Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Statistical Design of RS-Loaded Lipid-Based Nanovesicles
2.3. Preparation of Lipid-Based Nanovesicles Loaded with RS
2.4. Characterization of RS-Loaded Lipid-Based Nanovesicles
2.4.1. Measurement of VS and ZP
2.4.2. Measurement of EE%
2.5. Optimization of RS-Loaded Lipid-Based Nanovesicles
2.6. Characterization of Optimum RS-Loaded Lipid-Based Nanovesicles
2.6.1. Transmission Electron Microscopy (TEM) Study
2.6.2. Differential Scanning Calorimetry (DSC)
2.6.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.6.4. In Vitro Release Study
2.7. Preliminary Study for Preparation of Intranasal RS-Loaded Glycethosomal In Situ Gels
2.8. Characterization of RS-Loaded Glycethosomal In Situ Gels
2.8.1. Gelation Time Determination
2.8.2. Gel Strength Assessment
2.8.3. pH Evaluation
2.8.4. Viscosity Evaluation
2.8.5. Spreadability Evaluation
2.8.6. Mucoadhesive Strength Measurement
2.8.7. In Vitro Release Study
2.9. Characterization of Optimized RS-Loaded Glycethosomal In Situ Gel Formulation
2.9.1. Ex Vivo Drug Permeation Study
2.9.2. Histopathological Investigation
2.9.3. In Vivo Evaluation
Animals
Induction of Schizophrenia in Rats
Calculation of Pharmacokinetic Parameters
Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Statistical Analysis by BBD
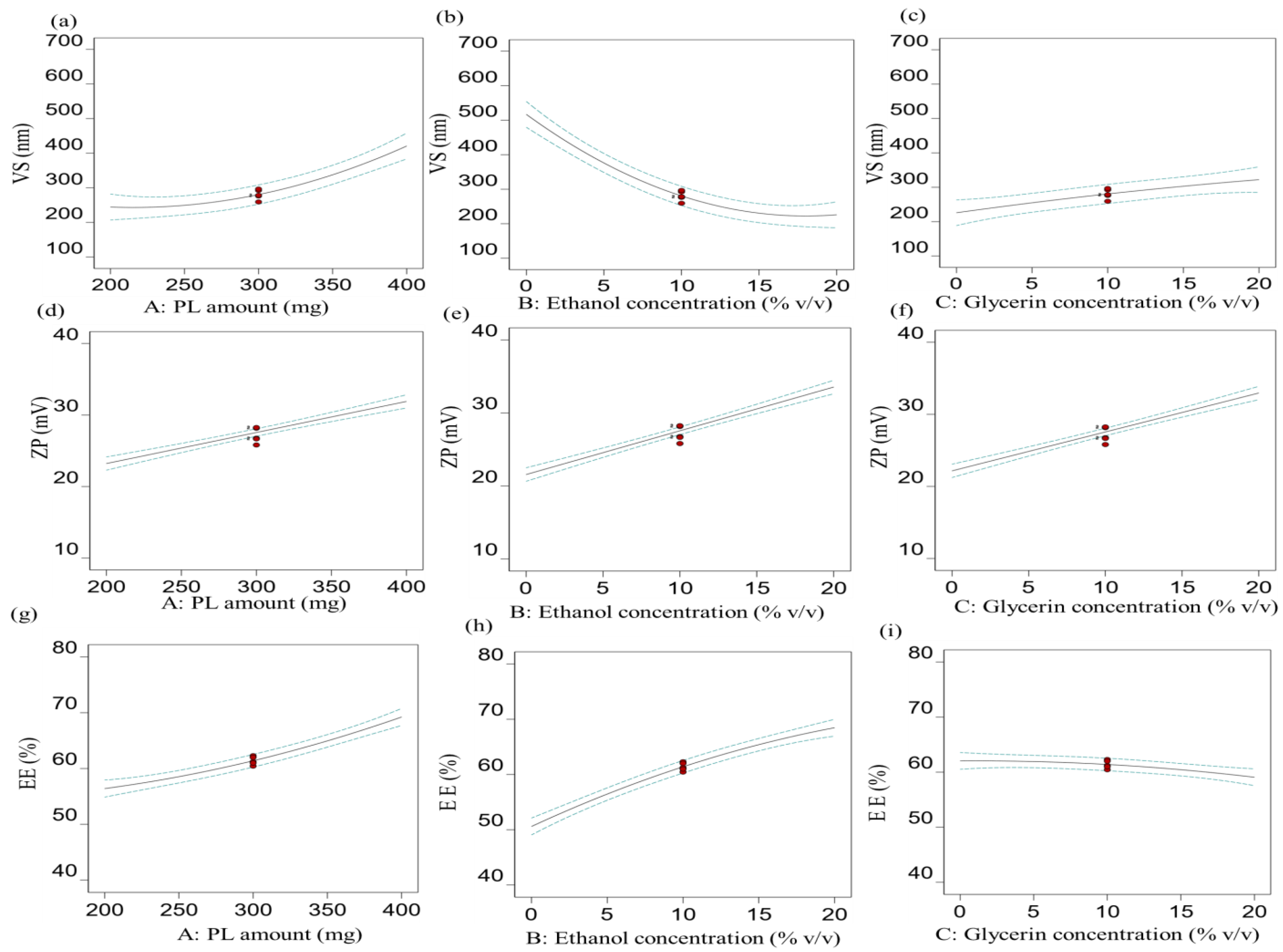
3.1.1. Effect of Independent Factors on VS (R1)
3.1.2. Effect of Independent Factors on ZP (R2)
3.1.3. Effect of Independent Factors on EE% (R3)
3.1.4. Optimization Technique
3.2. Characterization of Optimized RS-Loaded Glycethosomal formulation
3.2.1. TEM Study
3.2.2. DSC Study
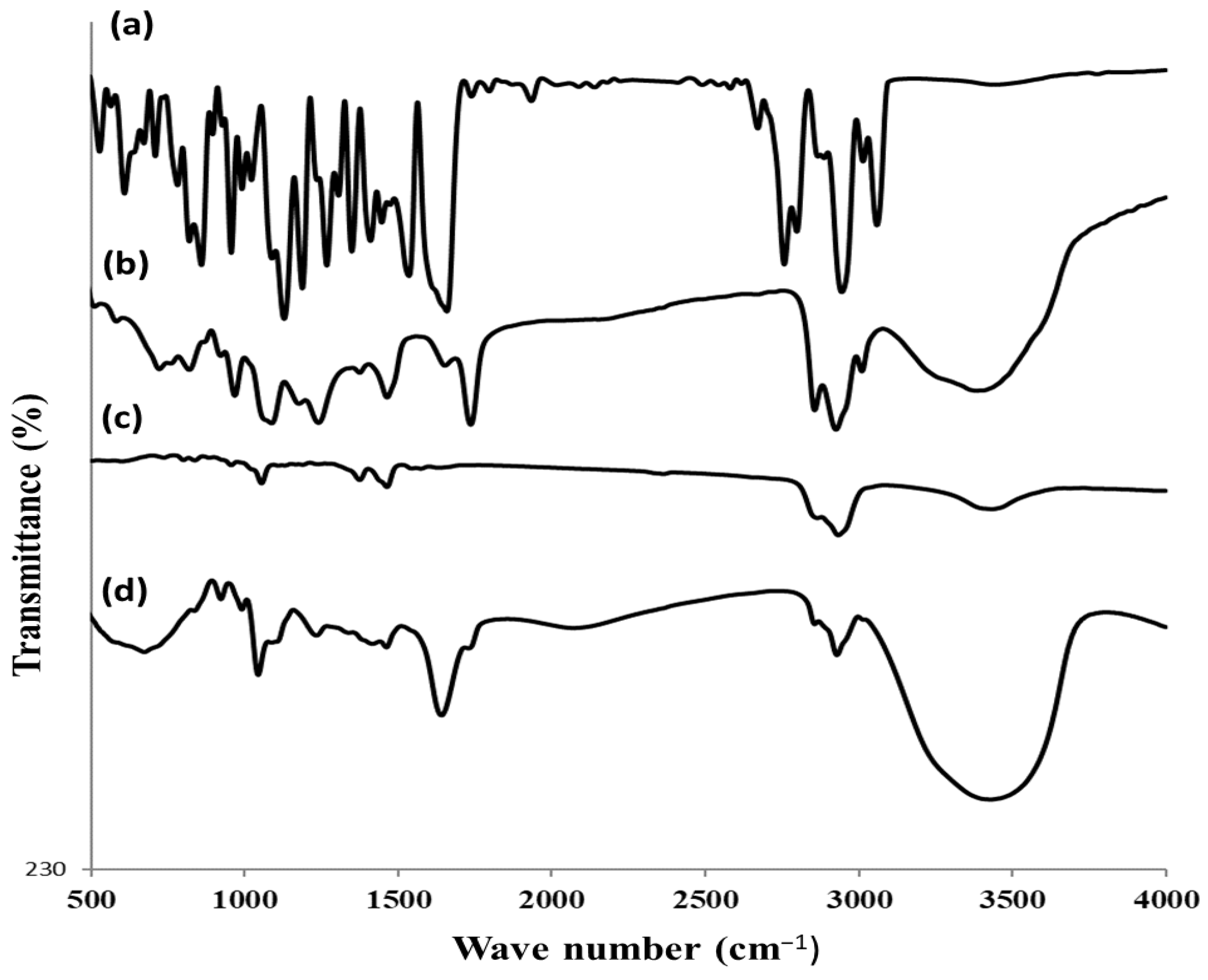
3.2.3. FTIR Study
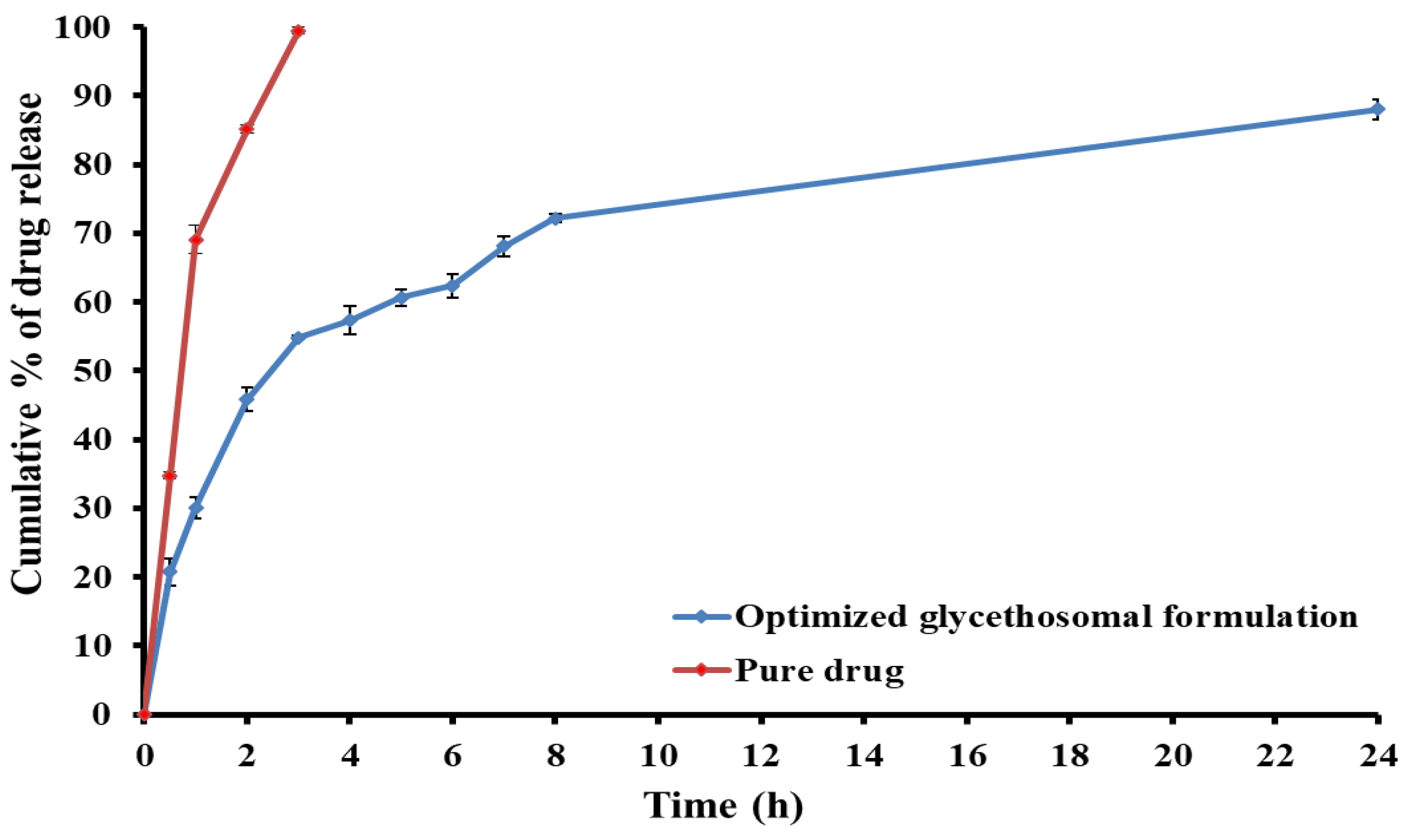
3.2.4. In Vitro Release Study
3.3. Development of RS-Loaded Glycethosomal In Situ Gel Formulations
3.4. Characterization of RS-Loaded Glycethosomal In Situ Gel Formulations
3.4.1. Determination of pH
3.4.2. Viscosity Determination
3.4.3. Spreadability Determination
3.4.4. Mucoadhesive Strength Study
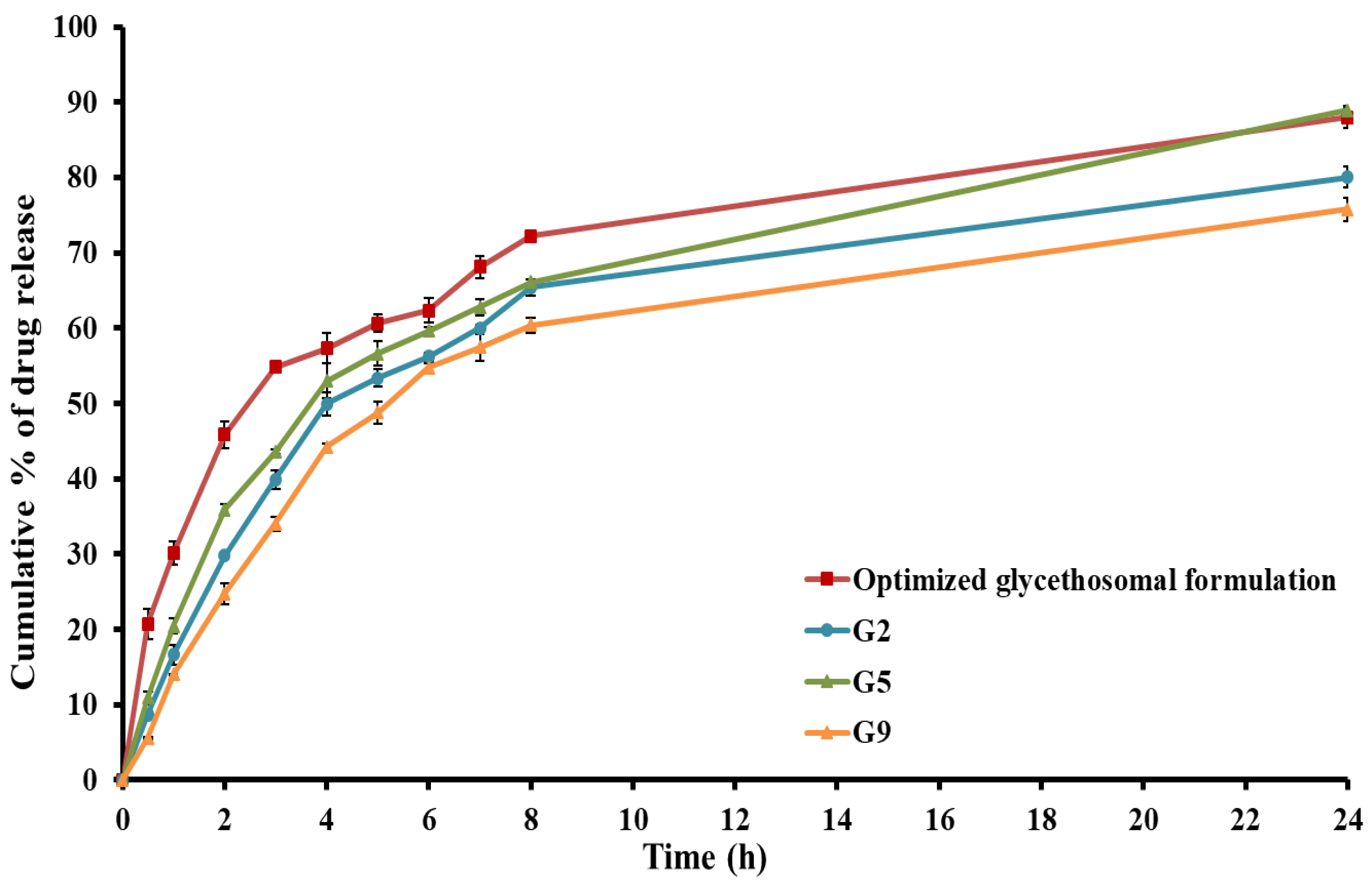
3.4.5. In Vitro Release Study
3.5. Characterization of Optimized RS-Loaded Glycethosomal In Situ Gel formulation
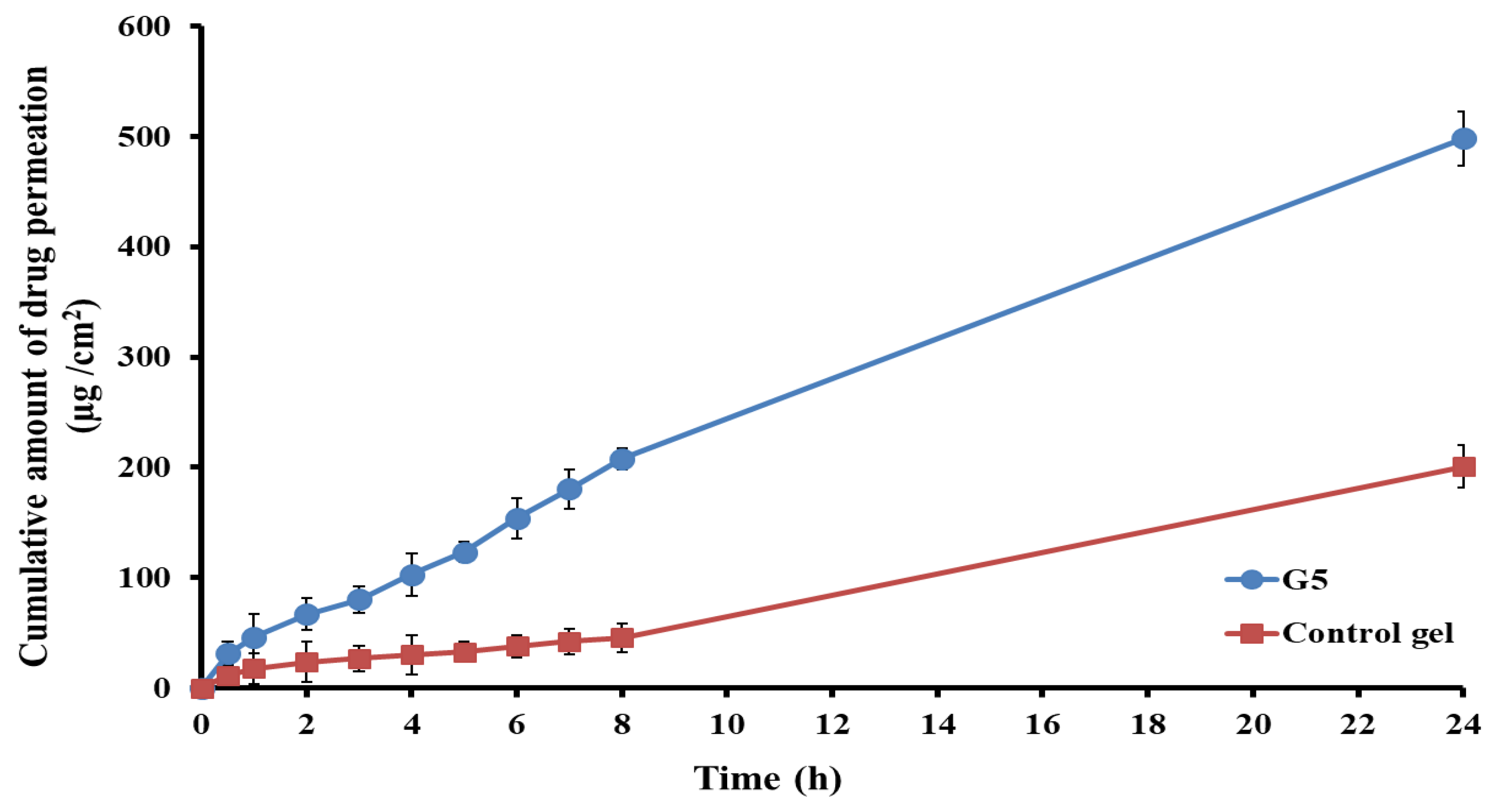
3.5.1. Ex Vivo Permeability Study
3.5.2. Histopathological Study
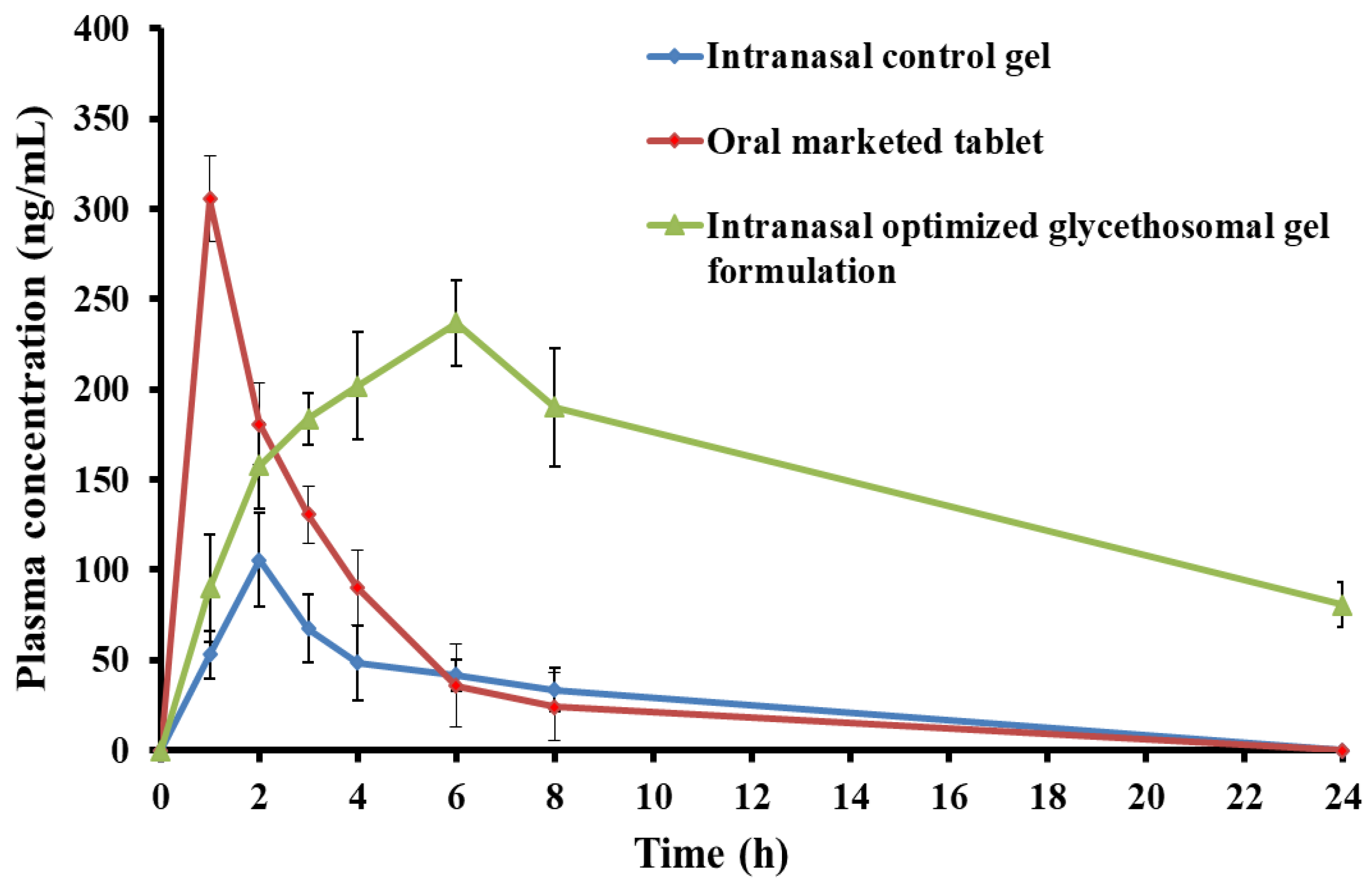
3.5.3. In Vivo Pharmacokinetic Study
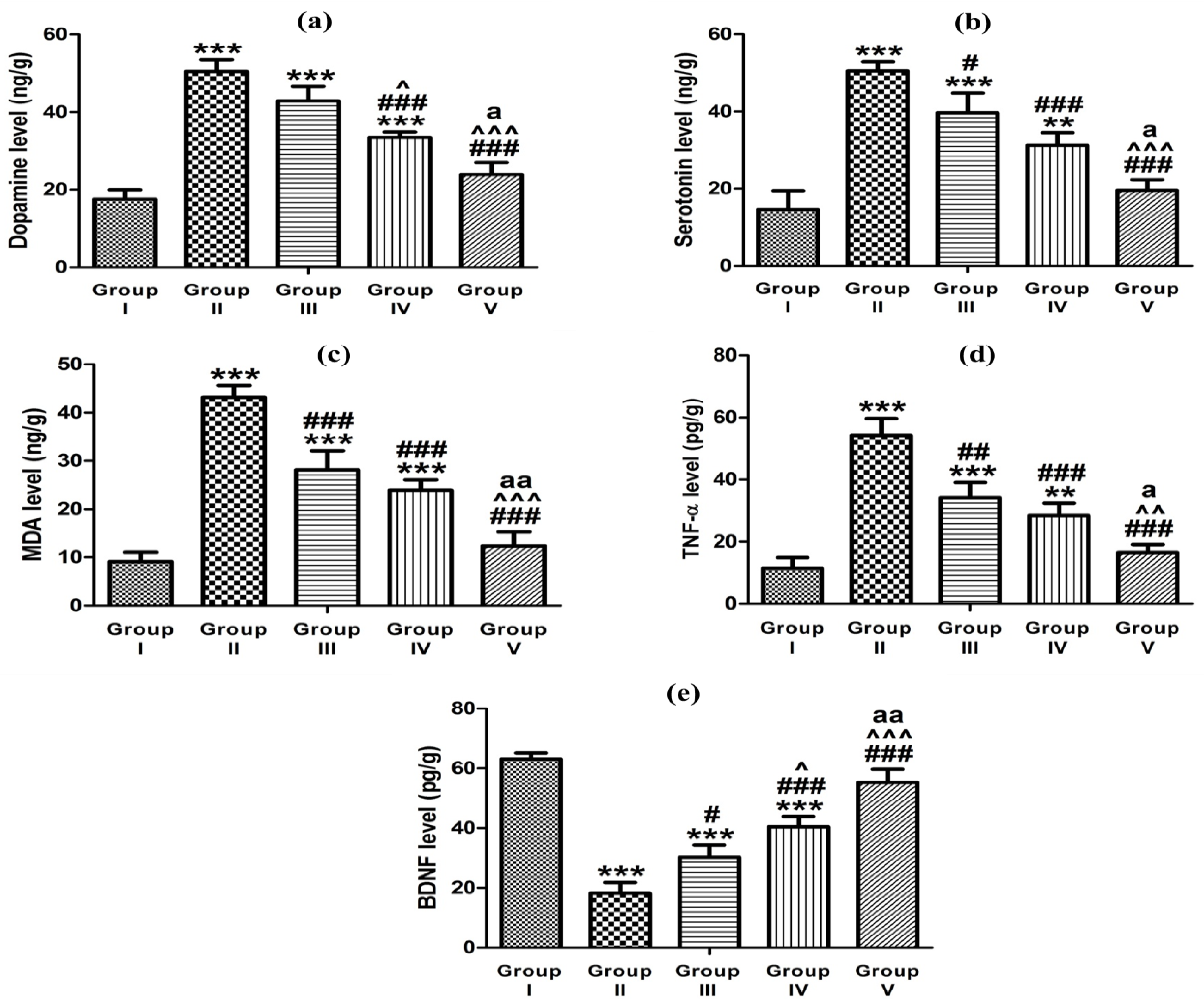
3.5.4. Effect of Optimized Gel Formulation on Ketamine-Induced Hippocampal Neurotransmitters’ Content, Oxidative Stress, Inflammation, and BDNF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Report of the International Pilot Study of Schizophrenia; World Health Organization: Geneva, Switzerland, 1973. [Google Scholar]
- Uttl, L.; Petrasek, T.; Sengul, H.; Svojanovska, M.; Lobellova, V.; Vales, K.; Radostova, D.; Tsenov, G.; Kubova, H.; Mikulecka, A. Chronic MK-801 application in adolescence and early adulthood: A spatial working memory deficit in adult Long-Evans rats but no changes in the hippocampal NMDA receptor subunits. Front. Pharmacol. 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Gener, T.; Campo, A.T.; Alemany-González, M.; Nebot, P.; Delgado-Sallent, C.; Chanovas, J.; Puig, M.V. Serotonin 5-HT1A, 5-HT2A and dopamine D2 receptors strongly influence prefronto-hippocampal neural networks in alert mice: Contribution to the actions of risperidone. Neuropharmacology 2019, 158, 107743. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.R.; Chogtu, B.; Bhattacharjee, D.; Agarwal, S. Extrapyramidal symptoms probably related to risperidone treatment: A case series. Ann. Neurosci. 2017, 24, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.J.; Salah, S.; Halim, S.A.A.; Badr-Eldin, S.M. Investigating the potential of utilizing glycerosomes as a novel vesicular platform for enhancing intranasal delivery of lacidipine. Int. J. Pharm. 2020, 582, 119302. [Google Scholar] [CrossRef] [PubMed]
- Ashtikar, M.; Nagarsekar, K.; Fahr, A. Transdermal delivery from liposomal formulations–Evolution of the technology over the last three decades. J. Control. Release 2016, 242, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.; Badr-Eldin, S.M. Development of an optimized avanafil-loaded invasomal transdermal film: Ex vivo skin permeation and in vivo evaluation. Int. J. Pharm. 2019, 570, 118657. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Abdel Hakim, L.F. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box–Behnken statistical design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef]
- Melis, V.; Manca, M.L.; Bullita, E.; Tamburini, E.; Castangia, I.; Cardia, M.C.; Valenti, D.; Fadda, A.M.; Peris, J.E.; Manconi, M. Inhalable polymer-glycerosomes as safe and effective carriers for rifampicin delivery to the lungs. Colloids Surfaces. B Biointerfaces 2016, 143, 301–308. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Desy Arya, I.F.; Wahab, H.A.; Nurzanah, D. Comparative Study of In Situ Gel Formulation Based on the Physico-Chemical Aspect: Systematic Review. Gels 2023, 9, 645. [Google Scholar] [CrossRef]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638. [Google Scholar]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Abdelnabi, D.M.; Elghamry, H.A. Response Surface Methodology for Optimization of Buspirone Hydrochloride-Loaded In Situ Gel for Pediatric Anxiety. Gels 2022, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- Abou-Taleb, H.A.; Khallaf, R.A.; Abdel-Aleem, J.A. Intranasal niosomes of nefopam with improved bioavailability: Preparation, optimization, and in-vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 3501–3516. [Google Scholar] [CrossRef]
- Abedullahh, M.H. Box-behnken design for development and optimization of acetazolamide microspheres. Int. J. Pharm. Sci. Res. 2014, 5, 1228–1239. [Google Scholar]
- Abdallah, M.H.; Lila, A.S.A.; Anwer, M.K.; Khafagy, E.-S.; Mohammad, M.; S Soliman, M. Formulation, development and evaluation of ibuprofen loaded nano-transferosomal gel for the treatment of psoriasis. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Das, P.J.; Dey, S.; Nayak, A.K.; Roy, P.K.; Chakrabarti, S.; Marbaniang, D.; Das, S.K.; Ray, S.; Chattopadhyay, P. Development and optimization of besifloxacin hydrochloride loaded liposomal gel prepared by thin film hydration method using 32 full factorial design. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124071. [Google Scholar] [CrossRef]
- Younes, N.F.; Habib, B.A. Augmented local skin accumulation efficiency of sertaconazole nitrate via glycerosomal hydrogel: Formulation, statistical optimization, ex vivo performance and in vivo penetration. J. Drug Deliv. Sci. Technol. 2022, 72, 103364. [Google Scholar] [CrossRef]
- Hasan, A.A.; Sabry, S.A.; Abdallah, M.H.; El-Damasy, D.A. Formulation and in vitro characterization of poly (DL-lactide-co-glycolide)/eudragit RLPO or RS30D nanoparticles as an oral carrier of levofloxacin hemihydrate. Pharm. Dev. Technol. 2016, 21, 655–663. [Google Scholar]
- Choi, H.G.; Jung, J.H.; Ryu, J.M.; Yoon, S.J.; Oh, Y.K.; Kim, C.K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wong, Y.C.; Zuo, Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, E.; Yener, G. Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, D.M.; Abdallah, M.H.; Elghamry, H.A. Buspirone hydrochloride loaded in situ nanovesicular gel as an anxiolytic nasal drug delivery system: In vitro and animal studies. AAPS PharmSciTech 2019, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.M.; Ibrahim, M.M.; Abdallah, M.H.; Mahdy, M.A. Sulpiride microemulsions as antipsychotic nasal drug delivery systems: In-vitro and pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2016, 36, 10–22. [Google Scholar] [CrossRef]
- Alzubaidi, A.F.; El-Helw, A.-R.M.; Ahmed, T.A.; Ahmed, O.A. The use of experimental design in the optimization of risperidone biodegradable nanoparticles: In vitro and in vivo study. Artif. Cells Nanomed. Biotechnol. 2017, 45, 313–320. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Eissa, R.G.; El-Megrab, N.A.; El-Nahas, H.M. Morphological characterization of optimized risperidone-loaded in-situ gel forming implants with pharmacokinetic and behavioral assessments in rats. J. Drug Deliv. Sci. Technol. 2021, 61, 102195. [Google Scholar] [CrossRef]
- Ahmed, H.I.; Abdel-Sattar, S.A.; Zaky, H.S. Vinpocetine halts ketamine-induced schizophrenia-like deficits in rats: Impact on BDNF and GSK-3β/β-catenin pathway. Naunyn-Schmiedebergs Arch. Pharmacol. 2018, 391, 1327–1338. [Google Scholar] [CrossRef]
- Habib, B.A.; Sayed, S.; Elsayed, G.M. Enhanced transdermal delivery of ondansetron using nanovesicular systems: Fabrication, characterization, optimization and ex-vivo permeation study-Box-Cox transformation practical example. Eur. J. Pharm. Sci. 2018, 115, 352–361. [Google Scholar] [CrossRef]
- Sathyamoorthy, N.; Magharla, D.; Chintamaneni, P.; Vankayalu, S. Optimization of paclitaxel loaded poly (ε-caprolactone) nanoparticles using Box Behnken design. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 362–373. [Google Scholar] [CrossRef]
- Teja, S.; Damodharan, N. 23 full factorial model for particle size optimization of methotrexate loaded chitosan nanocarriers: A design of experiments (DoE) approach. BioMed Res. Int. 2018, 2018, 7834159. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of anti-hypertensive effect of carvedilol. J. Liposome Res. 2019, 29, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Sakdiset, P.; Amnuaikit, T.; Pichayakorn, W.; Pinsuwan, S. Formulation development of ethosomes containing indomethacin for transdermal delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 760–768. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Khan, N.; Alfaleh, M.A.; Asfour, H.Z.; Riadi, Y.; Bilgrami, A.L.; Akhter, M.H. Plumbagin-loaded glycerosome gel as topical delivery system for skin cancer therapy. Polymers 2021, 13, 923. [Google Scholar] [CrossRef]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. Biomed Res. Int. 2017, 2017, 8310979. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, A.A.; Alanazi, M.A.; Munahhi, L.A.; Hamroon, J.D.; Mortagi, Y.; Qushawy, M.; Soliman, G.M. Binary ethosomes for the enhanced topical delivery and antifungal efficacy of ketoconazole. OpenNano 2023, 11, 100145. [Google Scholar] [CrossRef]
- Naguib, M.J.; Hassan, Y.R.; Abd-Elsalam, W.H. 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int. J. Pharm. 2021, 607, 121010. [Google Scholar] [CrossRef]
- Ramkar, S.; Kaurav, M.; Sudheesh, M.; Pandey, R.S. Enhanced skin penetration of Finasteride loaded DMSO-liposomes for the treatment of androgenic alopecia: Comparison with conventional liposomes. Drug Dev. Ind. Pharm. 2023, 49, 52–61. [Google Scholar] [CrossRef]
- Barupal, A.K.; Gupta, V.; Ramteke, S. Preparation and Characterization of Ethosomes for Topical delivery of Aceclofenac. Indian J. Pharm. Sci. 2010, 72, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Sambhakar, S.; Paliwal, S.K.; Sharma, S.; Sati, B.; Singh, B. Formulation and development of risperidone loaded niosomes for improved bioavailability: In vitro and in vivo study. Acta Pol. Pharm.–Drug Res. 2017, 74, 1859–1873. [Google Scholar]
- Abdulla, N.A.; Balata, G.F.; El-Ghamry, H.A.; Gomaa, E. Intranasal delivery of Clozapine using nanoemulsion-based in-situ gels: An approach for bioavailability enhancement. Saudi Pharm. J. 2021, 29, 1466–1485. [Google Scholar] [CrossRef]
- Ghazwani, M.; Vasudevan, R.; Kandasamy, G.; Manusri, N.; Devanandan, P.; Puvvada, R.C.; Veeramani, V.P.; Paulsamy, P.; Venkatesan, K.; Chidmabaram, K. Formulation of Intranasal Mucoadhesive Thermotriggered In Situ Gel Containing Mirtazapine as an Antidepressant Drug. Gels 2023, 9, 457. [Google Scholar] [CrossRef]
- Naeem, M.; Rahman, N.U.; TAVARES, G.; Barbosa, S.F.; Chacra, N.B.; Loebenberg, R.; Sarfraz, M.K. Physicochemical, in vitro and in vivo evaluation of flurbiprofen microemulsion. An. Acad. Bras. Ciências 2015, 87, 1823–1831. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson’s disease model. BioMed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.C.; Builders, P.F.; Agubata, C.O.; Attama, A.A. Development of ethosomal vesicular carrier for topical application of griseofulvin: Effect of ethanol concentration. J. Pharm. Investig. 2019, 49, 27–36. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A mucoadhesive, thermoreversible in situ nasal gel of geniposide for neurodegenerative diseases. PLoS ONE 2017, 12, e0189478. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Verma, S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci. World J. 2014, 2014, 280928. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Rahman, N.; Ahmad, S.; Ali, M.; Ahmad, I. Enhance transdermal delivery of flurbiprofen via microemulsions: Effects of different types of surfactants and cosurfactants. DARU J. Pharm. Sci. 2011, 19, 433. [Google Scholar]
- Zhu, C.; Zhang, Y.; Wu, T.; He, Z.; Guo, T.; Feng, N. Optimizing glycerosome formulations via an orthogonal experimental design to enhance transdermal triptolide delivery. Acta Pharm. 2022, 72, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; Aburahma, M.H. Ufasomes nano-vesicles-based lyophilized platforms for intranasal delivery of cinnarizine: Preparation, optimization, ex-vivo histopathological safety assessment and mucosal confocal imaging. Pharm. Dev. Technol. 2016, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Said, M.; Elmenoufy, G.A. Evaluation of quetiapine fumarate and its solid lipid nanoparticles as antipsychotic drug in rat model of schizophrenia. Biomed. Res. Ther. 2017, 4, 1480–1497. [Google Scholar]
- Xu, Y.; Deng, C.; Zheng, Y.; Liu, N.; Fu, B. Applying vinpocetine to reverse synaptic ultrastructure by regulating BDNF-related PSD-95 in alleviating schizophrenia-like deficits in rat. Compr. Psychiatry 2019, 94, 152122. [Google Scholar] [CrossRef]
- Ben-Azu, B.; Aderibigbe, A.O.; Eneni, A.-E.O.; Ajayi, A.M.; Umukoro, S.; Iwalewa, E.O. Morin attenuates neurochemical changes and increased oxidative/nitrergic stress in brains of mice exposed to ketamine: Prevention and reversal of schizophrenia-like symptoms. Neurochem. Res. 2018, 43, 1745–1755. [Google Scholar] [CrossRef]
- Farkhakfar, A.; Hassanpour, S.; Zendehdel, M. Resveratrol plays Neuroprotective role on ketamine-induced schizophrenia-like behaviors and oxidative damage in mice. Neurosci. Lett. 2023, 813, 137436. [Google Scholar] [CrossRef]
- Hendouei, N.; Farnia, S.; Mohseni, F.; Salehi, A.; Bagheri, M.; Shadfar, F.; Barzegar, F.; Hoseini, S.D.; Charati, J.Y.; Shaki, F. Alterations in oxidative stress markers and its correlation with clinical findings in schizophrenic patients consuming perphenazine, clozapine and risperidone. Biomed. Pharmacother. 2018, 103, 965–972. [Google Scholar] [CrossRef]
- Jiang, N.; Jingwei, L.; Wang, H.; Huang, H.; Wang, Q.; Zeng, G.; Li, S.; Liu, X. Ginsenoside 20 (S)-protopanaxadiol attenuates depressive-like behaviour and neuroinflammation in chronic unpredictable mild stress-induced depressive rats. Behav. Brain Res. 2020, 393, 112710. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Dalimi, A.; Ghaffarifar, F.; Nader-Mohammadi, M.; Molavi, P.; Dadkhah, M.; Molaei, S. Berberine improves inhibitory avoidance memory impairment of Toxoplasma gondii-infected rat model of ketamine-induced schizophrenia. BMC Complement. Med. Ther. 2023, 23, 303. [Google Scholar] [CrossRef] [PubMed]



| Independent Variables | Symbol | Level of Variation | |
|---|---|---|---|
| Low | High | ||
| PL amount (mg) | A | 200 | 400 |
| Ethanol concentration (% v/v) | B | 0 | 20 |
| Glycerin concentration (% v/v) | C | 0 | 20 |
| Dependent variables | Symbol | Constraint | |
| VS (nm) | R1 | Minimize | |
| ZP (mV) | R2 | Maximize | |
| EE% (%) | R3 | Maximize | |
| Type of Formulation | Run (e) | A: PL Amount (mg) | B: Ethanol Concentration (% v/v) | C: Glycerin Concentration (% v/v) | R1: VS (nm) | R2: ZP (mV) | R3: EE% (%) |
|---|---|---|---|---|---|---|---|
| Glycerosome (a) | F1 | 400 | 0 | 10 | 646.80 ± 31.65 | 25.30 ± 1.95 | 57.86 ± 0.87 |
| Glycethosome (b) | F2 | 300 | 20 | 20 | 231.30 ± 19.18 | 39.25 ± 0.42 | 67.20 ± 1.15 |
| Glycethosome | F3 | 400 | 20 | 10 | 410.50 ± 14.08 | 37.86 ± 0.99 | 78.16 ± 1.68 |
| Glycethosome | F4 | 200 | 10 | 20 | 328.90 ± 20.11 | 27.76 ± 1.51 | 55.37 ± 0.99 |
| Glycethosome | F5 | 300 | 10 | 10 | 292.30 ± 12.63 | 26.66 ± 1.05 | 62.29 ± 1.05 |
| Ethosome (c) | F6 | 400 | 10 | 0 | 352.20 ± 11.61 | 26.16 ± 1.10 | 70.30 ± 1.81 |
| Glycethosome | F7 | 300 | 10 | 10 | 259.20 ± 15.94 | 26.76 ± 0.94 | 61.23 ± 0.85 |
| Glycerosome | F8 | 300 | 0 | 20 | 571.70 ± 26.81 | 27.91 ± 0.95 | 47.62 ± 1.65 |
| Glycethosome | F9 | 300 | 10 | 10 | 276.20 ± 10.13 | 28.15 ± 1.22 | 62.01 ± 1.12 |
| Glycethosome | F10 | 200 | 20 | 10 | 163.10 ± 7.45 | 28.56 ± 0.75 | 60.58 ± 0.57 |
| Ethosome | F11 | 200 | 10 | 0 | 180.90 ± 16.73 | 19.57 ± 1.15 | 57.05 ± 1.10 |
| Ethosome | F12 | 300 | 20 | 0 | 189.70 ± 20.19 | 28.66 ± 0.88 | 69.11 ± 1.41 |
| Glycerosome | F13 | 200 | 0 | 10 | 473.70 ± 10.08 | 17.26 ± 0.65 | 47.22 ± 1.95 |
| Glycethosome | F14 | 400 | 10 | 20 | 442.30 ± 16.88 | 38.58 ± 0.81 | 65.23 ± 1.81 |
| Liposome (d) | F15 | 300 | 0 | 0 | 466.50 ± 29.15 | 15.92 ± 1.31 | 50.83 ± 0.89 |
| Glycethosome | F16 | 300 | 10 | 10 | 278.50 ± 18.05 | 25.81 ± 0.91 | 60.45 ± 1.25 |
| Glycethosome | F17 | 300 | 10 | 10 | 296.50 ± 9.93 | 28.25 ± 1.05 | 60.99 ± 1.31 |
| Gel Base | Tsol-gel (°C) |
|---|---|
| P407 (17% w/w) | >60 |
| P407 (18% w/w) | 44.67 ± 0.47 |
| P407 (19% w/w) | 37.00 ± 0.82 |
| P407 (20% w/w) | 33.67 ± 0.49 |
| Formulation | Gel Base | Mucoadhesive Gent | Gel/Glycethosome Ratio (w/w) | Gelation Time (sec) | Gel Strength (sec) |
|---|---|---|---|---|---|
| G1 | P407 (20% w/w) | Carbopol 940 (0.1% w/w) | 4:1 | No gelation | - |
| 5:1 | No gelation | - | |||
| 6:1 | 30.28 ± 0.66 | 22.34 ± 0.85 | |||
| G2 | P407 (20% w/w) | Carbopol 940 (0.2% w/w) | 2:1 | No gelation | - |
| 3:1 | 35.47 ± 0.92 | 18.30 ± 1.12 | |||
| 4:1 | 20.12 ± 0.15 | 29.23 ± 1.01 | |||
| G3 | P407 (20% w/w) | Carbopol 940 (0.3% w/w) | 2:1 | No gelation | - |
| 3:1 | 44.37 ± 1.02 | 23.25 ± 0.91 | |||
| 4:1 | 10.80 ± 0.79 | > 50 | |||
| G4 | P407 (20% w/w) | HPMC-K4M (0.7% w/w) | 4:1 | No gelation | - |
| 5:1 | 36.73 ± 0.84 | 23.27 ± 0.94 | |||
| 6:1 | 24.35 ± 1.12 | 27.40 ± 0.34 | |||
| G5 | P407 (20% w/w) | HPMC-K4M (1% w/w) | 2:1 | No gelation | - |
| 3:1 | No gelation | - | |||
| 4:1 | 12.36 ± 0.19 | 32.74 ± 0.64 | |||
| G6 | P407 (20% w/w) | HPMC-K4M (1.2% w/w) | 2:1 | No gelation | - |
| 3:1 | 44.37 ± 1.02 | 39.84 ± 0.43 | |||
| 4:1 | 10.80 ± 0.79 | > 50 | |||
| G7 | P407 (20% w/w) | PVP K30 (0.5% w/w) | 4:1 | No gelation | - |
| 5:1 | 38.45 ± 0.59 | 12.92 ± 1.23 | |||
| 6:1 | 27.01 ± 0.93 | 15.75 ± 0.78 | |||
| G8 | P407 (20% w/w) | PVP K30 (0.7% w/w) | 4:1 | No gelation | - |
| 5:1 | 36.28 ± 0.73 | 16.19 ± 0.18 | |||
| 6:1 | 20.63 ± 1.93 | 20.13 ± 0.90 | |||
| G9 | P407 (20% w/w) | PVP K30 (1% w/w) | 3:1 | No gelation | - |
| 4:1 | 34.23 ± 0.27 | 30.10 ± 0.19 | |||
| 5:1 | 10.38 ± 0.91 | 35.27 ± 1.10 |
| Formulation | Gel Base | Mucoadhesive Agent | Gel/Glycethosome Ratio (w/w) | pH | Viscosity (cP) | Spreadability (cm) | Mucoadhesive Strength (dyne/cm2) |
|---|---|---|---|---|---|---|---|
| G2 | P407 (20% w/w) | Carbopol 940 (0.2% w/w) | 4:1 | 6.41 ± 0.04 | 15,366.67 ± 169.97 | 3.00 ± 0.08 | 4799.04 ± 63.71 |
| G5 | P407 (20% w/w) | HPMC-K4M (1% w/w) | 4:1 | 6.58 ± 0.12 | 11,133.33 ± 47.14 | 3.26 ± 0.05 | 4163.23 ± 38.97 |
| G9 | P407 (20% w/w) | PVP K30 (1% w/w) | 5:1 | 6.74 ± 0.02 | 16,366.67 ± 309.12 | 2.53 ± 0.12 | 5197.24 ± 53.05 |
| Permeation Parameter | Optimized Glycethosomal Gel (G5) | Control Gel |
|---|---|---|
| Jss (μg/cm2·h−1) | 22.11 ± 3.37 | 4.07 ± 1.12 |
| Kp × 10−3 (cm/h) | 11.06 ± 1.75 | 2.04 ± 0.58 |
| Er | 5.43 | - |
| Parameters | Control Gel | Oral Marketed Tablet | Optimized Glycethosomal Gel |
|---|---|---|---|
| Cmax (ng/mL) | 105.54 ± 25.74 | 305.51 ± 23.85 | 236.87 ± 23.67 |
| tmax (h) | 2 | 1 | 6 |
| Kel (h−1) | 0.092 ± 0.02 | 0.37 ± 0.12 | 0.057 ± 0.01 |
| t1/2 (h) | 7.52 ± 0.33 | 1.88 ± 0.61 | 12.08 ± 0.87 |
| AUC0-t (ng·mL−1·h) | 442.74 ± 36.53 | 1106.39 ± 55.14 | 3610.84 ± 69.38 |
| AUC0-∞ (ng·mL−1·h) | 807.86 ± 40.79 | 1172.39 ± 48.71 | 5018.35 ± 53.46 |
| AUMC0-∞ (ng·mL−1·h2) | 8411.58 ± 73.24 | 2932.79 ± 64.41 | 92,519.05 ± 95.11 |
| MRT (h) | 10.41 ± 0.81 | 2.50 ± 0.14 | 18.44 ± 0.96 |
| Relative bioavailability (%) | - | 249.89 | 815.57 |
| Animal Groups | Dopamine (ng/g Tissue) | Serotonin (ng/g Tissue) | MDA (ng/g Tissue) | TNF-α (pg/g Tissue) | BDNF (pg/g Tissue) |
|---|---|---|---|---|---|
| Group I | 17.52 ± 2.46 | 14.62 ± 4.83 | 9.10 ± 1.96 | 11.43 ± 3.42 | 63.19 ± 1.96 |
| Group II | 50.43 ± 3.15 | 50.46 ± 2.49 | 43.17 ± 2.39 | 54.27 ± 5.42 | 18.26 ± 3.51 |
| Group III | 42.86 ± 3.73 | 39.69 ± 5.10 | 28.15 ± 3.97 | 34.12 ± 4.90 | 30.20 ± 4.11 |
| Group IV | 33.49 ± 1.39 | 31.24 ± 3.26 | 23.95 ± 2.11 | 28.38 ± 4.01 | 40.41 ± 3.56 |
| Group V | 23.92 ± 3.06 | 19.57 ± 2.73 | 12.38 ± 2.93 | 16.45 ± 2.66 | 55.27 ± 4.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.H.; El-Horany, H.E.-S.; El-Nahas, H.M.; Ibrahim, T.M. Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route. Pharmaceutics 2023, 15, 2521. https://doi.org/10.3390/pharmaceutics15112521
Abdallah MH, El-Horany HE-S, El-Nahas HM, Ibrahim TM. Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route. Pharmaceutics. 2023; 15(11):2521. https://doi.org/10.3390/pharmaceutics15112521
Chicago/Turabian StyleAbdallah, Marwa H., Hemat El-Sayed El-Horany, Hanan M. El-Nahas, and Tarek M. Ibrahim. 2023. "Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route" Pharmaceutics 15, no. 11: 2521. https://doi.org/10.3390/pharmaceutics15112521
APA StyleAbdallah, M. H., El-Horany, H. E.-S., El-Nahas, H. M., & Ibrahim, T. M. (2023). Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route. Pharmaceutics, 15(11), 2521. https://doi.org/10.3390/pharmaceutics15112521







