1. Introduction
The CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system has emerged as a flexible gene editing tool for both cell engineering and therapeutic applications [
1,
2,
3,
4,
5]. For Cas9-mediated genome editing to be effective, different components need to be delivered inside the nucleus of target cells. These include the Cas9 protein, a guide RNA that targets the Cas9 nuclease to a specific sequence in the genomic DNA and, in case of gene correction or insertion, a DNA template to enable precise gene editing via homologous recombination. The sheer size of these components, combined with the need for the successful delivery of each component in optimal stoichiometric ratios, is the prime reason why effective delivery has proven to be difficult. Viral vectors can be constructed to carry genetic information to encode for the Cas9 protein as well as the single guide RNA as a means of intracellular delivery of CRISPR-Cas. However, because expression from viral promoters is often strong and long-lasting, this can give rise to unintended off-target effects and potential immunogenic responses [
6,
7,
8]. More transient systems are therefore desired.
Direct delivery of the Cas9:sgRNA ribonucleoprotein complex (RNP) offers several advantages. First, it is fast, as it does not depend on transcription, translation and subsequent assembly into an active RNP inside the cell, which can often be suboptimal due to different degradation profiles of the Cas9 protein and the sgRNA. Second, its transient nature reduces the chance of off-target cutting events and potential immune responses [
9]. Third, it avoids potential risks of insertional mutagenesis, which is often associated with viral vectors [
10].
To this end, different strategies have recently been explored for the delivery of Cas9 RNP into cells, including electroporation [
11] and induced osmotic transduction (iTOP) [
12], as well as the use of chemical transfection reagents [
13] and non-viral vectors [
9,
14,
15,
16,
17,
18]. However, developing synthetic vectors for the direct delivery of CRISPR-Cas RNP with high efficiency has been challenging. Such synthetic vectors should avidly complex or encapsulate Cas9 RNP in order to protect it from premature proteolytic degradation, and it should trigger cellular uptake, endosomal escape and vector disassembly to enable the free RNP to be transported into the nucleus through the nuclear pore complexes.
Cell-penetrating peptides (CPPs) have been explored for the delivery of Cas9 RNPs in various cell types [
19,
20,
21,
22,
23]. CPPs are short (5–30 amino acids), polycationic or amphipathic peptides that facilitate the cellular uptake of different types of cargoes, such as small molecules, proteins and plasmid DNA, as well as nanoparticles [
6,
24]. The mechanism of action of CPPs is not fully understood but is generally believed to follow two uptake routes: (1) direct translocation across the cell membrane and (2) endocytosis after binding to cell surface heparan sulphates [
6,
25]. Within endocytic compartments, CPPs interact with endosomal membranes, often triggered by changes in pH in the endosomes, which results in endosome destabilization and partial cargo release [
6,
26].
Initial studies on CPP-mediated Cas9 RNP delivery used covalent attachment of polycationic CPPs to Cas9 to enhance its intracellular delivery, however, with moderate success. Low levels of Cas9-mediated insertion/deletion polymorphisms (indels) were observed at relatively high doses of Cas9-CPP conjugates [
21,
27,
28]. Because covalent attachment might negatively influence RNP activity, either by modifying active site residues [
29] or by interfering with sgRNA binding, non-covalent approaches based on electrostatic complexation of Cas9 RNP with cationic CPPs have recently been explored [
22,
23,
30]. For stable complex formation with Cas9 RNP, cationic CPPs have been modified with one or more lipid tails [
30]. It is worth emphasizing that in some of these studies these levels of efficiency were achieved by conducting experiments in conditions that are conducive to enhanced transfection rates, involving the use of Opti-MEM (a reduced serum medium). However, it is crucial to acknowledge that the effectiveness might be influenced when applying the system to cell types that are typically resistant to transfection or when working within a serum-rich environment. Compared to covalent methods, these studies have shown a remarkable increase in indel efficiencies, which could be further increased via the addition of PVA-PEG to the medium [
22].
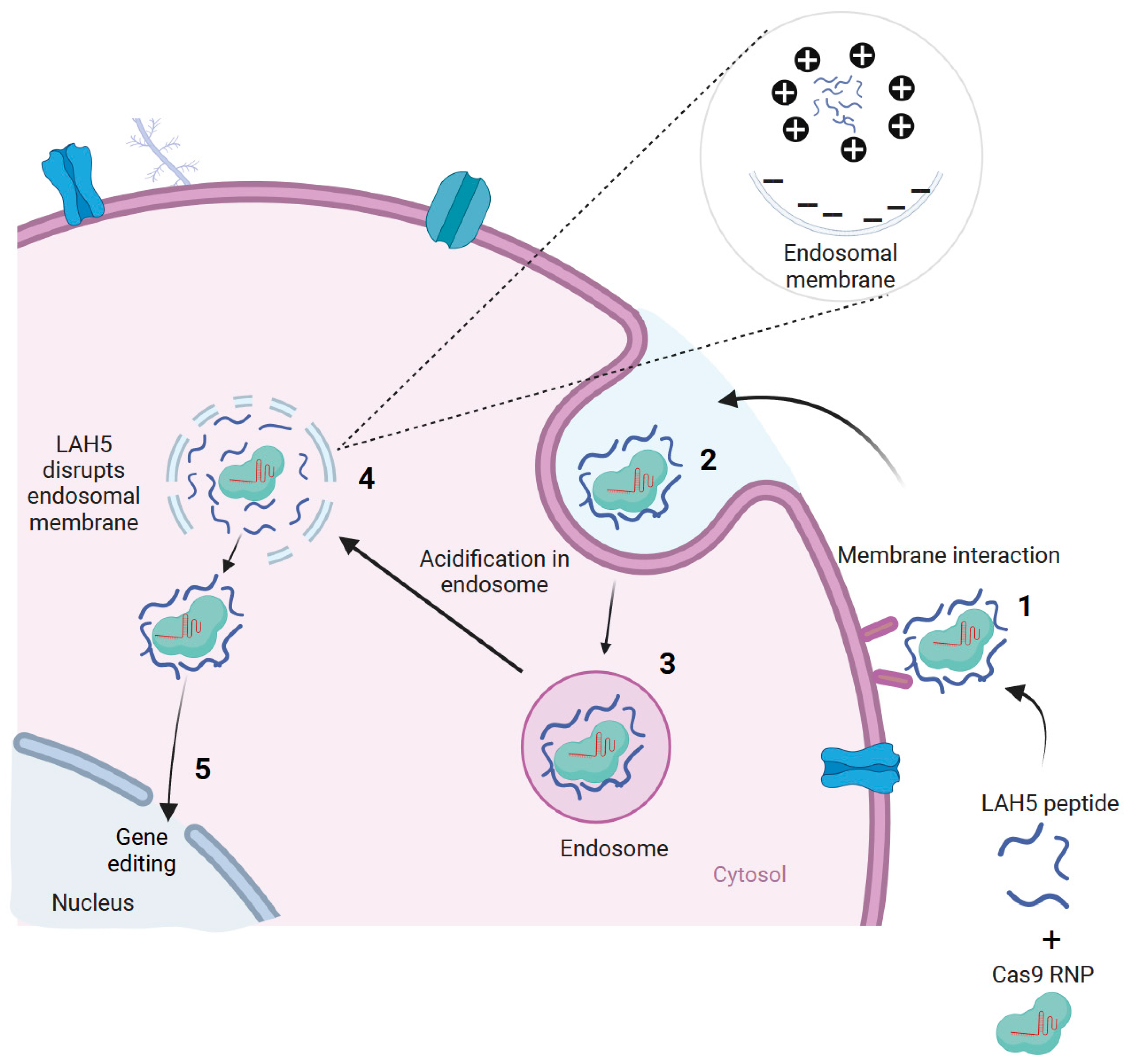
We previously screened a large number of different CPPs, both cationic and amphipathic, for their capacity to transfect pDNA into various cell lines [
31]. From a total of >90 CPPs, only five showed robust transfection efficiencies in the presence of serum without compromising cell viability. One of these peptides, LAH5 (KKALLALALHHLAHLAHHLALALKKA), initially described by Kichler et al. (2003), showed the highest transfection efficiency of all those that were tested. This amphipathic peptide is rich in histidine residues, which are thought to be responsible for their capability to induce endosomal escape (
Figure 1) [
32].
In this study, the aim was to determine if LAH5 is also suitable for the delivery of Cas9 RNP to a variety of cells in the culture. The use of the LAH5 peptide for Cas9 RNP delivery or therapeutic protein delivery applications has not been explored previously. We show for the first time that LAH5 peptides form nanocomplexes with Cas9 RNP that are efficiently internalized by a variety of different cell lines. The functional delivery of Cas9 RNP is demonstrated in HEK293T cells expressing a fluorescent Cas9 reporter construct using different ratios of RNP/LAH5 nanocomplexes. Next, CCR5 gene-targeted indels are demonstrated in various cell lines, including primary fibroblasts. Finally, we demonstrate that the co-delivery of an ssDNA HDR template with Cas9 RNP/LAH5 nanocomplexes results in efficient gene correction. To the best of our knowledge, this is the first study to show that CPPs can deliver these three different components at the same time, which are crucial for CRISPR-Cas-mediated gene correction.
2. Materials and Methods
2.1. General Reagents
All chemicals were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands) unless otherwise specified. spCas9 and spCas9-GFP protein were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands). Modified sgRNA sequences were obtained from Sigma-Aldrich (Haverhill, UK, sequences given in
Table S1) and stored in DNAse and RNAse-free water from IDT (Integrated DNA Technologies Inc. Leuven Belgium). DNA oligonucleotides (
Table S2), HDR template (
Table S3), ATTO-550-labeled tracRNA, non-labeled crRNA (
Table S4) and Alexa-fluor-647-labeled HDR template (
Table S3) were purchased from IDT. Lipofectamine CRISPRMAX and Lipofectamine3000™ were obtained from Thermo Fisher Scientific (Waltham, MA, USA, ABD). T7 endonuclease and Q5 high-fidelity 2X master mix were purchased from New England Biolabs (Ipswich, MA, USA). The 6His-CM18-PTD4 [
19], S10 [
23], L17E [
33] and LAH5: KKALLALALHHLAHLAHHLALALKKA peptides were purchased from Synpeptide (Shanghai, China). DNA extraction and PCR purification kits were purchased from QIAGEN Benelux B.V. (Venlo, The Netherlands). TAE buffer was purchased from Biorad (Lunteren, The Netherlands).
2.2. Cas9 RNP/LAH5 Nanocomplex Formation and Particle Sizing
Cas9 and sgRNA stocks were diluted in nuclease-free water (Thermo Scientific, Waltham, MA, USA). The LAH5 peptide, supplied as a powder, was dissolved in nuclease-free water with 0.1% (v/v) acetic acid at a final peptide concentration of 750 µM. sgRNA (0.75 µM) and Cas9 (0.75 µM) dissolved in HEPES buffer with a pH of 7.4 were mixed via pipetting and were incubated for 10 min at room temperature to allow the formation of the Cas9 ribonucleoprotein (RNP) complex. Subsequently, an equal volume of LAH5 (37.5 µM to 187.5 µM) was added to the RNP and incubated for 10 min at RT to enable nanocomplex formation. The obtained molar ratios of Cas9:sgRNA:LAH5 varied between 1:1:50 and 1:1:250. Following nanocomplex formation, samples were diluted in HEPES buffer with a pH of 7.4; then, 100 µL of the sample was transferred into a lowest sizing cell cuvette (Malvern, Malvern, UK), and size distribution was measured with a Malvern Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK). Subsequently, formulated nanocomplexes were diluted 8× times in 10 mM HEPES buffer with a pH of 7.4; then, the zeta potential was measured with Zetasizer Nano Z (Malvern ALV CGS-3, Worcestershire, UK). All measurements were performed in triplicate.
2.3. Electrophoretic Mobility Shift Assay (EMSA)
Peptide-mediated complexation was determined by performing a fluorescence-based electrophoretic mobility shift assay by using a non-stained and non-denaturing 1.5% agarose gel in TAE buffer with a pH of 8.3. For this, Cas9-GFP (Merck), ATTO-550-labeled tracrRNA, unlabeled crRNA (IDT), Alexa-647-labeled HDR template (IDT) and unlabeled LAH5 peptides were used. Labeled components and unlabeled LAH5 peptides were formulated at various molar ratios as described above. All labeled components were diluted such that the final concentration reached 1 µM when loaded on gel. All formulated nanocomplexes were adjusted to a total volume of 10 µL by adding nuclease-free water. Then, 2 µL of 40% Glycerol was added, after which samples were loaded into the wells of the gel. The agarose gel was run at 170 V for 15 min. Gel images were captured by ChemiDocTM XRS+ (Bio-Rad, Lunteren, The Netherlands), and fluorescent images were merged and analyzed with ChemiDocTM XRS+ (Bio-Rad) with Image lab software version 5.0.
2.4. Cell Lines and Cell Culture
HEK293T (CRL-3216), HeLa (CCL-2), HEPG2 (HB-8065) and primary fibroblast cells (CCL-186) were all obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) through LGC Standards (Molsheim, France). The HEK293T stoplight cell line was constructed as described previously [
34]. HEK293T stoplight cells, HEK293T HDR stoplight cells, HEK293T and primary fibroblast cells were cultured in a high-glucose DMEM medium, and HeLa and HEPG2 cells were cultured in Eagle’s minimum essential medium. The culture medium was supplemented with 10% (
v/
v) FBS (Sigma Zwijndrecht, The Netherlands), and cells were cultured at 37 °C and 5% CO
2. Cell culture plastics, unless otherwise specified, were purchased from Greiner Bio-One (Alphen aan de Rijn, The Netherlands).
2.5. Generation of HEK293T HDR Stoplight Reporter Cell Line
The HDR fluorescent stoplight reporter construct was synthesized by Integrated DNA Technologies (Leuven, Belgium). The reporter construct was cloned into a pHAGE2-MCS-EF1a-IRES-NeoR plasmid using NotI and BamHI restriction enzymes and a NEB Quick Ligation Kit (all from New England Biolabs, MA, USA). The pHAGE2-EF1a-HDR stoplight-IRES-NeoR plasmid was then used for lentiviral production and transduction. HEK293T cells were transfected with pHAGE2-EF1a-HDR Stoplight-IRES-NeoR, MD2.G plasmid and PSPAX2 plasmid (Addgene #12259 and #12260, respectively) at a 2:1:1 ratio using 3 µg of 25 kDa linearized PEI per µg of DNA. The transfection medium was removed after 24 h and replaced with DMEM with 10% FBS. After 48 h, the lentiviral supernatant was harvested, and cells were cleared via a 5 min 500× g centrifugation step, followed by 0.45 µm syringe filter filtration. HEK293T cells were transduced with lentiviral stocks overnight with 8 µg/µL polybrene (Sigma, Zwijndrecht, The Netherlands). After 24 h, the transduction medium was removed and replaced with a culture medium supplemented with 1500 µg/mL G418. After 5 days, the concentration of G418 was lowered to a maintenance concentration of 1000 µg/mL G418. After two weeks of culturing in the presence of selection antibiotics, the reporter cells were sorted for mCherry+eGFP− fluorescent signals on a BD FACSAria III cell sorter.
2.6. Transfection Experiments
All cell types used in this study were plated at 30,000 cells per well in a 96-well plate (Greiner M0687-100EA) and were incubated for 24 h at 37 °C and 5% CO2 to reach a confluency of 50%. Next, cells were transfected with Cas-CPP or Cas-HDR-CPP. As a positive control for transfection, spCas9 RNP complexed with CRISPRMAX was used according to the manufacturer’s instructions (Thermo Fisher Scientific). Cas-CPP nanocomplexes were prepared following RNP formation. Increasing concentrations of LAH5 peptide were mixed with RNP by keeping the ratio of RNP:LAH5 between 1:50 and 1:250. Then, Cas-CPP mixtures were incubated for 10 min to form nanocomplexes. For HDR experiments after the preparation of RNPs, various concentrations of HDR template were added to the preformed spCas9 RNP to obtain multiple ratios of 1:1, 1:2 and 1:4, and they were incubated for 5 min at room temperature. Finally, LAH5 peptide was added to the spCas9 RNP and HDR template mixtures and incubated for 10 min to form Cas-HDR-CPP nanocomplexes. Opti-MEM was added to reach a total volume of 160 μL before removing culture medium and adding the nanocomplexes to the cells at the indicated concentrations. Cas9, sgRNA and HDR template concentrations were 20 nM at a 1:1:1 molar ratio in the 96-well plate. A duration of 24 h after transfection, cells were washed 2 times with fresh media. Cells were incubated for another 24 h at 37 °C and 5% CO2. In case DNA extraction was needed for genetic analysis (T7 endonuclease and TIDE), the transfection experiments were performed in 48-well plates (Greiner M8937-100EA) to ensure sufficient amounts of DNA for post-analysis.
2.7. Stoplight Gene Editing and Correction Assays
To assess the efficiency of delivery at the cellular level, upon transfection with Cas-CPP nanocomplexes, two different reporter cell lines, HEK293T NHEJ stoplight cells [
34] and HEK293T HDR stoplight cells, were used. A duration of 48 h after transfection, gene editing and gene correction efficiencies were assessed using flow cytometry analysis.
2.8. Flow Cytometry to Determine Gene Editing and Gene Correction Efficiencies in HEK293T Stoplight and HEK 293T HDR Stoplight Cells
Cells were harvested by washing twice with PBS, followed by a trypsinization step, after which cells were fixed in 1% paraformaldehyde. Cells were washed in PBS and transferred to a BD Falcon U-bottom 96-well plate (Becton Dickinson, Franklin Lakes, NJ, USA) for flow cytometry analysis. Reporter fluorescence was detected via flow cytometry using BD FACS CANTO II (Becton Dickinson, Franklin Lakes, NJ, USA). mCherry was measured using the PerCP-Cy5-5-A channel of the flow cytometer, and eGFP fluorescence was determined in the FITC channel. The flow cytometry results were analyzed with Flowlogic software (Inivai Technologies, Mentone, Australia, version 8.7). The gating strategy used for the flow cytometry analyses, both for the HEK293T stoplight and HEK293T HDR stoplight cells, is specified in
Figures S6 and S7. Both gene editing and gene correction efficiency, depending on the cell line, were identified as the number of mCherry
+ cells expressing eGFP, as described previously [
34].
2.9. Confocal Microscopy
HEK293T stoplight cells were plated at a quantity of 30,000 cells per well in a 96-well imaging plate (Greiner CellStar #655090). After 24 h of incubation, cells were treated with different concentrations of Cas-CPP and Cas-HDR-CPP nanocomplexes. After 24 h of incubation at 37 °C and 5% CO2, cells were washed with 100 μL of high-glucose DMEM medium supplemented with 10% FBS. Following this, cells were incubated for another 24 h at 37 °C and 5% CO2. Cell nuclei were stained by adding 2 µg/mL Hoechst 33342 in a complete cell culture medium as the final concentration and incubated for 30 min, after which cells were imaged using the Yokogawa CV7000 Confocal Microscope. (Yokogawa Corporation, Tokyo, Japan).
2.10. Cytotoxicity Assays
The cytotoxicity of the RNP/LAH5 peptide nanocomplexes was evaluated via an MTS (cell viability) assay [
35]. Cells were seeded into 96-well plates with 50% confluency and were incubated for 24 h at 37 °C and 5% CO
2. Cas-CPP nanocomplexes (prepared at molar ratios of 1:50 to 1:1000) were added at a concentration of 20 nM of RNP per well in a total volume of 100 μL of Opti-MEM. A duration of 24 h post-transfection, cytotoxicity was determined with the CellTiter 96
® AQueous One Solution Cell Proliferation Assay (MTS) (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The absorbance was measured at 490 nm on a Bio-Rad iMark microplate reader model 1681130. The relative cell viability was calculated by setting the absorbance value of untreated cells to 100%, and the absorbance value for those treated with 1% Triton X-100 was set to 0%.
2.11. Cell Uptake Assay
Cas9-GFP (Sigma), ATTO 550 tracrRNA (IDT) and crRNA (IDT), specific for the HPRT housekeeping gene and Alexa-647-labeled single-stranded HDR template, were used to prepare fluorescent Cas-CPP nanocomplexes for cellular uptake experiments. First, ATTO 550 tracrRNA (IDT) and crRNA (IDT) were mixed at a 1:1 ratio to form an ATTO-550-labeled sgRNA complex. Then, the uptake of these components was tested with and without complexation with Cas9-GFP (10 nM) and ATTO 550 sgRNA (10 nM) (RNP) with 3 μM LAH5 peptide at a 1:150 ratio (m/m). A complex consisting of 10 nM Cas9-GFP, ATTO 550 sgRNA (RNP) and Alexa 647 HDR template at equimolar concentrations was prepared following the previously described procedure. Additionally, the labeled RNP and labeled HDR template were mixed with 3 µM LAH5 peptide at a ratio of 1:1:150 (m/m). Following the preparation of the nanocomplexes, HeLa cells were transfected in a 96-well black plate. A duration of 24 h after transfection, the nuclei of HeLa cells were stained by supplementing the complete cell medium with Hoechst 33342 dye at a final concentration of 2 µg/mL, followed by incubation for 30 min. Microscopy images were recorded at 60× magnification using the Yokogawa CV7000s confocal microscope. In addition, HeLa cells were transfected with a CD63-eGFP plasmid using Lipofectamine 3000 according to the manufacturer’s protocol. A duration of 48 h after plasmid transfection, cells were treated with Cas9/ATTO 550 sgRNA(RNP) with and without LAH5 complexation, as described above.
2.12. T7 Endonuclease Assay
A T7E1 assay was performed to detect the insertion/deletion (indel) frequency after gene editing [
36]. Genomic DNA was extracted from the cells 48 h after transfection with LAH5 peptide/RNP nanocomplexes using the Qiagen DNeasy Blood & Tissue Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. PCR was performed using the primers designed for the sgRNA target locus (
Table S2) using Q5
® Hot Start High-Fidelity 2X Master Mix (New England Biolabs, MA, USA). Afterward, PCR products were purified using the QIAquick PCR Purification kit (Qiagen GmbH, Hilden, Germany). PCR products were denatured at 95 °C for 10 min in the presence of NEBuffer 2 (New England Biolabs), and they were annealed by slowly lowering the temperature (95–85 °C at 2 °C per second and 85–25 °C at 1 °C per second). Subsequently, re-annealed PCR products were incubated with 5U T7E1 enzyme (New England Biolabs, MA, USA) at 37 °C for 18 min to cut heteroduplexes. DNA products were run on a 2% agarose gel in TAE buffer with a pH of 8.3 (Biorad). The indel frequency was calculated by determining the intensities of cleaved and uncleaved bands based on a densitometry analysis using ImageJ version 1.53t.
2.13. TIDE Analysis (Tracking of Indels via Decomposition)
Genomic DNA was isolated, and the sgRNA target genomic region was amplified via PCR using the same methods as described in the section on the T7E1 assay (primers are listed in
Table S2). The PCR products were purified using the QIAquick PCR Purification kit (Qiagen GmbH, Hilden, Germany) and were submitted for unidirectional Sanger sequencing (Macrogen Europe). Afterward, Sanger sequence chromatograms of purified PCR products were used for a TIDE analysis (
http://tide.nki.nl, accessed on 6 November 2022) [
37]. Gene modification frequencies were determined using the sequencing chromatogram from negative control cells as a reference and by comparing the sequence chromatogram from treated samples. During the analysis, parameters were set to detect a maximum indel size of 15 nucleotides. The decomposition frame was set to the default parameters [
37].
2.14. Statistical Analysis
Statistical analysis was performed using GraphPad Prism (9.4.1). The statistical analysis methods that were used are specified under the specific figure legends. Values in all experiments are represented as means ± standard errors of the mean (SDs) of at least three independent experiments performed in duplicate. An increase in the delivery efficiency was considered significant at * p < 0.05 using an analysis of variance (ANOVA) with Dunnett’s multiple comparisons test or an ANOVA with Bonferroni’s multiple comparisons test.
4. Discussion
For efficient genome editing, the intracellular delivery of CRISPR-Cas9 components is essential. This report investigates a promising delivery strategy, wherein CPPs are employed as the chosen vehicle for effective delivery. Peptide-based delivery offers an advantage in which pre-assembled Cas9 RNP can be complexed and delivered and, depending on the peptide carrier used, induce endosomal escape for the cytosolic delivery of the complexes. In this report, we repurposed the LAH5 amphipathic peptide, initially developed for the transfection of pDNA [
32] for CRISPR-Cas9 RNP delivery to a variety of human cells in vitro. We demonstrated that LAH5 peptide can form stable nanocomplexes with Cas9 RNP and with Cas9 RNP combined with an ssDNA template for homology-directed repair. We are the first to demonstrate that Cas9 RNP and an ssDNA template can be stably co-entrapped inside LAH5 peptide nanocomplexes. The electrophoretic mobility shift assay (EMSA) demonstrates that, at a molar excess >50 for LAH5:Cas9 RNP, stable nanocomplexes can be formed via electrostatic complexation. Even though hydrophobic clustering of LAH5 peptide itself cannot be excluded, as was demonstrated before [
38], and the formed nanocomplexes are polydisperse, we observed stable sizes of the nanocomplexes at 1:200 and 1:250 RNP/LAH5 ratios and confirmed this interaction via an EMSA assay. Moreover, elevating the concentration of the LAH5 peptide in the RNP/peptide nanocomplex results in the formation of smaller complexes. This reduction in size can be attributed to the shift in the equilibrium between LAH5 peptides that are free in the solution and LAH5 peptides that are complexed with RNPs. As a result, potentially exposed negative surfaces are better shielded by cationic LAH5 peptides, leading to nanocomplexes that are less prone to aggregating into larger structures. It is also worth noting that the protein and nucleotide labeling strategies that are employed to visualize these molecules within EMSA assays may have some effects on particle complexation, which is an inherent challenge in such assays. However, we believe that these effects are limited, as Cas9 GFP tagging barely affects its isoelectric point—the main determinant for peptide nanocomplexation—shifting from 9.0 to 8.8, according to ExPASy isoelectric point analysis. Moreover, ATTO labels are commonly employed to label sgRNAs while retaining their functionality, suggesting that, after this modification, the secondary structure (and thus its complexation with Cas9) remains intact. It is important to note that further biophysical and structural characterization of LAH5 nanocomplexes is needed to elucidate the stoichiometry of each of the components in the nanocomplexes, as well as the topology of the supramolecular multicomponent assemblies. It is at present unclear what the dynamics of release are for each of the cargoes. It is also unclear if LAH5 is accessible on the surface of the nanocomplexes for membrane interaction or if it needs to be released first in order to be membrane-active. Nevertheless, because we demonstrated that these multiple components can be complexed by LAH5, this approach offers the opportunity to efficiently deliver multiple components that are needed for gene editing with a single delivery system. To show a proof of concept for this, we demonstrated that the co-delivery of an ssDNA template with Cas9 RNP led to targeted gene correction in a newly developed reporter cell line. To our knowledge, this is the first time that HDR-mediated targeted gene correction via the delivery of all components with a peptide-based delivery system was demonstrated. A recent publication by Foss et al. elegantly showed the CPP-mediated delivery of CRISPR-Cas9 RNP into human lymphocytes for gene knock-out, but it relied on the co-delivery of an AAV vector carrying the HDR template for the targeted insertion of a gene construct encoding chimeric antigen receptors [
39]. However, the study did not demonstrate peptide-mediated RNP/additional delivery of DNA templates for targeted HDR-mediated gene repair. In another recent report, an amphiphilic peptide-mediated delivery system was used for the delivery of RNPs without an additional DNA template to edit primary human lymphocytes [
40]. In the context of our study, it is noteworthy to emphasize the uniqueness of our findings, as we successfully demonstrated the exclusive ability of a peptide-based system to co-deliver both RNP and HDR templates. This distinctive aspect sets our study apart from the existing literature in the field.
It is crucial to acknowledge that the high editing efficiencies observed in this study may not necessarily translate to scenarios requiring the co-delivery of larger DNA fragments to facilitate HDR-directed repair or correction in non-dividing cells. A notable limitation of synthetic delivery systems employing cell-penetrating peptides (CPPs) likely lies in their inability to actively facilitate nuclear trafficking, despite their effectiveness in intracellular delivery. Consequently, further investigation is needed to assess the suitability of CPP-based delivery strategies for HDR in non-dividing or slow-dividing cells, similar to polyplexes and lipoplexes [
41,
42].
When comparing our results with previous publications on CPP-assisted CRISPR-Cas delivery, it is important to note that our LAH5-based transfection was active at much lower concentrations of Cas9 RNP compared to several recently published peptide-mediated delivery studies [
39,
40]. In fact, the optimal concentrations at which we observed editing (20 nM of Cas9) are 10–1000-fold lower than these published results [
39,
40]. This observation may be attributed to the high histidine content that was present in our cell-penetrating peptide (CPP), which possesses a more favorable pKa for effective endosomal escape compared to peptides with lower histidine residue compositions in their sequences [
43]. This has obvious advantages, as high concentrations of Cas9 RNP may cause innate immune responses by engaging pattern recognition receptors such as TLR3, RIG-I and PKR, which in turn may lead to Cas9-specific adaptive responses [
44,
45]. Being able to lower the dose may reduce such undesired immune effects. Interestingly, despite a negative zeta potential at a pH of 7.4, we observed fast uptake and delivery of our LAH5-RNP complexes, demonstrating efficient cargo delivery after cell exposure of only 3 h (
Figure S4). Whereas a negative charge of nanoparticles generally leads to decreased endosomal uptake, other factors such as charge density and hydrophobicity are of equal importance and may explain these results [
46]. Work by Kichler et al. showed through the use of (ATR)-FTIR spectroscopy that the LAH4 family exhibits alpha-helical confirmations with all positive histidine residues located on one side of the helix [
32]. Further NMR spectroscopy indicated that the interactions of these CPPs with membranes were modulated by these histidine-rich side-chains. Moreover, at a pH level of 5.4, the nanocomplexes underwent a shift toward a positive charge (
Figure 3C). This characteristic empowers the RNP: LAH5 nanocomplexes to proficiently interfere with endosomal membranes, capitalizing on the pH disparity. It is worth emphasizing that the pH level experiences fluctuations during the process of endosomal uptake, and the acidic milieu within the endosome plays a pivotal role in facilitating this phenomenon.
Furthermore, the robustness of our peptide-based transfection was demonstrated by showing gene editing in a variety of human cell types. Even though the editing efficiencies varied from 20 to 70%, in all cases, sufficiently high levels of editing were achieved to be therapeutically relevant. LAH5 peptide demonstrated significantly superior performance compared to CRISPRMAX at shorter incubation times. This observation strongly suggests that the cytosolic delivery of Cas9 RNP occurs more rapidly when facilitated by LAH5 peptide as compared to complexation with a lipid-based transfection agent.
This work demonstrates that the amphipathic LAH5 peptide can efficiently deliver Cas9 RNP with and without HDR template in a variety of cell types by means of nanocomplex formation and concomitant intracellular delivery of its cargo. The editing efficiencies were unprecedented, showing high levels of gene editing at Cas9 RNP concentrations as low as 5–10 nM.
We do not expect that the current nanocomplex formulation is stable enough for direct in vivo applications. To make nanocomplexes, a large molar excess of LAH5 peptide was needed. This points to a weak interaction with Cas9 RNP. Intravenous injection would quickly dilute out the free peptide, leading to a change in the equilibrium and potential dissociation of the nanocomplexes. Nevertheless, future research will investigate nanocomplexes’ stability under simulated in vivo conditions (whole serum) to obtain more insights into the stability of these complexes. For now, LAH5-peptide-assisted delivery of Cas9 RNP could be very useful, e.g., for the ex vivo modification of NK or T cells, as it relies on a single peptide that can be easily synthesized under good manufacturing practice (GMP).