Porphyrin as a Cryoprotectant for Graphene Oxide-Coated Gold Nanorods to Produce Conjugated Product with Improved Stability and Opto-Phototherapeutic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation Techniques
2.3. Synthesis of Graphene Oxide
2.4. Synthesis of Gold Nanorods
2.5. Synthesis of Graphene Oxide-Coated Gold Nanorods
2.6. Synthesis of Meso-Tetra-(4-sulfonatophenyl)porphyrin (TPPS4)
2.7. Conjugation of TPPS4 to GO@AuNRs
2.8. Freeze-Drying of AuNRs, GO@AuNRs, and GO@AuNRs-TPPS4
2.9. Photothermal Evaluation
2.10. Fluorescence Quantum Yield Measurements
2.11. Singlet Oxygen Quantum Yield Evaluation
3. Results
3.1. Characterisation of Gold Nanorods, Graphene Oxide, and Graphene Oxide-Anchored Gold Nanorods Conjugated to TPPS4
3.2. Lyophilisation of Nanocomposite Conjugated to Porphyrin
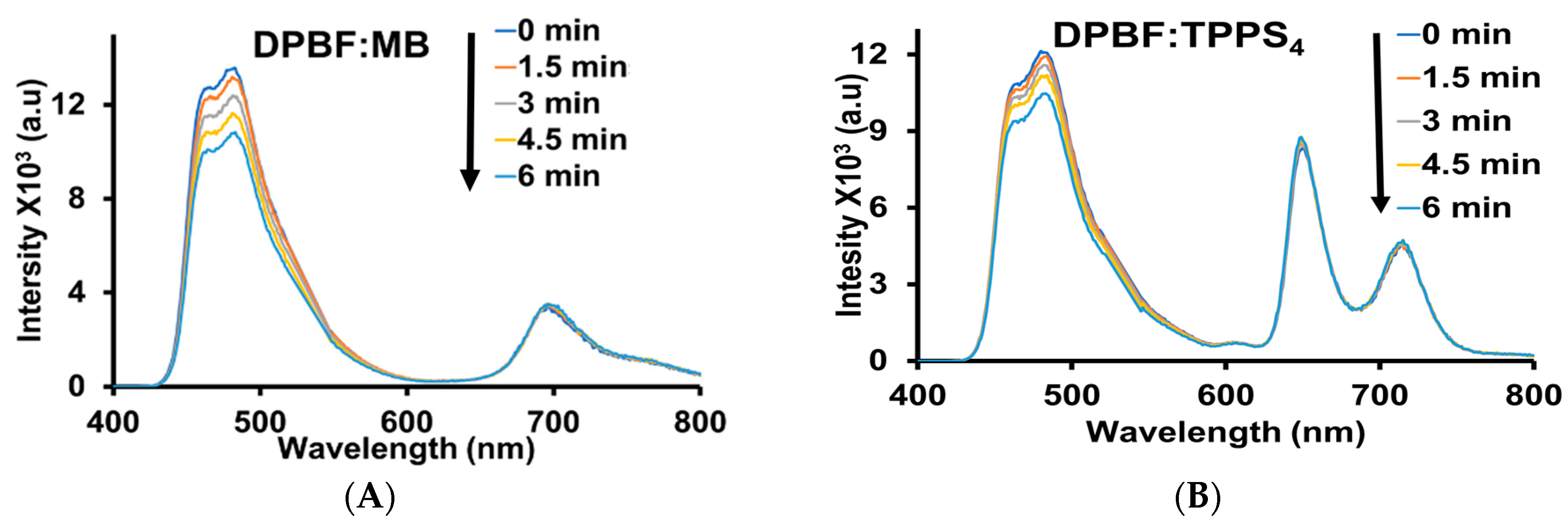
3.3. Lifetime, Quantum Yield, and Singlet Oxygen Generation Profiling
3.4. Photothermal Profiling Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.; Perry, H.L.; Wilton-Ely, J.D. Strategies for the functionalisation of gold nanorods to reduce toxicity and aid clinical translation. Nanotheranostics 2021, 5, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Shi, J.; Zhu, B.; Li, J.; Cao, S. Gold nanorods/graphene oxide nanosheets immobilized by polydopamine for efficient remotely triggered drug delivery. J. Mater. Sci. 2020, 55, 14530–14543. [Google Scholar] [CrossRef]
- Requejo, K.I.; Liopo, A.; Zubarev, E.R. Gold Nanorods Synthesis with Small Thiolated Molecules. Langmuir 2020, 36, 3758–3769. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, J.; Cui, S.; Zhu, H.; An, W.; Fu, Q.; Shao, C.; Yao, A.; Chen, B.; Shi, D. In situ synthesis of graphene oxide/gold nanorods theranostic hybrids for efficient tumor computed tomography imaging and photothermal therapy. Nano Res. 2017, 10, 37–48. [Google Scholar] [CrossRef]
- Khan, M.S.; Pandey, S.; Bhaisare, M.L.; Gedda, G.; Talib, A.; Wu, H.-F. Graphene oxide@ gold nanorods for chemo-photothermal treatment and controlled release of doxorubicin in mice Tumor. Colloids Surf. B Biointerfaces 2017, 160, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ni, H.; Jin, X.; Yuan, J. Graphene oxide wrapped gold nanorods for enhanced photo-thermal stability. RSC Adv. 2015, 5, 54971–54977. [Google Scholar] [CrossRef]
- Dembereldorj, U.; Choi, S.Y.; Ganbold, E.O.; Song, N.W.; Kim, D.; Choo, J.; Lee, S.Y.; Kim, S.; Joo, S.W. Gold Nanorod-Assembled PEGylated Graphene-Oxide Nanocomposites for Photothermal Cancer Therapy. Photochem. Photobiol. 2014, 90, 659–666. [Google Scholar] [CrossRef]
- Duman, F.D.; Sebek, M.; Thanh, N.T.; Loizidou, M.; Shakib, K.; MacRobert, A.J. Enhanced photodynamic therapy and fluorescence imaging using gold nanorods for porphyrin delivery in a novel in vitro squamous cell carcinoma 3D model. J. Mater. Chem. B 2020, 8, 5131–5142. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Monteiro, C.S.; Chaves, C.R.; Sáfar, G.A.M.; Moreira, R.L.; Pinheiro, M.V.B.; Martins, D.C.S.; Ladeira, L.O.; Krambrock, K. Hybrid systems based on gold nanostructures and porphyrins as promising photosensitizers for photodynamic therapy. Colloids Surf. B Biointerfaces 2017, 150, 297–307. [Google Scholar] [CrossRef]
- Hlapisi, N.; Motaung, T.E.; Linganiso, L.Z.; Oluwafemi, O.S.; Songca, S.P. Encapsulation of gold nanorods with porphyrins for the potential treatment of cancer and bacterial diseases: A critical review. Bioinorg. Chem. Appl. 2019, 2019, 7147128. [Google Scholar] [CrossRef]
- Lebepe, T.C.; Parani, S.; Ncapayi, V.; Maluleke, R.; Mbaz, G.I.M.; Fanoro, O.T.; Varghese, J.R.; Komiya, A.; Kodama, T.; Oluwafemi, O.S. Graphene Oxide-Gold Nanorods Nanocomposite-Porphyrin Conjugate as Promising Tool for Cancer Phototherapy Performance. Pharmaceuticals 2021, 14, 1295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wu, H.; Yao, S.Q.; Xu, Q.-H.; Xu, G.Q. Nanocomposites Containing Gold Nanorods and Porphyrin-Doped Mesoporous Silica with Dual Capability of Two-Photon Imaging and Photosensitization. Langmuir 2010, 26, 14937–14942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Yuan, D.; Zhang, H.Z.; Lu, Y.D.; Wang, N.; Zou, H.Y.; Wang, J. Rapid detection of heparin by gold nanorods and near-infrared fluorophore ensemble based platform via nanometal surface energy transfer. Sens. Actuators B Chem. 2018, 274, 318–323. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, J.J.; Zhang, H.Z.; Wang, N.; Zou, H.Y.; Huang, C.Z.; Wang, J. Highly selective detection of spermine in human urine via a nanometal surface energy transfer platform. Talanta 2018, 188, 218–224. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, Y.; Lu, X.; Thai, T.; Lee, N.A.; Bach, U.; Gooding, J.J. Biocompatible gold nanorods: One-step surface functionalization, highly colloidal stability, and low cytotoxicity. Langmuir 2015, 31, 4973–4980. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Aqabani, H.; Hikmat, S.; Abu-Dahab, R. Colloidal Stability and Cytotoxicity of Polydopamine-Conjugated Gold Nanorods against Prostate Cancer Cell Lines. Molecules 2021, 26, 1299. [Google Scholar] [CrossRef]
- Hamaly, M.A.; Abulateefeh, S.R.; Al-Qaoud, K.M.; Alkilany, A.M. Freeze-drying of monoclonal antibody-conjugated gold nanorods: Colloidal stability and biological activity. Int. J. Pharm. 2018, 550, 269–277. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Abulateefeh, S.R.; Mills, K.K.; Bani Yaseen, A.I.; Hamaly, M.A.; Alkhatib, H.S.; Aiedeh, K.M.; Stone, J.W. Colloidal Stability of Citrate and Mercaptoacetic Acid Capped Gold Nanoparticles upon Lyophilization: Effect of Capping Ligand Attachment and Type of Cryoprotectants. Langmuir 2014, 30, 13799–13808. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.A.; Panfilova, E.V.; Pylaev, T.E.; Bibikova, O.A.; Staroverov, S.A.; Bogatyrev, V.A.; Dykman, L.A.; Khlebtsov, N.G. New types of nanomaterials: Powders of gold nanospheres, nanorods, nanostars, and gold-silver nanocages. Nanotechnol. Russ. 2013, 8, 209–219. [Google Scholar] [CrossRef]
- Zagami, R.; Franco, D.; Pipkin, J.D.; Antle, V.; De Plano, L.; Patanè, S.; Guglielmino, S.; Monsù Scolaro, L.; Mazzaglia, A. Sulfobutylether-β-cyclodextrin/5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphine nanoassemblies with sustained antimicrobial phototherapeutic action. Int. J. Pharm. 2020, 585, 119487. [Google Scholar] [CrossRef]
- Ham, H.; Khai, T.V.; Park, N.H.; So, D.S.; Lee, J.W.; Na, H.G.; Kwon, Y.J.; Cho, H.Y.; Kim, H.W. Freeze-drying-induced changes in the properties of graphene oxides. Nanotechnology 2014, 25, 235601. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Adronov, A. Noncovalent functionalization and solubilization of carbon nanotubes by using a conjugated Zn–porphyrin polymer. Chem. Eur. J. 2006, 12, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Ncapayi, V.; Parani, S.; Sakho, E.H.M.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Synthesis of fluorescent CuInS2/ZnS quantum dots—Porphyrin conjugates for photodynamic therapy. MRS Commun. 2018, 8, 398–403. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [PubMed]
- Sakho, E.H.M.; Oluwafemi, O.S.; Thomas, S.; Kalarikkal, N. Dynamic energy transfer in non-covalently functionalized reduced graphene oxide/silver nanoparticle hybrid (NF-RGO/Ag) with NF-RGO as the donor material. J. Mater. Sci. Mater. Electron. 2017, 28, 2651–2659. [Google Scholar] [CrossRef]
- Lebepe, T.C.; Oluwafemi, O.S. Photothermal Conversion Profiling of Large-Scaled Synthesized Gold Nanorods Using Binary Surfactant with Hydroquinone as a Reducing Agent. Nanomaterials 2022, 12, 1723. [Google Scholar] [CrossRef]
- Tsolekile, N.; Ncapayi, V.; Obiyenwa, G.K.; Matoetoe, M.; Songca, S.; Oluwafemi, O.S. Synthesis of meso-tetra-(4-sulfonatophenyl) porphyrin (TPPS4)–CuInS/ZnS quantum dots conjugate as an improved photosensitizer. Int. J. Nanomed. 2019, 14, 7065. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Dong, X.; Cheng, W.-Y.; Duan, H.; Cheung, P.C.K. In vitro and in vivo photothermal cancer therapeutic effects of gold nanorods modified with mushroom β-Glucan. J. Agric. Food Chem. 2018, 66, 4091–4098. [Google Scholar] [CrossRef]
- Huang, P.; Wang, S.; Wang, X.; Shen, G.; Lin, J.; Wang, Z.; Guo, S.; Cui, D.; Yang, M.; Chen, X. Surface Functionalization of Chemically Reduced Graphene Oxide for Targeted Photodynamic Therapy. J. Biomed. Nanotechnol. 2015, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Field, R.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef]
- Wu, L.; Feng, L.; Ren, J.; Qu, X. Electrochemical detection of dopamine using porphyrin-functionalized graphene. Biosens. Bioelectron. 2012, 34, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.; De Luca, G.; Romeo, A.; Castriciano, M.A.; Scolaro, L.M. Spectroscopic investigation on porphyrins nano-assemblies onto gold nanorods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Mondal, D.; Martin, J.; Kane, R.S. Photoactivated antimicrobial activity of carbon nanotube−porphyrin conjugates. Langmuir 2010, 26, 17369–17374. [Google Scholar] [CrossRef]
- Geng, J.; Jung, H.-T. Porphyrin functionalized graphene sheets in aqueous suspensions: From the preparation of graphene sheets to highly conductive graphene films. J. Phys. Chem. C 2010, 114, 8227–8234. [Google Scholar] [CrossRef]
- Geng, J.; Kong, B.-S.; Yang, S.B.; Jung, H.-T. Preparation of graphene relying on porphyrin exfoliation of graphite. Chem. Commun. 2010, 46, 5091–5093. [Google Scholar] [CrossRef]
- Wang, A.; Yu, W.; Xiao, Z.; Song, Y.; Long, L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, C. A 1, 3-dipolar cycloaddition protocol to porphyrin-functionalized reduced graphene oxide with a push-pull motif. Nano Res. 2015, 8, 870–886. [Google Scholar] [CrossRef]
- Wang, A.; Yu, W.; Huang, Z.; Zhou, F.; Song, J.; Song, Y.; Long, L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, L. Covalent functionalization of reduced graphene oxide with porphyrin by means of diazonium chemistry for nonlinear optical performance. Sci. Rep. 2016, 6, 23325. [Google Scholar] [CrossRef]
- Chen, Q.; Brambilla, L.; Daukiya, L.; Mali, K.S.; De Feyter, S.; Tommasini, M.; Müllen, K.; Narita, A. Synthesis of Triply Fused Porphyrin-Nanographene Conjugates. Angew. Chem. Int. Ed. 2018, 57, 11233–11237. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J.; Hu, Z.; Hasan, M.; Vargas, E.; Pantoya, M.; Wang, S. Thermal-Recoverable Tough Hydrogels Enhanced by Porphyrin Decorated Graphene Oxide. Nanomaterials 2019, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Gacka, E.; Burdzinski, G.; Marciniak, B.; Kubas, A.; Lewandowska-Andralojc, A. Interaction of light with a non-covalent zinc porphyrin–graphene oxide nanohybrid. Phys. Chem. Chem. Phys. 2020, 22, 13456–13466. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Renjini, S.; Nancy, T.M.; Kumary, V.A. Electrochemical synthesis of thin-layered graphene oxide-poly (CTAB) composite for detection of morphine. J. Appl. Electrochem. 2020, 50, 41–50. [Google Scholar] [CrossRef]
- Mao, B.; Calatayud, D.G.; Mirabello, V.; Hodges, B.J.; Martins, J.A.R.; Botchway, S.W.; Mitchels, J.M.; Pascu, S.I. Interactions between an Aryl Thioacetate-Functionalized Zn(II) Porphyrin and Graphene Oxide. Adv. Funct. Mater. 2016, 26, 687–697. [Google Scholar] [CrossRef]







| Sample | PLQY (%) | τ1 (ns) | τ2 (ns) | χ2 | τavg (ns) |
|---|---|---|---|---|---|
| TPPS4 | 1.14 | 2.36 (4.86%) | 9.85 (95.14%) | 1.28 | 6.11 |
| GO@AuNRs-TPPS4 | 0.31 | 4.86 (1.89%) | 8.63 (98.11%) | 1.30 | 6.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebepe, T.C.; Maluleke, R.; Mgedle, N.; Oluwafemi, O.S. Porphyrin as a Cryoprotectant for Graphene Oxide-Coated Gold Nanorods to Produce Conjugated Product with Improved Stability and Opto-Phototherapeutic Properties. Pharmaceutics 2023, 15, 2538. https://doi.org/10.3390/pharmaceutics15112538
Lebepe TC, Maluleke R, Mgedle N, Oluwafemi OS. Porphyrin as a Cryoprotectant for Graphene Oxide-Coated Gold Nanorods to Produce Conjugated Product with Improved Stability and Opto-Phototherapeutic Properties. Pharmaceutics. 2023; 15(11):2538. https://doi.org/10.3390/pharmaceutics15112538
Chicago/Turabian StyleLebepe, Thabang Calvin, Rodney Maluleke, Nande Mgedle, and Oluwatobi Samuel Oluwafemi. 2023. "Porphyrin as a Cryoprotectant for Graphene Oxide-Coated Gold Nanorods to Produce Conjugated Product with Improved Stability and Opto-Phototherapeutic Properties" Pharmaceutics 15, no. 11: 2538. https://doi.org/10.3390/pharmaceutics15112538





