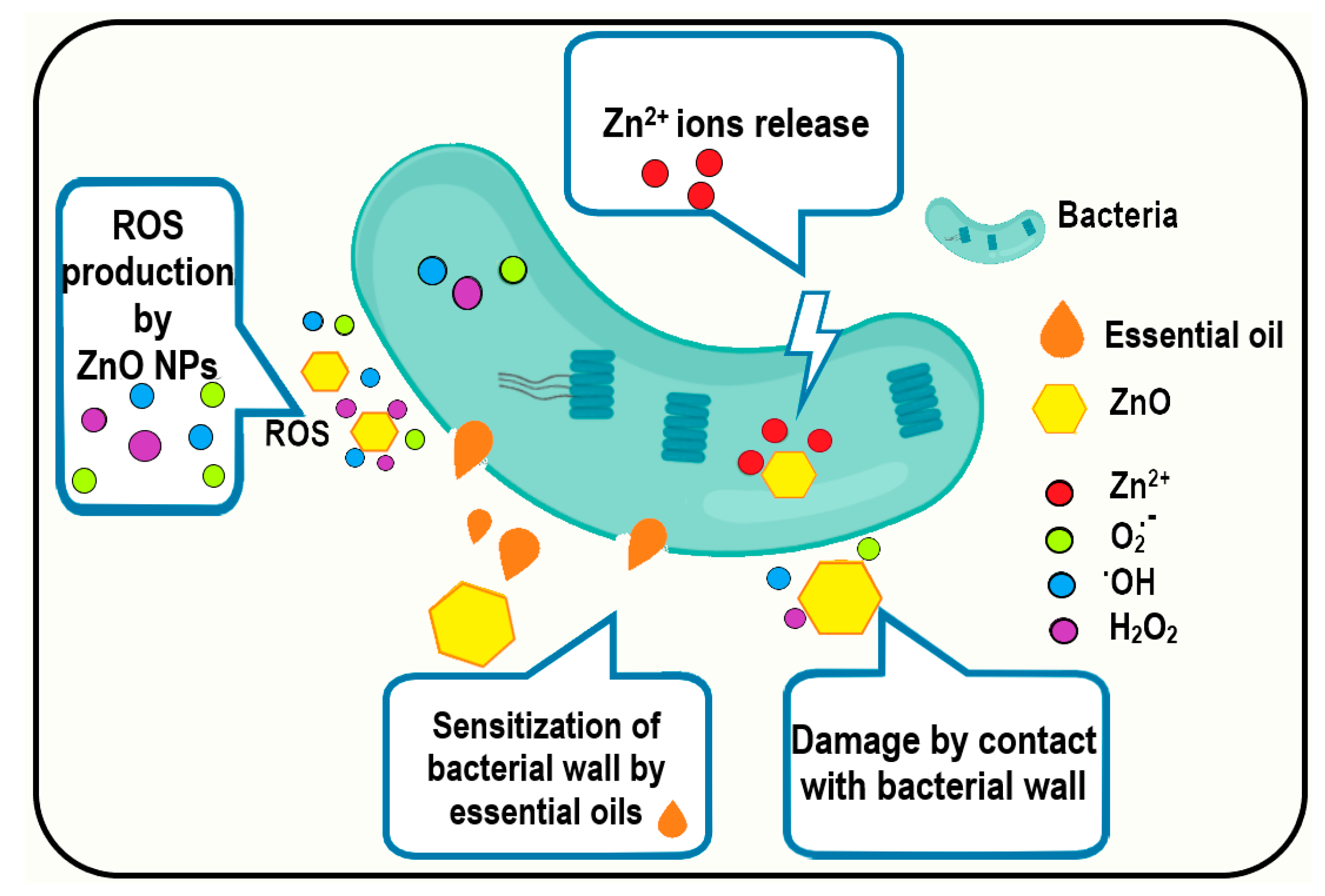
3.5. Antibacterial Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deaconu, M.; Nicu, I.; Tincu, R.; Brezoiu, A.M.; Mitran, R.A.; Vasile, E.; Matei, C.; Berger, D. Tailored doxycycline delivery from mcm-41-type silica carriers. Chem. Pap. 2018, 72, 1869–1880. [Google Scholar] [CrossRef]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Control of biofilm-associated infections by signaling molecules and nanoparticles. Int. J. Pharm. 2016, 510, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive surfaces of polylactide and silver nanoparticles for the prevention of microbial contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Mogosanu, G.D.; Grumezescu, A.M.; Huang, K.S.; Bejenaru, L.E.; Bejenaru, C. Prevention of microbial communities: Novel approaches based natural products. Curr. Pharm. Biotechnol. 2015, 16, 94–111. [Google Scholar] [CrossRef]
- Feger, G.; Angelov, B.; Angelova, A. Prediction of amphiphilic cell-penetrating peptide building blocks from protein-derived amino acid sequences for engineering of drug delivery nanoassemblies. J. Phys. Chem. B 2020, 124, 4069–4078. [Google Scholar] [CrossRef]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.N.; Yetmez, M. Zno applications and challenges. Curr. Org. Chem. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.C.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial activity of solvothermal obtained zno nanoparticles with different morphology and photocatalytic activity against a dye mixture: Methylene blue, rhodamine b and methyl orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Spoiala, A.; Ilie, C.I.; Trusca, R.D.; Oprea, O.C.; Surdu, V.A.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Ditu, L.M. Zinc oxide nanoparticles for water purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Ficai, D.; Oprea, O.; Ficai, A.; Holban, A.M. Metal oxide nanoparticles: Potential uses in biomedical applications. Curr. Proteomics 2014, 11, 139–149. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the alcohols on the zno synthesis and its properties: The photocatalytic and antimicrobial activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Preda, M.D.; Popa, M.L.; Neacsu, I.A.; Grumezescu, A.M.; Ginghina, O. Antimicrobial clothing based on electrospun fibers with zno nanoparticles. Int. J. Mol. Sci. 2023, 24, 1629. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Holban, A.M.; Ficai, A.; Grumezescu, A.M. Zinc oxide nanostrucures: New trends in antimicrobial therapy. In Nanostructures for Antimicrobial Therapy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 503–514. [Google Scholar]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with zno nanoparticles and citronella essential oil-a novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Pavaloiu, R.D.; Sha’at, F.; Bubueanu, C.; Deaconu, M.; Neagu, G.; Sha’at, M.; Anastasescu, M.; Mihailescu, M.; Matei, C.; Nechifor, G.; et al. Polyphenolic extract from sambucus ebulus l. Leaves free and loaded into lipid vesicles. Nanomaterials 2020, 10, 56. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Natural compounds for preventing ear, nose, and throat-related oral infections. Plants 2021, 10, 1847. [Google Scholar] [CrossRef] [PubMed]
- Francikowski, J.; Baran, B.; Cup, M.; Janiec, J.; Krzyzowski, M. Commercially available essential oil formulas as repellents against the stored-product pest alphitobius diaperinus. Insects 2019, 10, 96. [Google Scholar] [CrossRef]
- Go, E.J.; Song, K.B. Effect of java citronella essential oil addition on the physicochemical properties of gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020, 29, 909–915. [Google Scholar] [CrossRef]
- Garcia-Fajardo, J.A.; Flores-Mendez, D.A.; Suarez-Jacobo, A.; Torres-Martinez, L.G.; Granados-Vallejo, M.; Corona-Gonzalez, R.I.; Guatemala-Morales, G.M.; Arriola-Guevara, E. Separation of d-limonene and other oxygenated compounds from orange essential oil by molecular distillation and fractional distillation with a wiped film evaporator. Processes 2023, 11, 991. [Google Scholar] [CrossRef]
- Hollingsworth, R.G. Limonene, a citrus extract, for control of mealybugs and scale insects. J. Econ. Entomol. 2005, 98, 772–779. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Saracila, M.; Turcu, R.P.; Dumitru, M. Effect of basil, thyme and sage essential oils as phytogenic feed additives on production performances, meat quality and intestinal microbiota in broiler chickens. Agriculture 2023, 13, 874. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jimenez, A.; Kenny, J.M.; Garrigos, M.C. Controlled release of thymol from poly(lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef]
- Bailen, M.; Illescas, C.; Quijada, M.; Martinez-Diaz, R.A.; Ochoa, E.; Gomez-Munoz, M.T.; Navarro-Rocha, J.; Gonzalez-Coloma, A. Anti-trypanosomatidae activity of essential oils and their main components from selected medicinal plants. Molecules 2023, 28, 1467. [Google Scholar] [CrossRef]
- Diass, K.; Merzouki, M.; Elfazazi, K.; Azzouzi, H.; Challioui, A.; Azzaoui, K.; Hammouti, B.; Touzani, R.; Depeint, F.; Gotor, A.A.; et al. Essential oil of lavandula officinalis: Chemical composition and antibacterial activities. Plants 2023, 12, 1571. [Google Scholar] [CrossRef]
- Todorova, D.; Yavorov, N.; Lasheva, V.; Damyanova, S.; Kostova, I. Lavender essential oil as antibacterial treatment for packaging paper. Coatings 2023, 13, 32. [Google Scholar] [CrossRef]
- Rochin-Medina, J.J.; Mendoza-Lopez, I.A.; Campo, N.C.D.; Bastidas-Bastidas, P.J.; Ramirez, K. Activity of plant essential oils against clinically and environmentally isolated salmonella enterica serotypes: In vitro assays and molecular docking. Lett. Appl. Microbiol. 2023, 76, ovad045. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Sirbu, I.O.; Covasa, M. The use of biopolymers as a natural matrix for incorporation of essential oils of medicinal plants. Gels-Basel 2022, 8, 756. [Google Scholar] [CrossRef]
- Brah, A.S.; Armah, F.A.; Obuah, C.; Akwetey, S.A.; Adokoh, C.K. Toxicity and therapeutic applications of citrus essential oils (ceos): A review. Int. J. Food Prop. 2023, 26, 301–326. [Google Scholar] [CrossRef]
- Zambito, Y.; Piras, A.M.; Fabiano, A. Bergamot essential oil: A method for introducing it in solid dosage forms. Foods 2022, 11, 3860. [Google Scholar] [CrossRef]
- He, S.K.; Wang, Y.F. Antimicrobial and antioxidant effects of kappa-carrageenan coatings enriched with cinnamon essential oil in pork meat. Foods 2022, 11, 2885. [Google Scholar] [CrossRef]
- Ogwaro, B.A.; O’Gara, E.A.; Hill, D.J.; Gibson, H. A study of the antimicrobial activity of combined black pepper and cinnamon essential oils against escherichia fergusonii in traditional african yoghurt. Foods 2021, 10, 2847. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L.; Gupta, T.B.; Bronlund, J. Manuka oil vs. Rosemary oil: Antimicrobial efficacies in wagyu and commercial beef against selected pathogenic microbes. Foods 2023, 12, 1333. [Google Scholar] [CrossRef]
- Olivas-Mendez, P.; Chavez-Martinez, A.; Santellano-Estrada, E.; Asorey, L.G.; Sanchez-Vega, R.; Renteria-Monterrubio, A.L.; Chavez-Flores, D.; Tirado-Gallegos, J.M.; Mendez-Zamora, G. Antioxidant and antimicrobial activity of rosemary (Rosmarinus officinalis) and garlic (Allium sativum) essential oils and chipotle pepper oleoresin (Capsicum annum) on beef hamburgers. Foods 2022, 11, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Seljak, K.B.; Kocbek, P.; Gasperlin, M. Mesoporous silica nanoparticles as delivery carriers: An overview of drug loading techniques. J. Drug Deliv. Sci. Technol. 2020, 59, 101906. [Google Scholar] [CrossRef]
- Sima, S.; Secuianu, C. The effect of functional groups on the phase behavior of carbon dioxide binaries and their role in ccs. Molecules 2021, 26, 3733. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of extraction methods, health benefits, and encapsulation and delivery using microemulsions and nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Racovita, R.C.; Ciuca, M.D.; Catana, D.; Comanescu, C.; Ciocirlan, O. Microemulsions of nonionic surfactant with water and various homologous esters: Preparation, phase transitions, physical property measurements, and application for extraction of tricyclic antidepressant drugs from aqueous media. Nanomaterials 2023, 13, 2311. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Ficai, D.; Ilie, C.I.; Trusca, R.D.; Surdu, V.A.; Oprea, O.C.; Mirt, A.L.; Vasilievici, G.; Semenescu, A.; et al. Increasing bioavailability of trans-ferulic acid by encapsulation in functionalized mesoporous silica. Pharmaceutics 2023, 15, 660. [Google Scholar] [CrossRef]
- Voicu, G.; Oprea, O.; Vasile, B.S.; Andronescu, E. Antibacterial activity of zinc oxide—Gentamicin hybrid material. Dig. J. Nanomater. Biostructures 2013, 8, 1191–1203. [Google Scholar]
- Alandiyjany, M.N.; Abdelaziz, A.S.; Abdelfattah-Hassan, A.; Hegazy, W.A.H.; Hassan, A.A.; Elazab, S.T.; Mohamed, E.A.A.; El-Shetry, E.S.; Saleh, A.A.; ElSawy, N.A.; et al. Novel in vivo assessment of antimicrobial efficacy of ciprofloxacin loaded mesoporous silica nanoparticles against salmonella typhimurium infection. Pharmaceuticals 2022, 15, 357. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial activities of chitosan derivatives. Pharmaceutics 2021, 13, 1639. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.S. Potential novel food-related and biomedical applications of nanomaterials combined with bacteriocins. Pharmaceutics 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Bactericidal activity of non-cytotoxic cationic nanoparticles against clinically and environmentally relevant pseudomonas spp. Isolates. Pharmaceutics 2021, 13, 1411. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C.; Banat, I.M. Recent advances in biomedical, therapeutic and pharmaceutical applications of microbial surfactants. Pharmaceutics 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Purcar, V.; Somoghi, R.; Nitu, S.G.; Nicolae, C.A.; Alexandrescu, E.; Gifu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchis, R.; Caprarescu, S.; et al. The effect of different coupling agents on nano-zno materials obtained via the sol-gel process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Prodan, G.; Matei, C.; Moscalu, F.; Diacon, A. Influence of synthesis route on the structure and properties of zinc oxide nanoparticles functionalized with anthocyanins from raw vegetable extracts. ECS J. Solid State Sci. Technol. 2017, 6, P870–P878. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Matei, C.; Prodan, G.; Aonofriesei, F.; Radu, M.D.; Moscalu, F. New composite nanomaterials with antimicrobial and photocatalytic properties based on silver and zinc oxide. J. Inorg. Organomet. Polym. 2019, 29, 2072–2082. [Google Scholar] [CrossRef]
- Rogozea, E.A.; Olteanu, N.L.; Petcu, A.R.; Lazar, C.A.; Meghea, A.; Mihaly, M. Extension of optical properties of ZnO/SiO2 materials induced by incorporation of au or nio nanoparticles. Opt. Mater. 2016, 56, 45–48. [Google Scholar] [CrossRef]
- Popa, M.L.; Preda, M.D.; Neacsu, I.A.; Grumezescu, A.M.; Ginghina, O. Traditional vs. Microfluidic synthesis of zno nanoparticles. Int. J. Mol. Sci. 2023, 24, 1875. [Google Scholar] [CrossRef]
- Imran, H.J.; Hubeatir, K.A.; Aadim, K.A. Synthesis and characterization of zno nanoparticles by pulsed laser ablation in liquid using different wavelengths for antibacterial application. Int. J. Nanoelectron. Mater. 2023, 16, 345–358. [Google Scholar]
- Oprea, O.; Andronescu, E.; Vasile, B.S.; Voicu, G.; Covaliu, C. Synthesis and characterization of zno nanopowder by non-basic route. Dig. J. Nanomater. Biostructures 2011, 6, 1393–1401. [Google Scholar]
- Zhang, C.J.; Tu, Q.M.; Francis, L.F.; Kortshagen, U.R. Band gap tuning of films of undoped zno nanocrystals by removal of surface groups. Nanomaterials 2022, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, A.; Matei, C.; Diacon, A.; Moscalu, F.; Berger, D. Novel zno-biochar nanocomposites obtained by hydrothermal method in extracts of ulva lactuca collected from black sea. Ceram. Int. 2023, 49, 10003–10013. [Google Scholar] [CrossRef]
- Motelica, L.; Popescu, A.; Razvan, A.G.; Oprea, O.; Trusca, R.D.; Vasile, B.S.; Dumitru, F.; Holban, A.M. Facile use of zno nanopowders to protect old manual paper documents. Materials 2020, 13, 5452. [Google Scholar] [CrossRef] [PubMed]
- Babayevska, N.; Przysiecka, L.; Nowaczyk, G.; Jarek, M.; Jarvekulg, M.; Kangur, T.; Janiszewska, E.; Jurga, S.; Iatsunskyi, I. Fabrication of gelatin-zno nanofibers for antibacterial applications. Materials 2021, 14, 103. [Google Scholar] [CrossRef]
- Shang, C.Y.; Bu, J.Y.; Song, C. Preparation, antimicrobial properties under different light sources, mechanisms and applications of TiO2: A review. Materials 2022, 15, 5820. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Azizi, S.; Sintwa, N.; Mokalane, K.; Mohale, K.C.; Mudau, F.N.; Maaza, M. Effect of optimized precursor concentration, temperature, and doping on optical properties of zno nanoparticles synthesized via a green route using bush tea (Athrixia phylicoides dc.) leaf extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Hema, N.; Vijaya, P.P. In vitro biocompatibility and antimicrobial activities of zinc oxide nanoparticles (zno nps) prepared by chemical and green synthetic route- a comparative study. Bionanoscience 2020, 10, 112–121. [Google Scholar]
- Pholnak, C.; Sirisathitkul, C.; Suwanboon, S.; Harding, D.J. Effects of precursor concentration and reaction time on sonochemically synthesized zno nanoparticles. Mater. Res-Ibero-Am. J. 2014, 17, 405–411. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Siva Kumari, B.; Venkateswara Rao, K.; Prabhu, Y.T. Beneficial role of zinc oxide nanoparticles on green crop production. Int. J. Multidiscip. Adv. Res. Trends 2015, 2, 273–282. [Google Scholar]
- Bazari, M.; Najmoddin, N. The effect of cationic, anionic and nonionic surfactants on morphology and antibacterial properties of zinc oxide. Materia 2022, 27, e13172. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Sapio, L.; Illiano, M.; Caiafa, I.; Naviglio, S. Chemical analysis and anti-proliferative activity of campania thymus vulgaris essential oil. J. Essent. Oil Res. 2017, 29, 461–470. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.Q.; Amoroso, V.; Acma, F.; Guiang, M.M.; Wu, H. Comparison of production of bioactive components in zanthoxylum nitidum taproots from different regions in southern china. Biomed. Chromatogr. 2023, 37, e5602. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using gc-ms and ftir spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Wany, A.; Kumar, A.; Nallapeta, S.; Jha, S.; Nigam, V.K.; Pandey, D.M. Extraction and characterization of essential oil components based on geraniol and citronellol from java citronella (Cymbopogon winterianus jowitt). Plant Growth Regul. 2014, 73, 133–145. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Trusca, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative antimicrobial chitosan/zno/ag nps/citronella essential oil nanocomposite—Potential coating for grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Lewczyk, D.C.; Cohan, J.W.; Goetz, M.L.; Trafford, B.L.; Fuller, R.L.; Sparks, J.R. Kinetic treatment of evaporation via thermogravimetric analysis: The case of d-limonene. Ind. Eng. Chem. Res. 2020, 59, 15069–15074. [Google Scholar] [CrossRef]
- Lin, X.C.; Cao, S.; Sun, J.Y.; Lu, D.L.; Zhong, B.L.; Chun, J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef]
- Miya, G.; Nyalambisa, M.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical profiling, toxicity and anti-inflammatory activities of essential oils from three grapefruit cultivars from kwazulu-natal in south africa. Molecules 2021, 26, 3387. [Google Scholar] [CrossRef]
- Porte, A.; Godoy, R.L.O. Chemical composition of thymus vulgaris l. (thyme) essential oil from the rio de janeiro state (brazil). J. Serbian Chem. Soc. 2008, 73, 307–310. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Bialon, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical composition of the essential oil of the new cultivars of lavandula angustifolia mill. Bred in ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef] [PubMed]
- Cebi, N.; Erarslan, A. Determination of the antifungal, antibacterial activity and volatile compound composition of citrus bergamia peel essential oil. Foods 2023, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Falah, F.; Arab, F.L.; Vasiee, M.; Yazdi, F.T. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of cinnamomum zeylanicum bark essential oil. Evid.-Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar]
- Kamel, D.G.; Mansour, A.I.A.; El-Diin, M.A.H.N.; Hammam, A.R.A.; Mehta, D.; Abdel-Rahman, A.M. Using rosemary essential oil as a potential natural preservative during stirred-like yogurt making. Foods 2022, 11, 1993. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Khaef, S.; Yari, A.; Nikeafshar, S.; Fathi, M.; Ghambari, S. Chemical composition analysis of the essential oil of mentha piperita l. From kermanshah, iran by hydrodistillation and hs/spme methods. J. Anal. Sci. Technol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Indriyani, N.N.; Al Anshori, J.; Permadi, N.; Nurjanah, S.; Julaeha, E. Bioactive components and their activities from different parts of citrus aurantifolia (christm.) swingle for food development. Foods 2023, 12, 2036. [Google Scholar] [CrossRef]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (christm.) swingle) essential oils: Volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef]
- Gao, S.J.; Liu, G.Z.; Li, J.G.; Chen, J.; Li, L.N.; Li, Z.; Zhang, X.L.; Zhang, S.M.; Thorne, R.F.; Zhang, S.Z. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of staphylococcus aureus and candida species. Front. Cell. Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Ene, V.L.; Vasile, B.S.; Andronescu, E.; Holban, A.M. Antibacterial biodegradable films based on alginate with silver nanoparticles and lemongrass essential oil-innovative packaging for cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Zehra, A.; Wani, S.M.; Jan, N.; Bhat, T.A.; Rather, S.A.; Malik, A.R.; Hussain, S.Z. Development of chitosan-based biodegradable films enriched with thyme essential oil and additives for potential applications in packaging of fresh collard greens. Sci. Rep. 2022, 12, 16923. [Google Scholar] [CrossRef]
- Shin, J.; Naskar, A.; Ko, D.; Kim, S.; Kim, K.S. Bioconjugated thymol-zinc oxide nanocomposite as a selective and biocompatible antibacterial agent against staphylococcus species. Int. J. Mol. Sci. 2022, 23, 6770. [Google Scholar] [CrossRef]
- Osaili, T.M.; Albiss, B.A.; Al-Nabulsi, A.A.; Alromi, R.F.; Olaimat, A.; Al-Holy, M.; Savvaidis, I.; Holley, R. Effects of metal oxide nanoparticles with plant extract on viability of foodborne pathogens. J. Food Saf. 2019, 39, e12681. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Hosseini, H.M.; Fooladi, A.A.I. Antibacterial activity of agar-based films containing nisin, cinnamon eo, and zno nanoparticles. J. Food Saf. 2018, 38, e12440. [Google Scholar] [CrossRef]
- Sami, R. Effects of chitosan-zinc oxide nano coating supplemented with bergamot essential oil on postharvest shelf life of table grapes (Vitisvinifera L., red globe). Mater. Express 2023, 13, 89–97. [Google Scholar] [CrossRef]
- Radulescu, M.; Andronescu, E.; Cirja, A.; Holban, A.M.; Mogoanta, L.; Balseanu, T.A.; Catalin, B.; Neagu, T.P.; Lascar, I.; Florea, D.A.; et al. Antimicrobial coatings based on zinc oxide and orange oil for improved bioactive wound dressings and other applications. Rom. J. Morphol. Embryol. 2016, 57, 107–114. [Google Scholar]
- Sepulveda, F.A.; Rivera, F.; Loyo, C.; Canales, D.; Moreno-Serna, V.; Benavente, R.; Rivas, L.M.; Ulloa, M.T.; Gil-Castell, O.; Ribes-Greus, A.; et al. Poly (lactic acid)/d-limonene/zno bio-nanocomposites with antimicrobial properties. J. Appl. Polym. Sci. 2022, 139, 51542. [Google Scholar] [CrossRef]
- Jiang, L.H.; Han, Y.H.; Xu, J.; Wang, T. Preparation and study of cellulose-based zno nps@hec/c-?-cd/menthol hydrogel as wound dressing. Biochem. Eng. J. 2022, 184, 108488. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Stoleru, E.; Rosca, I.; Serban, A.; Nita, L.E.; Rusu, A.G.; Ghilan, A.; Macsim, A.M.; Mititelu-Tartau, L. Development of a new polymer network system carrier of essential oils. Biomed. Pharmacother. 2022, 149, 112919. [Google Scholar] [CrossRef]
- Rapa, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.M.; Predescu, C. Bioactive collagen hydrolysate-chitosan/essential oil electrospun nanofibers designed for medical wound dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Macsim, A.M.; Tudorachi, N.; Rosca, I.; Stoica, I.; Tampu, D.; Aflori, M.; Doroftei, F. Synthesis of poly(ethylene brassylate-co-squaric acid) as potential essential oil carrier. Pharmaceutics 2021, 13, 477. [Google Scholar] [CrossRef]

















| Sample Label | Essential Oil Used |
|---|---|
| ZnO | - |
| ZnO_Cit | Citronella |
| ZnO_Ora | Orange |
| ZnO_Thy | Thyme |
| ZnO_Lav | Lavender |
| ZnO_Grap | Grapefruit |
| ZnO_Berg | Bergamot |
| ZnO_Cin | Cinnamon |
| ZnO_Ros | Rosemary |
| ZnO_Mint | Minzol |
| ZnO_Lime | Limette |
| No | Bacteria Strains | Gram-Positive | Gram-Negative |
|---|---|---|---|
| 1 | Listeria monocytogenes, ATCC 19114 | Yes | |
| 2 | Staphylococcus aureus, ATCC 25923 | Yes | |
| 3 | Salmonella typhimurium, ATCC 14028 | Yes | |
| 4 | Escherichia coli, ATCC 25922 | Yes |
| Unit Cell | a = b [Å] | c [Å] | V [Å3] | c/a | Microstrain (%) | Average Crystallite Size (nm) | Dislocation Density (δ) ×10−4 |
|---|---|---|---|---|---|---|---|
| ZnO | 3.25081 | 5.20661 | 47.65065 | 1.60163 | 0.26 ± 0.10 | 34.45 ± 4.93 | 8.43 |
| Sample/ Wavenumber (cm−1) | ZnO | ZnO_ Cit | ZnO_ Ora | ZnO_ Thy | ZnO_ Lav | ZnO_ Grap | ZnO_ Berg | ZnO_ Cin | ZnO_ Ros | ZnO_ Mint | ZnO_ Lime |
|---|---|---|---|---|---|---|---|---|---|---|---|
| –OH | 3401 | 3340 | 3414 | 3374 | 3392 | 3423 | 3406 | 3379 | 3423 | 3379 | 3388 |
| –Csp2–H | - | - | - | 3020 | 3006 | 3010 | 3011 | 3027 | - | - | 3009 |
| –Csp3–H CH3 asym CH2 asym CH3 sym CH2 sym | - - - - - | 2965 2920 2854 2833 | 2959 2926 2868 2854 | 2958 2923 2868 -tail | 2962 2925 2868 2855 | 2961 2927 2871 2855 | 2965 2921 2872 2856 | 2975 2930 2842 2813 | 2959 2927 2873 2854 | 2952 2918 2868 2847 | 2962 2923 2855 2832 |
| C=O | - | 1735 | 1740 | 1706 | 1735 | 1735 | 1735 | 1733 | 1743 | 1706/38 | - |
| C=C–C stretching | - | 1674 | 1620 | 1620 | 1622/74 | 1620 | 1622 | 1622/73 | 1620 | 1620 | 1620 |
| - | 1572 | 1584 | 1584 | 1575 | 1572 | 1572 | 1572 | 1584 | 1584 | 1584 | |
| C–O–str C–OHdef | - | 1121 | - | - | 1117 | 1121 | 1115 | 1121 | 1045 | 1045 | 1045 |
| 1020 | 1024 | 1024 | 1020 | 1020 | 1020 | 1025 | 1024 | 1024 | 1024 | ||
| −bending | 615/673 | 612/677 | 614/680 | 612/677 | 613/682 | 613/680 | 612/678 | 606/688 | 613/677 | 612/678 | 614/678 |
| Zn–Ostr | 458 | 456 | 420 | 418 | 424 | 420 | 422 | 455 | 456 | 455 | 417 |
| Sample | Mass Loss (%) RT-225 °C | Endothermic Peak (°C) | Mass Loss (%) 225–500 °C | Exothermic Peak (°C) | Residual Mass (%) | Estimated Load (%) |
|---|---|---|---|---|---|---|
| ZnO | 0.11% | 70.1 | 1.03% | 328.2 | 98.46% | - |
| ZnO_Cit | 4.00% | 111.9 | 2.25% | 340.0 | 93.54% | 5.00% |
| ZnO_Ora | 0.56% | 117.2 | 2.10% | 337.6 | 97.04% | 1.44% |
| ZnO_Thy | 10.66% | 140.1 | 1.46% | 332.1 | 87.65% | 10.98% |
| ZnO_Lav | 9.25% | 118.3 | 1.63% | 334.6 | 88.91% | 9.70% |
| ZnO_Grap | 0.80% | 113.2 | 2.39% | 342.3 | 96.56% | 1.93% |
| ZnO_Berg | 4.57% | 115.6 | 2.27% | 343.4 | 92.94% | 5.61% |
| ZnO_Cin | 14.22% | 167.8 | 2.41% | 349.2 | 83.08% | 15.62% |
| ZnO_Ros | 2.26% | 108.5 | 1.76% | 338.2 | 95.73% | 2.77% |
| ZnO_Mint | 12.42% | 145.9 | 1.28% | 333.6 | 86.06% | 12.60% |
| ZnO_Lime | 2.72% | 111.7 | 1.64 | 336.1 | 95.41% | 3.10% |
| Sample | Gram-Positive Bacteria | Gram-Negative Bacteria | ||
|---|---|---|---|---|
| L. monocytogenes | S. aureus | S. typhimurium | E. coli | |
| ZnO_Cit | 12.3 ± 0.6 b | 12.0 ± 1.0 b | 16.3 ± 0.6 b | 14.7 ± 0.6 d |
| ZnO_Ora | 11.0 ± 0.0 a | 8.7 ± 0.6 a | 15.0 ± 0.0 a | 10.7 ± 0.6 a |
| ZnO_Thy | 13.7 ± 0.6 c | 14.3 ± 0.6 c | 18.3 ± 0.6 c | 15.7 ± 0.6 d |
| ZnO_Lav | 10.7 ± 0.0 a | 12.7 ± 0.6 b | 14.7 ± 0.0 a | 11.7 ± 0.6 b |
| ZnO_Grap | 10.7 ± 0.6 a | 9.0 ± 0.0 a | 14.3 ± 0.0 a | 10.3 ± 0.6 a |
| ZnO_Berg | 13.3 ± 0.6 b,c | 12.7 ± 0.6 b | 13.0 ± 1.0 d | 12.0 ± 0.0 b |
| ZnO_Cin | 13.7 ± 0.6 c | 12.0 ± 0.6 b | 16.0 ± 0.0 b | 13.3 ± 0.6 c |
| ZnO_Ros | 11.0 ± 0.0 a | 12.3 ± 0.6 b | 11.7 ± 0.6 d | 13.0 ± 1.0 b,c |
| ZnO_Mint | 11.3 ± 0.6 a | 12.0 ± 0.0 b | 12.0 ± 1.0 d | 11.7 ± 0.6 b |
| ZnO_Lime | 10.3 ± 0.6 a | 9.3 ± 0.6 a | 12.3 ± 0.6 d | 10.3 ± 0.6 a |
| ZnO | 10.7 ± 0.6 a | 8.7 ± 0.6 a | 14.7 ± 0.6 a | 10.3 ± 0.6 a |
| Citronella | 22.0 ± 1.0 | 16.3 ± 0.6 | 9.7 ± 0.6 | 37.7 ± 1.2 |
| Orange | 13.0 ± 1.0 | 9.7 ± 0.6 | 10.0 ± 0.0 | 9.7 ± 0.6 |
| Thyme | 24.3 ± 0.6 | 57.7 ± 0.6 | 55.3 ± 0.6 | 59.0 ± 1.0 |
| Lavender | 12.0 ± 0.0 | 20.3 ± 0.6 | 15.0 ± 1.0 | 17.0 ± 1.0 |
| Grapefruit | 12.3 ± 0.6 | 12.7 ± 0.6 | 11.7 ± 0.6 | 12.3 ± 0.6 |
| Bergamot | 8.0 ± 0.0 | 7.3 ± 0.6 | 8.7 ± 0.6 | 6.7 ± 0.6 |
| Cinnamon | 30.7 ± 0.6 | 31.7 ± 0.6 | 22.0 ± 1.0 | 15.3 ± 0.6 |
| Rosemary | 9.0 ± 0.0 | 9.0 ± 0.0 | 8.3 ± 0.6 | 11.7 ± 0.6 |
| Minzol | 15.0 ± 1.0 | 15.0 ± 1.0 | 13.0 ± 1.0 | 19.3 ± 0.6 |
| Limette | 7.3 ± 0.6 | 11.0 ± 1.0 | 9.0 ± 0.0 | 10.0 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics 2023, 15, 2470. https://doi.org/10.3390/pharmaceutics15102470
Motelica L, Vasile B-S, Ficai A, Surdu A-V, Ficai D, Oprea O-C, Andronescu E, Mustățea G, Ungureanu EL, Dobre AA. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics. 2023; 15(10):2470. https://doi.org/10.3390/pharmaceutics15102470
Chicago/Turabian StyleMotelica, Ludmila, Bogdan-Stefan Vasile, Anton Ficai, Adrian-Vasile Surdu, Denisa Ficai, Ovidiu-Cristian Oprea, Ecaterina Andronescu, Gabriel Mustățea, Elena Loredana Ungureanu, and Alina Alexandra Dobre. 2023. "Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils" Pharmaceutics 15, no. 10: 2470. https://doi.org/10.3390/pharmaceutics15102470
APA StyleMotelica, L., Vasile, B.-S., Ficai, A., Surdu, A.-V., Ficai, D., Oprea, O.-C., Andronescu, E., Mustățea, G., Ungureanu, E. L., & Dobre, A. A. (2023). Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics, 15(10), 2470. https://doi.org/10.3390/pharmaceutics15102470