Utility of a Novel Micro-Spraying Device for Intranasal Administration of Drug Solutions to Mice
Abstract
:1. Introduction
1.1. Background
1.2. Rationale
1.3. Objectives
2. Materials and Methods
2.1. Reagents and Materials
2.2. Animals
2.3. Intranasal Distribution of Trypan Blue Solution
2.4. Measurement of the Amount of [14C]-Inulin to the Brain and Olfactory Bulb after Intranasal Administration
2.5. Statistical Analysis
3. Results
3.1. Adhesion of 0.4% Trypan Blue Solution to the Nasal Cavity of Mice
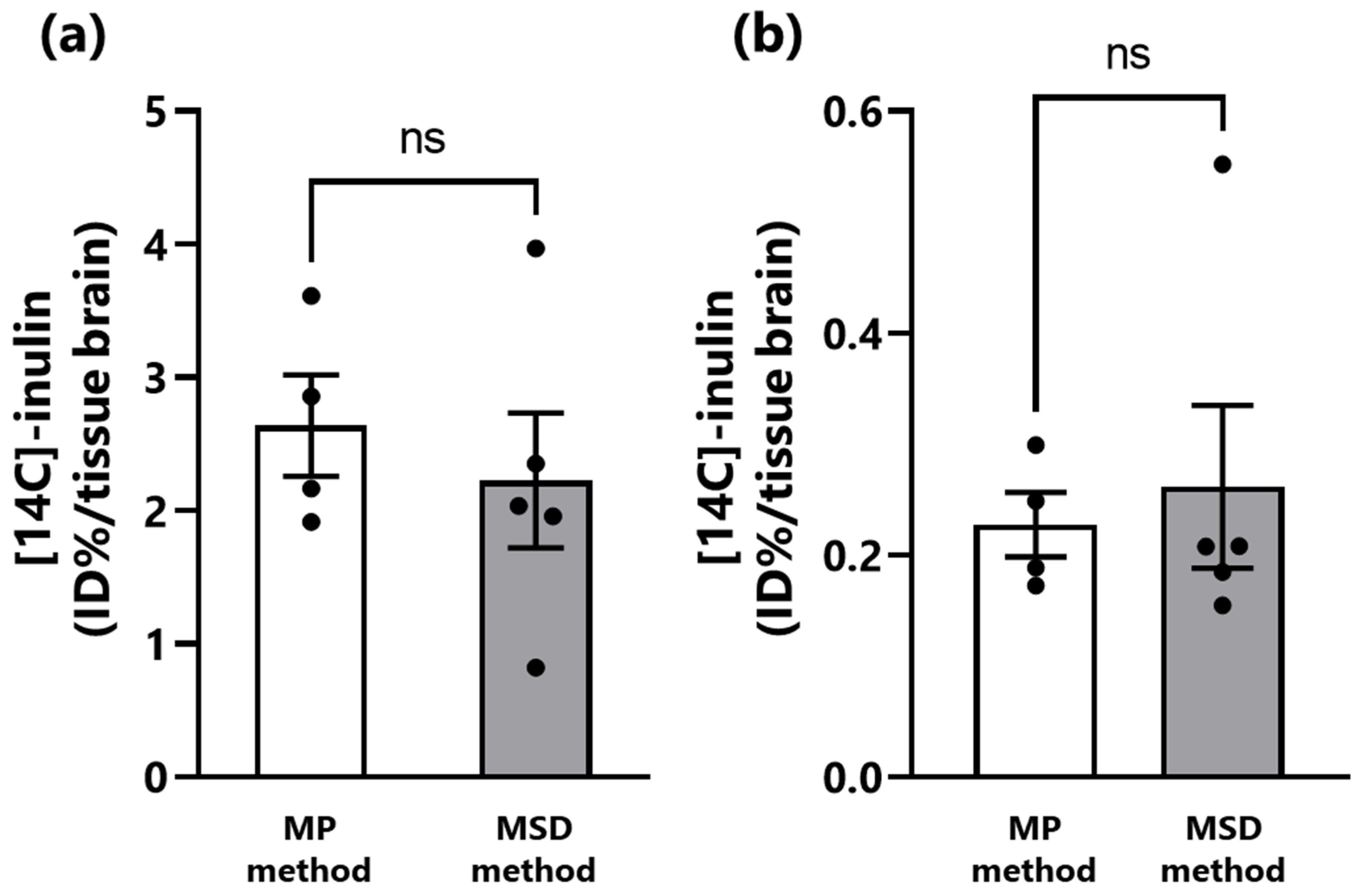
3.2. Transfer of Inulin from the Nasal Cavity to the Brain of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | micropipette |
| MSD | micro-spraying device |
| N2B | nose-to-brain |
References
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Joukhadar, C.; Schenk, B.; Kaehler, S.T.; Kollenz, C.J.; Bauer, P.; Muller, M.; Eichler, H.G. A replicate study design for testing bioequivalence: A case study on two desmopressin nasal spray preparations. Eur. J. Clin. Pharmacol. 2003, 59, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef]
- Chapman, C.D.; Frey, W.H., 2nd; Craft, S.; Danielyan, L.; Hallschmid, M.; Schioth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Kanazawa, T.; Akiyama, F.; Kakizaki, S.; Takashima, Y.; Seta, Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials 2013, 34, 9220–9226. [Google Scholar] [CrossRef]
- Suico, J.G.; Hövelmann, U.; Zhang, S.; Shen, T.; Bergman, B.; Sherr, J.; Zijlstra, E.; Frier, B.M.; Plum-Mörschel, L. Glucagon administration by nasal and intramuscular routes in adults with type 1 diabetes during insulin-induced hypoglycaemia: A randomised, open-label, crossover study. Diabetes Ther. 2020, 11, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Minko, T. Nanotherapeutics for nose-to-brain drug delivery: An approach to bypass the blood brain barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Kanazawa, T.; Fukuda, M.; Suzuki, N.; Suzuki, T. Novel methods for intranasal administration under inhalation anesthesia to evaluate nose-to-brain drug delivery. J. Vis. Exp. 2018, 14, 141. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., 2nd. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, M.J.; de Lange, E.C. Emerging insights for translational pharmacokinetic and pharmacokinetic-pharmacodynamic studies: Towards prediction of nose-to-brain transport in humans. AAPS J. 2015, 17, 493–505. [Google Scholar] [CrossRef]
- Suzuki, N.; Kanazawa, T.; Suzuki, T. Intranasal administration. Drug Deliv. Syst. 2020, 35, 76–80. [Google Scholar] [CrossRef]
- Fukuda, M.; Kanazawa, T.; Iioka, S.; Oguma, T.; Iwasa, R.; Masuoka, S.; Suzuki, N.; Kosuge, Y.; Suzuki, T. Quantitative analysis of inulin distribution in the brain focused on nose-to-brain route via olfactory epithelium by reverse esophageal cannulation. J. Control. Release 2021, 332, 493–501. [Google Scholar] [CrossRef]
- Kurano, T.; Kanazawa, T.; Ooba, A.; Masuyama, Y.; Maruhana, N.; Yamada, M.; Iioka, S.; Ibaraki, H.; Kosuge, Y.; Kondo, H.; et al. Nose-to-brain/spinal cord delivery kinetics of liposomes with different surface properties. J. Control. Release 2022, 344, 225–234. [Google Scholar] [CrossRef]
- Kurano, T.; Kanazawa, T.; Iioka, S.; Kondo, H.; Kosuge, Y.; Suzuki, T. Intranasal administration of N-acetyl-L-cysteine combined with cell-penetrating peptide-modified polymer nanomicelles as a potential therapeutic approach for amyotrophic lateral sclerosis. Pharmaceutics 2022, 14, 2590. [Google Scholar] [CrossRef]
- Ullah, I.; Chung, K.; Beloor, J.; Lee, S.K.; Kumar, P. A Positioning Device for the placement of mice during intranasal siRNA delivery to the central nervous system. J. Vis. Exp. 2019, 150, e59201. [Google Scholar] [CrossRef]
- Hanson, L.R.; Fine, J.M.; Svitak, A.L.; Faltesek, K.A. Intranasal administration of CNS therapeutics to awake mice. J. Vis. Exp. 2013, 74, 4440. [Google Scholar] [CrossRef]
- Toray Precision, Inc. Homepage. Available online: https://www.tpc.toray/product/nozzle/noz_011.html#link_plan (accessed on 12 July 2023).
- Kumar, N.N.; Gautam, M.; Lochhead, J.J.; Wolak, D.J.; Ithapu, V.; Singh, V.; Thorne, R.G. Relative vascular permeability and vascularity across different regions of the rat nasal mucosa: Implications for nasal physiology and drug delivery. Sci. Rep. 2016, 6, 31732. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997. Available online: https://imagej.nih.gov/ij/ (accessed on 1 April 2023).
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug. Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Lalatsa, A.; Schatzlein, A.G.; Uchegbu, I.F. Strategies to deliver peptide drugs to the brain. Mol. Pharm. 2014, 11, 1081–1093. [Google Scholar] [CrossRef]
- Shinde, S.C.; Mahale, N.B.; Chaudhari, S.R.; Thorat, R.S. Recent advances in brain targeted drug delivery system: A review. World J. Pharm. Res. 2015, 4, 542–549. [Google Scholar]
- Singh, A.K.; Mishra, S.K.; Mishra, G.; Maurya, A.; Awasthi, R.; Yadav, M.K.; Atri, N.; Pandey, P.K.; Singh, S.K. Inorganic clay nanocomposite system for improved cholinesterase inhibition and brain pharmacokinetics of donepezil. Drug Dev. Ind. Pharm. 2020, 46, 8–19. [Google Scholar] [CrossRef]
- Kamei, N.; Takeda-Morishita, M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J. Control. Release 2015, 197, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Shingaki, T.; Kanayama, Y.; Tanaka, M.; Zochi, R.; Hasegawa, K.; Watanabe, Y.; Takeda-Morishita, M. Visualization and quantitative assessment of the brain distribution of insulin through nose-to-brain delivery based on the cell-penetrating peptide noncovalent strategy. Mol. Pharm. 2016, 13, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, H.; Suzuki, N.; Suzuki, T. Application of ionic liquid to enhance the nose-to-brain delivery of etodolac. Eur. J. Pharm. Sci. 2022, 178, 106290. [Google Scholar] [CrossRef]
- Thorne, R.G.; Hanson, L.R.; Ross, T.M.; Tung, D.; Frey, W.H., II. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 2008, 152, 785–797. [Google Scholar] [CrossRef]
- Lu, C.T.; Zhao, Y.Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef]
- Krishnan, J.K.S.; Arun, P.; Chembukave, B.; Appu, A.P.; Vijayakumar, N.; Moffett, J.R.; Puthillathu, N.; Namboodiri, A.M.A. Effect of administration method, animal weight and age on the intranasal delivery of drugs to the brain. J. Neurosci. Methods 2017, 286, 16–21. [Google Scholar] [CrossRef]
- Flamm, J.; Hartung, S.; Gänger, S.; Maigler, F.; Pitzer, C.; Schindowski, K. Establishment of an olfactory region-specific intranasal delivery technique in mice to target the central nervous system. Front. Pharmacol. 2022, 12, 789780. [Google Scholar] [CrossRef]
- Le Guellec, S.; Ehrmann, S.; Vecellio, L. In-vitro-in vivo correlation of intranasal drug deposition. Adv. Drug Deliv. Rev. 2021, 170, 340–352. [Google Scholar] [CrossRef]
- Baldelli, A.; Boraey, M.A.; Oguzlu, H.; Cidem, A.; Rodriguez, A.P.; Ong, H.X.; Jiang, F.; Bacca, M.; Thamboo, A.; Traini, D.; et al. Engineered nasal dry powder for the encapsulation of bioactive compounds. Drug Discov. Today 2022, 27, 2300–2308. [Google Scholar] [CrossRef]
- Iwasaki, S.; Yamamoto, S.; Sano, N.; Tohyama, K.; Kosugi, Y.; Furuta, A.; Hamada, T.; Igari, T.; Fujioka, Y.; Hirabayashi, H.; et al. Direct drug delivery of low-permeable compounds to the central nervous system via intranasal administration in rats and monkeys. Pharm. Res. 2019, 36, 76. [Google Scholar] [CrossRef]
- McMartin, C.; Hutchinson, L.E.; Hyde, R.; Peters, G.E. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J. Pharm. Sci. 1987, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Parvathi, M. Intranasal drug delivery to brain: An overview. Int. J. Res. Pharm. Chem. 2012, 2, 889–895. [Google Scholar]
- Kushwaha, S.K.; Keshari, R.K.; Rai, A.K. Advances in nasal trans-mucosal drug delivery. J. Appl. Pharmaceut. Sci. 2011, 1, 21–28. [Google Scholar]
- Mathison, S.; Nagilla, R.; Kompella, U.B. Nasal route for direct delivery of solutes to the central nervous systems: Fact or fiction? J. Drug Target. 1998, 5, 415–441. [Google Scholar] [CrossRef] [PubMed]
- Laddha, U.D.; Tagalpallewar, A.A. Physicochemical, biopharmaceutical, and practical considerations for efficient nose-to-brain drug delivery. In Direct Nose-To-Brain Drug Delivery. Mechanism, Technological Advances, Applications, and Regulatory Updates; Pardeshi, C.V., Souto, E.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 39–54. [Google Scholar]
- Merkus, P.; Ebbens, F.A.; Muller, B.; Fokkens, W.J. Influence of anatomy and head position on intranasal drug deposition. Eur. Arch. Otorhinolaryngol. 2006, 263, 827–832. [Google Scholar] [CrossRef]
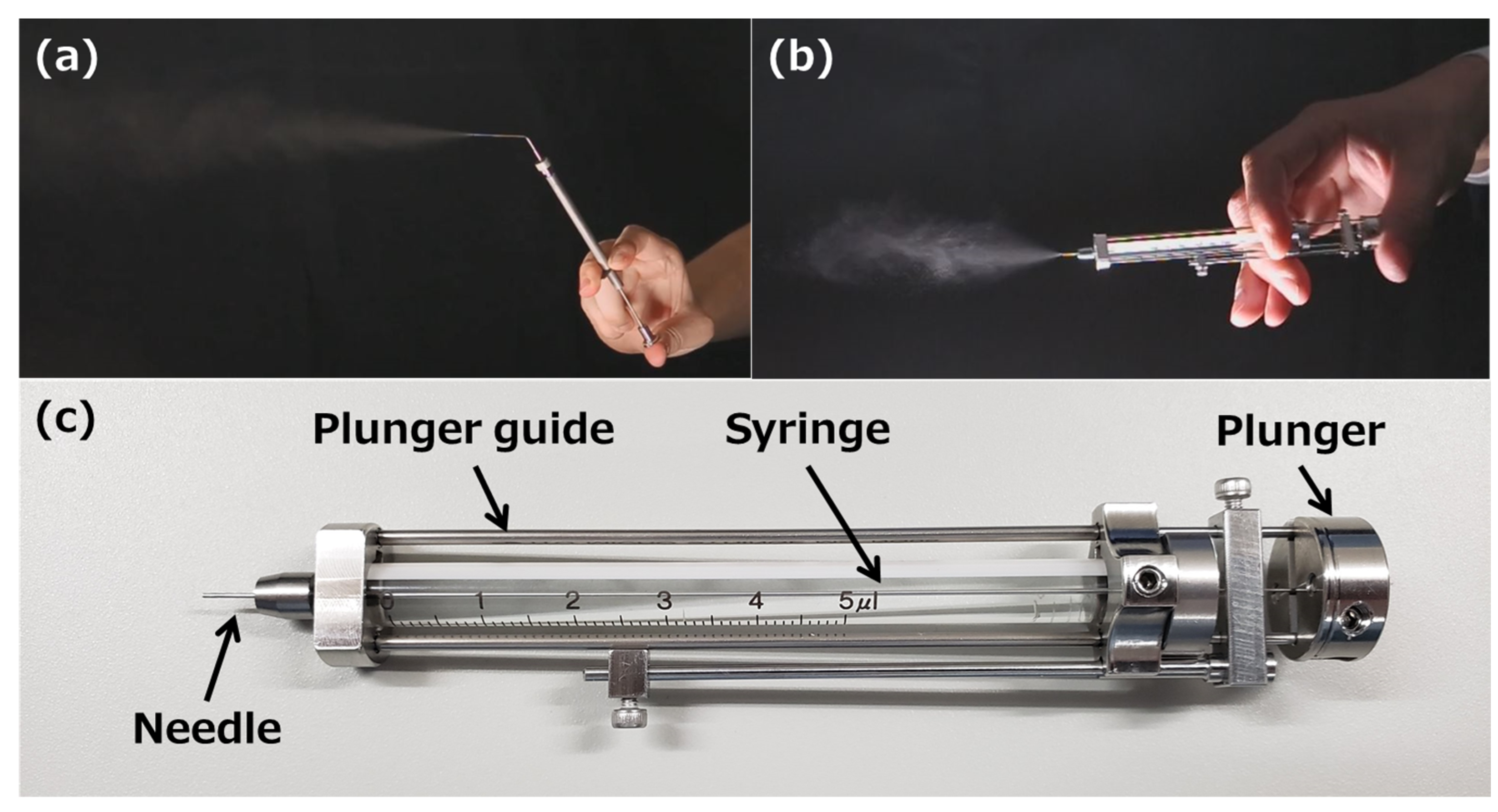


| Spray Characteristics | |
|---|---|
| Minimum spray volume | 1 μL |
| Maximum spray volume | 5 μL |
| Average spray angle | 43.8° |
| Average atomizing particle size | 12.97 μm |
| Needle size and device weight | |
| Sprayable viscosity at 20 °C | 1.000 mPa·s |
| Needle outer diameter | 0.52 mm |
| Needle length | 7.0 mm |
| Device weight | 45.5 g |
| Time for Administration (s) | n | Mean | S.E.M. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| MSD method | 57 | 55 | 51 | 57 | 63 | 57 | … | … | 56.67 | 3.54 |
| MP method | 197 | 191 | 194 | 191 | 190 | 192 | 193 | 194 | 192.75 | 2.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, N.; Tanigawa, H.; Nagatomo, T.; Miyagishi, H.; Kanazawa, T.; Suzuki, T.; Kosuge, Y. Utility of a Novel Micro-Spraying Device for Intranasal Administration of Drug Solutions to Mice. Pharmaceutics 2023, 15, 2553. https://doi.org/10.3390/pharmaceutics15112553
Suzuki N, Tanigawa H, Nagatomo T, Miyagishi H, Kanazawa T, Suzuki T, Kosuge Y. Utility of a Novel Micro-Spraying Device for Intranasal Administration of Drug Solutions to Mice. Pharmaceutics. 2023; 15(11):2553. https://doi.org/10.3390/pharmaceutics15112553
Chicago/Turabian StyleSuzuki, Naoto, Hiroaki Tanigawa, Taiki Nagatomo, Hiroko Miyagishi, Takanori Kanazawa, Toyofumi Suzuki, and Yasuhiro Kosuge. 2023. "Utility of a Novel Micro-Spraying Device for Intranasal Administration of Drug Solutions to Mice" Pharmaceutics 15, no. 11: 2553. https://doi.org/10.3390/pharmaceutics15112553
APA StyleSuzuki, N., Tanigawa, H., Nagatomo, T., Miyagishi, H., Kanazawa, T., Suzuki, T., & Kosuge, Y. (2023). Utility of a Novel Micro-Spraying Device for Intranasal Administration of Drug Solutions to Mice. Pharmaceutics, 15(11), 2553. https://doi.org/10.3390/pharmaceutics15112553







