Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis
Abstract
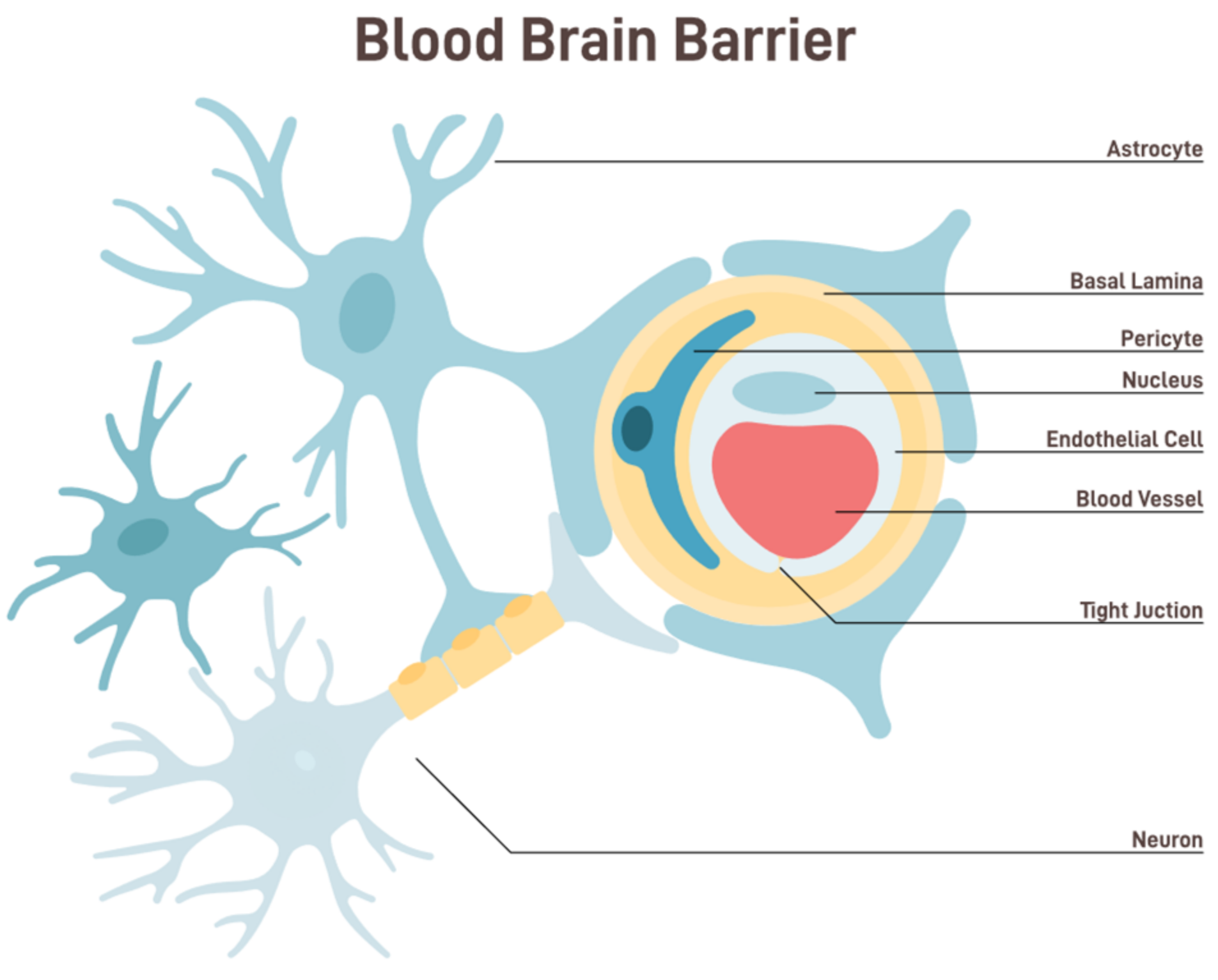
:1. Introduction
Selective Mechanism
2. Optimizing Entry
3. Device-Mediated Noninvasive Techniques
4. Brain Tumors
5. Neurodegenerative Disorders (NDs)
6. Current Techniques in Drug Delivery across the BBB
6.1. Direct Injections
6.2. Molecular Trojan Horses
6.3. Biochemical BBB Disruption
6.4. Nanoparticle-Mediated Delivery
6.5. Focused Ultrasound (FUS) with Microbubbles
6.6. Magnetic Resonance Imaging (MRI)
6.7. Electromagnetic Field Modulation
6.8. Vasoactive Chemicals
6.9. Gut Microbiome [134]
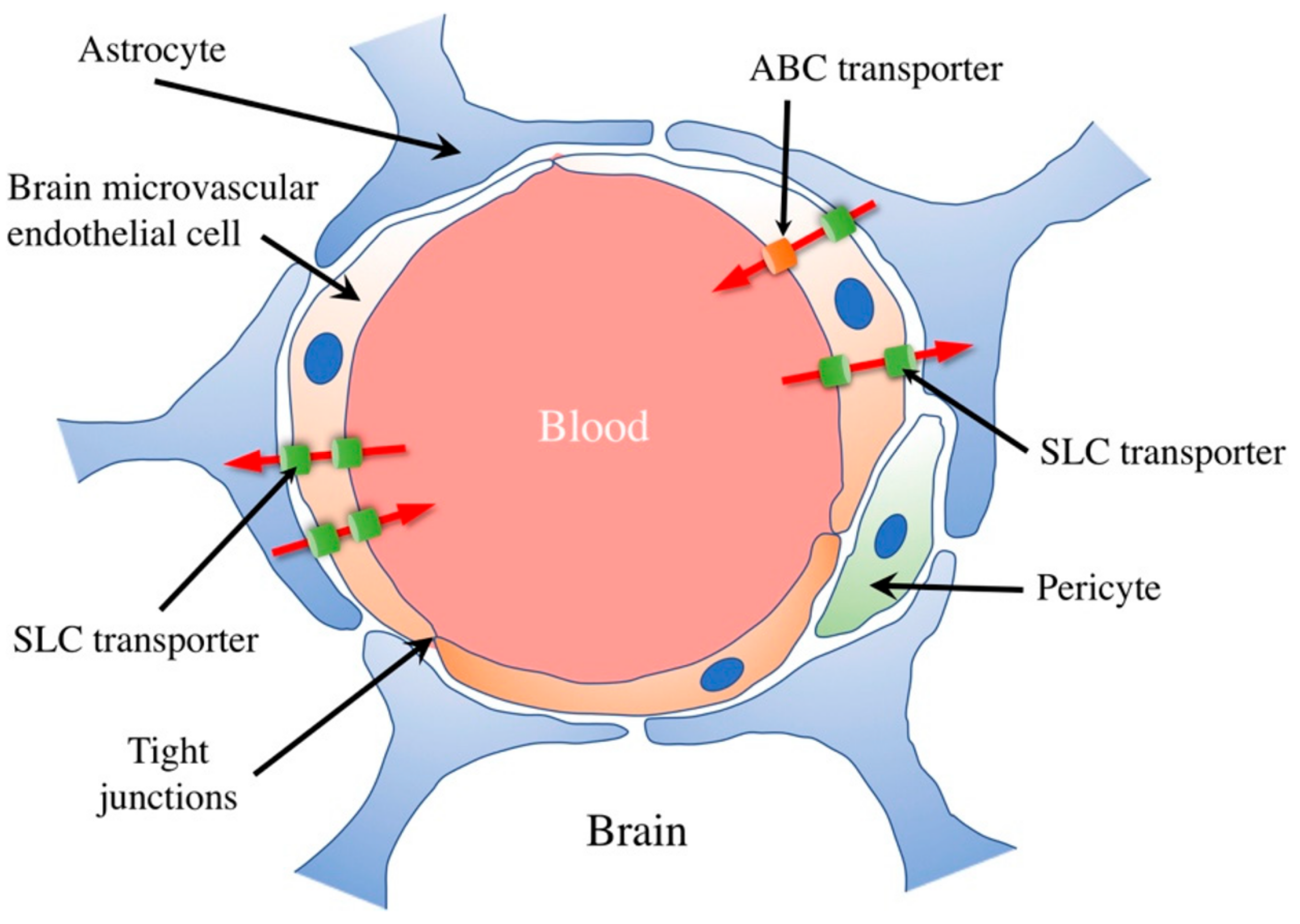
6.10. Surface Transporters
6.11. Penetrating Peptides
6.12. Extracellular Vesicles
6.13. Liposomes
6.14. Optical Imaging
6.15. Peptides
6.16. Antibodies
6.17. Intranasal
6.18. Circadian Rhythm [165]
6.19. Precision Medicine
6.20. Light-Induced Techniques
6.21. Radiofrequency (RF) Modulation
6.22. Thermal Techniques
7. Ex Vivo Modeling
7.1. Animal Modes
7.2. Organoid Model
7.3. The BBB Chip
8. Summary
8.1. Advantages
- Precision and Specificity: Techniques like FUS offer pinpoint accuracy in targeting specific brain regions [174]. This ensures that only the desired area is treated, reducing the risk of systemic side effects.
- Versatility: The non-invasive nature of these techniques makes them suitable for a wide range of applications, from delivering small-molecule drugs to larger molecules like antibodies or even genes.
- Minimally Disruptive: Unlike invasive methods, which can cause tissue damage or infection, non-invasive techniques are generally safer with minimal post-procedure complications.
- Repeatability: Given their non-destructive nature, these techniques can be applied repeatedly, allowing for chronic treatments or adjustments [175].
8.2. Challenges
- Understanding Long-term Effects: While initial studies are promising, the long-term effects of repeated BBB disruption or electromagnetic field exposure remain to be comprehensively understood [176].
- Optimization of Parameters: Each technique requires fine-tuning parameters to ensure efficacy without compromising safety. For instance, ultrasound’s right frequency and duration of the optimal wavelength for light-induced techniques are vital for success [177].
- Systemic Side Effects: Despite targeted delivery, there is a potential for drugs to diffuse from the target site, leading to unintended effects elsewhere in the brain or body.
- Technological Limitations: Current devices may not be optimized for deep brain structures or use in specific populations like children or older adults [178].
9. Future Perspectives
- Combination Therapies: Combining non-invasive techniques could further improve delivery efficacy. For instance, using FUS to enhance nanoparticle delivery across the BBB could combine the strengths of both methods [179].
- Advanced Monitoring: Integrating real-time imaging, like MRI, with drug delivery can provide immediate feedback, ensuring optimal delivery and minimizing potential risks [180].
- Personalized Approaches: As our understanding grows, it may be possible to tailor techniques to individual patients based on their unique anatomy, pathology, and therapeutic needs [181].
- Expansion to Other Diseases: While the current focus might be on neurological disorders, the potential exists to expand these techniques for other conditions, from brain tumors to systemic illnesses with CNS involvement [182].
Applications
10. Conclusions
Funding
Conflicts of Interest
References
- Gazzaniga, M.S.; Ivry, R.B.; Mangun, G.R. Cognitive Neuroscience: The Biology of the Mind; Norton & Company: New York, NY, USA, 2018. [Google Scholar]
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. Neuroscience: Exploring the Brain; Wolters Kluwer Health: Philadelphia, PA, USA, 2016. [Google Scholar]
- Abbott, N.J. Dynamics of CNS barriers: Evolution, differentiation, and modulation. Cell. Mol. Neurobiol. 2005, 25, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Saunders, N.R.; Habgood, M.D.; Dziegielewska, K.M.; Potter, A. Barrier mechanisms in the brain, I. Adult brain. Clin. Exp. Pharmacol. Physiol. 2016, 23, 137–146. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Mayhan, W.G. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Terasaki, T. Contribution of carrier-mediated transport systems to the BBB as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm. Nov. Drug Deliv. Syst. 2007, 10, 13–23. [Google Scholar]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Inflammatory mediators and modulation of BBB permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Pardridge, W.M. Targeted delivery of protein and gene medicines through the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.; Reiss, Y.; Plate, K.; Agalliu, D.; Constantin, G. Functional morphology of the BBB in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.; Betsholtz, C.; Zlokovic, B. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Yang, A.; Stevens, M.; Chen, M.; Lee, D.; Stähli, D.; Gate, D.; Contrepois, K.; Chen, W.; Iram, T.; Zhang, L.; et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 2020, 583, 425–430. [Google Scholar] [CrossRef]
- Profaci, C.; Munji, R.; Pulido, R.; Daneman, R. The BBB in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Banks, W. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Molecular biology of the blood-brain barrier. Mol. Biotechnol. 2006, 32, 103–120. [Google Scholar]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]
- Menei, P.; Daniel, V.; Montero-Menei, C.; Brouillard, M.; Pouplard-Barthelaix, A.; Benoit, J.P. Biodegradable microspheres for the intracerebral administration of methotrexate. J. Neurosurg. 1993, 78, 915–921. [Google Scholar]
- Sweeney, M.D.; Sagare, A.P. Vascular dysfunction in cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 98. [Google Scholar]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Neuwelt, E.; Abbott, N.J.; Abrey, L.; Banks, W.A.; Blakley, B.; Davis, T.; Engelhardt, B. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008, 7, 84–96. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M. Principles, clinical applications, and pitfalls of brain stimulation. Ann. Neurol. 2019, 85, 14–33. [Google Scholar]
- Reato, D.; Rahman, A. Effects of weak transcranial alternating current stimulation on brain activity—A review of known mechanisms from animal studies. Front. Hum. Neurosci. 2010, 7, 687. [Google Scholar] [CrossRef]
- Kobus, T.; Zervantonakis, I.K.; Zhang, Y.; McDannold, N.J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated BBB disruption. J. Control. Release 2016, 238, 281–288. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Bringas, J.R.; McKnight, T.R.; Wendland, M.F.; Mamot, C.; Drummond, D.C.; Berger, M.S. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004, 64, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Reuveni, T.; Motiei, M.; Popovtzer, R. Nanoparticles as computed tomography contrast agents: Current status and future perspectives. Nanomedicine 2015, 10, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.A.; Woodworth, G.F.; Sailor, K.A.; Shih, T.Y.; Xu, Q.; Swaminathan, G.; Hanes, J. A dense poly (ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012, 4, 149ra119. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., II. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Ghanouni, P.; Dobakhti, F.; Kennedy, A.M. Blood-brain barrier: From anatomy to astrocyte interactions. In Nanotechnology for Biomedical Imaging and Diagnostics: From Nanoparticle Design to Clinical Applications; Wiley: Hoboken, NJ, USA, 2015; pp. 375–402. [Google Scholar]
- Banks, W.A.; Sharma, P.; Bullock, K.M. Transport of protein-bound and free peptides, cyclic peptides, and modified neuropeptides across the blood-brain barrier. J. Alzheimer’s Dis. 2019, 68, 1617–1629. [Google Scholar]
- Rao, J.; Li, M.; Wang, Y.; Liu, B.; Zhao, C. Exosomes in CNS drug delivery. Adv. Healthc. Mater. 2020, 9, 1901868. [Google Scholar]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Non-invasive opening of BBB by focused ultrasound. Acta Neurochir. Suppl. 2016, 121, 243–247. [Google Scholar]
- LeWitt, P.A.; Lipsman, N.; Kordower, J.H.; Aebischer, P.; Chen, L.; Svendsen, C.N. Focused ultrasound opening of the blood-brain barrier for treatment of Parkinson’s disease. Mov. Disord. 2021, 36, 76–82. [Google Scholar] [CrossRef]
- Burger, M.C.; Glavis-Bloom, C.; Wang, A.; Gonzalez-Cuyar, L.F.; Oh, J.H. Implantable drug delivery devices for the treatment of neurologic disorders. Front. Neurosci. 2017, 11, 535. [Google Scholar]
- Bhardwaj, R.; Blanchard, A.D.; Jaffe, G.J. Drug delivery strategies for retinal diseases. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Biomaterials in drug delivery and tissue engineering: One laboratory’s experience. Acc. Chem. Res. 2000, 33, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Timbie, K.F.; Mead, B.P.; Price, R.J. Drug and gene delivery across the blood–brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging–guided focal opening of the BBB in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Nance, E.; Timbie, K.; Miller, G.W.; Song, J.; Louttit, C.; Klibanov, A.L.; Woodworth, G.F. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the BBB using MRI-guided focused ultrasound. J. Control. Release 2014, 189, 123–132. [Google Scholar] [CrossRef]
- Abbott, N.J.; Friedman, A. Overview and introduction: The BBB in health and disease. Epilepsia 2012, 53, 1–6. [Google Scholar] [CrossRef]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release 2015, 204, 60–68. [Google Scholar] [CrossRef]
- Zhou, B.; Xiong, Z.; Wang, P. Improved delivery for central nervous system diseases: Challenges and future prospects. Signal Transduct. Target. Ther. 2019, 4, 1–17. [Google Scholar]
- Mégevand, P.; Groppe, D.M.; Goldfinger, M.S. Novel applications of imaging in the diagnosis and management of neurological disease. Neurotherapeutics 2019, 16, 26–37. [Google Scholar]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.Z.; McDonnell, S.; Airan, R.D. Effects on the brain of a novel 40 Hz flicker from phosphene generation in patients with Alzheimer’s disease. PLoS ONE 2019, 14, e0218741. [Google Scholar]
- Zeng, Y.; Kurokawa, T. Wearable ultrasound devices for diagnostics and therapy. Bio-Des. Manuf. 2018, 1, 77–89. [Google Scholar]
- Alvarez-Erviti, L.; Couch, Y. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Ai, L.; Bansal, P.; Mueller, J.K. Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum. Brain Mapp. 2018, 39, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Park, J.; Vykhodtseva, N.; Zhang, Y.Z. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: Evaluation during tumor progression in a rat glioma model. Phys. Med. Biol. 2017, 62, 2428–2441. [Google Scholar] [CrossRef]
- Felistia, Y.; Wen, P.Y. Molecular Profiling and Targeted Therapies in Gliomas. Curr. Neurol. Neurosci. Rep. 2023, 23, 627–636. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2012, 131, EPE99–EPE107. [Google Scholar] [CrossRef]
- Bystritsky, A.; Korb, A.S.; Douglas, P.K. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011, 4, 125–136. [Google Scholar] [CrossRef]
- Burgess, A.; Hynynen, K. Drug delivery across the blood–brain barrier using focused ultrasound. Expert Opin. Drug Deliv. 2014, 11, 711–721. [Google Scholar] [CrossRef]
- Gooch, C.L.; Pracht, E.; Borenstein, A.R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81, 479–484. [Google Scholar] [CrossRef]
- Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 10 July 2023).
- Available online: https://www.parkinson.org/understanding-parkinsons/statistics (accessed on 10 July 2023).
- Li, H.; Liu, C.C.; Zheng, H.; Huang, T.Y. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer’s disease-conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl. Neurodegener. 2018, 7, 34. [Google Scholar] [CrossRef]
- Leinenga, G.; Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef]
- Jordão, J.F.; Ayala-Grosso, C.A.; Markham, K.; Huang, Y.; Chopra, R.; McLaurin, J.; Hynynen, K.; Aubert, I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS ONE 2010, 5, e10549. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.; Krinsky-McHale, S.J.; Zigman, W.B.; Schupf, N.; New York Aging Research Program. Adults with Down syndrome in randomized clinical trials targeting prevention of Alzheimer’s disease. Alzheimers Dement. 2022, 18, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Baseri, B.; Choi, J.J.; Deffieux, T.; Samiotaki, G.; Tung, Y.S.; Olumolade, O.; Konofagou, E.E. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the BBB using focused ultrasound and microbubbles. Phys. Med. Biol. 2012, 57, N65. [Google Scholar] [CrossRef]
- Fan, C.H.; Ting, C.Y.; Lin, C.Y.; Chan, H.L.; Chang, Y.C.; Chen, Y.Y.; Liu, H.L. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci. Rep. 2016, 6, 19579. [Google Scholar] [CrossRef]
- Martin, E.; Jeanmonod, D.; Morel, A.; Zadicario, E.; Werner, B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol. 2009, 66, 858–861. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Gonzalez, P.; Andres-Benito, P.; Ferrer, I.; Rodríguez, F.J. Wnt signaling alterations in the human spinal cord of amyotrophic lateral sclerosis cases: Spotlight on Fz2 and Wnt5a. Mol. Neurobiol. 2019, 56, 6777–6791. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the BBB and improves pathologic abnormalities and behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef]
- Islam, Y.; Leach, A.G.; Smith, J.; Pluchino, S.; Coxon, C.R.; Sivakumaran, M.; Downing, J.; Fatokun, A.A.; Teixidò, M.; Ehtezazi, T. Physiological and pathological factors affecting drug delivery to the brain by nanoparticles. Adv. Sci. 2021, 8, 2002085. [Google Scholar] [CrossRef]
- Cho, E.E.; Drazic, J.; Ganguly, M.; Stefanovic, B.; Hynynen, K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced BBB opening. J. Cereb. Blood Flow Metab. 2018, 38, 1260–1274. [Google Scholar]
- Pawar, B.; Vasdev, N.; Gupta, T.; Mhatre, M.; More, A.; Anup, N.; Tekade, R.K. Current Update on Transcellular Brain Drug Delivery. Pharmaceutics 2022, 14, 2719. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.P.; Hegde, M.; Shetty, S.S.; Rafic, T.; Mutalik, S.; Rao, B.S. Targeting receptor-ligand chemistry for drug delivery across blood-brain barrier in brain diseases. Life Sci. 2021, 274, 119326. [Google Scholar] [CrossRef]
- Betterton, R.D.; Davis, T.P.; Ronaldson, P.T. Organic Cation Transporter (OCT/OCTN) Expression at Brain Barrier Sites: Focus on CNS Drug Delivery. Handb. Exp. Pharmacol. 2021, 266, 301–328. [Google Scholar] [PubMed]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef]
- Lonser, R.R.; Walbridge, S.; Garmestani, K.; Butman, J.A.; Walters, H.A.; Vortmeyer, A.O.; Oldfield, E.H. Successful and safe perfusion of the primate brainstem: In vivo magnetic resonance imaging of macromolecular distribution during infusion. J. Neurosurg. 2002, 97, 905–913. [Google Scholar] [CrossRef]
- Saltzman, W.M. Drug Delivery: Engineering Principles for Drug Therapy; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Morrison, P.F.; Laske, D.W. Direct delivery of medications to the central nervous system. Clin. Pharmacokinet. 1994, 26, 85–100. [Google Scholar]
- Rubin, L.L.; Staddon, J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Moos, T.; Burkhart, A. Receptor-mediated drug delivery to the brain in the treatment of central nervous system diseases. J. Mol. Med. 2014, 92, 497–506. [Google Scholar]
- Chen, W.; Hu, Y.; Ju, D. Gene therapy for neurodegenerative disorders: Advances, insights and prospects. Acta Pharm. Sin. B 2020, 10, 1347–1359. [Google Scholar] [CrossRef]
- Rapoport, S.I. Osmotic opening of the blood-brain barrier: Principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.; Praminick, B.; Demopoulos, A.; McCrea, H.; Bodhinayake, I.; et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neuro-Oncol. 2016, 128, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, F.; Wang, K.; Zhong, Y.; Wei, X.; Wang, Q.; Zhang, H. Engineered Exosomes: A Promising Drug Delivery Strategy for Brain Diseases. Curr. Med. Chem. 2022, 29, 3111–3124. [Google Scholar] [CrossRef]
- Yue, Q.; Peng, Y.; Zhao, Y.; Lu, R.; Fu, Q.; Chen, Y.; Yang, Y.; Hai, L.; Guo, L.; Wu, Y. Dual-targeting for brain-specific drug delivery: Synthesis and biological evaluation. Drug Deliv. 2018, 25, 426–434. [Google Scholar] [CrossRef]
- Jiang, T.; Qiao, Y.; Ruan, W.; Zhang, D.; Yang, Q.; Wang, G.; Chen, Q.; Zhu, F.; Yin, J.; Zou, Y. Cation-Free siRNA Micelles as Effective Drug Delivery Platform and Potent RNAi Nanomedicines for Glioblastoma Therapy. Adv. Mater. 2021, 33, 2104779. [Google Scholar] [CrossRef]
- Ban, J.; Li, S.; Zhan, Q.; Li, X.; Xing, H.; Chen, N.; Long, L.; Hou, X.; Zhao, J.; Yuan, X. PMPC modified PAMAM dendrimer enhances brain tumor-targeted drug delivery. Macromol. Biosci. 2021, 21, 2000392. [Google Scholar] [CrossRef]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric nanoparticles in brain cancer therapy: A review of current approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Wu, J.; Hernandez, Y.; Miyasaki, K.; Kwon, E. Engineered nanomaterials that exploit BBB dysfunction for delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114820. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qian, J.; Zheng, S.; Changyi, Y.; Zhang, J.; Ju, S.; Zhu, J.; Li, C. Overcoming the Blood–Brain Barrier for Delivering Drugs into the Brain by Using Adenosine Receptor Nanoagonist. ACS Nano 2018, 12, 9968–9978. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Wilson Miller, G.; Suk, J.S.; Hanes, J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef]
- Timbie, K.F.; Mead, B.P. Drug delivery across the blood-brain barrier: Recent advances in the use of nanocarriers. Nanomedicine 2017, 12, 159–167. [Google Scholar]
- Mead, B.P.; Price, R.J. Targeted drug delivery to the brain using focused ultrasound: A review. J. Drug Target. 2016, 24, 871–882. [Google Scholar]
- Gorick, C.; Breza, V.; Nowak, K.; Cheng, V.; Fisher, D.; Debski, A.; Hoch, M.; Demir, Z.; Tran, N.; Schwartz, M.; et al. Applications of focused ultrasound-mediated BBB opening. Adv. Drug Deliv. Rev. 2022, 191, 114583. [Google Scholar] [CrossRef]
- Burgess, A.; Ayala-Grosso, C.; Ganguly, M.; Jordaõ, J.; Aubert, I.; Hynynen, K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE 2011, 6, e27877. [Google Scholar] [CrossRef]
- Diaz, R.; McVeigh, P.; O’Reilly, M.; Burrell, K.; Bebenek, M.; Smith, C.; Etame, A.; Zadeh, G.; Hynynen, K.; Wilson, B.; et al. Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: Potential for targeting experimental brain tumors. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1075–1087. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced BBB disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef]
- Nisbet, R.; Van der Jeugd, A.; Leinenga, G.; Evans, H.; Janowicz, P.; Götz, J. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain J. Neurol. 2017, 140, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.; Treat, L.; Dewey, J.; McDannold, N.; Hynynen, K.; Bacskai, B. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS ONE 2008, 3, e2175. [Google Scholar] [CrossRef] [PubMed]
- Th’evenot, E.; Jordão, J.; O’Reilly, M.; Markham, K.; Weng, Y.; Foust, K.; Kaspar, B.; Hynynen, K.; Aubert, I. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 2012, 23, 1144–1155. [Google Scholar] [CrossRef]
- Pineda-Pardo, J.; Gasca-Salas, C.; Fernández-Rodríguez, B.; Rodríguez-Rojas, R.; Del Alamo, M.; Obeso, I.; Hernández-Fernández, F.; Trompeta, C.; Martínez-Fernández, R.; Matarazzo, M.; et al. Striatal BBB opening in Parkinson’s disease dementia: A pilot exploratory study. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated BBB disruption: A safety study. J. Control. Release 2014, 204, 60–69. [Google Scholar] [CrossRef]
- Konofagou, E.E. Optimization of the ultrasound-induced BBB opening. Theranostics 2012, 2, 1223. [Google Scholar] [CrossRef]
- McDannold, N.; Arvanitis, C.D.; Vykhodtseva, N.; Livingstone, M.S. Temporary disruption of the BBB by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012, 72, 3652–3663. [Google Scholar] [CrossRef]
- Liu, J.; Chu, C.; Zhang, J.; Bie, C.; Chen, L.; Aafreen, S.; Xu, J.; Kamson, D.; van Zijl, P.; Walczak, P.; et al. Label-free assessment of mannitol accumulation following osmotic BBB opening ssing chemical exchange saturation transfer magnetic resonance imaging. Pharmaceutics 2022, 14, 2529. [Google Scholar] [CrossRef]
- Chu, C.; Jablonska, A.; Gao, Y.; Lan, X.; Lesniak, W.; Liang, Y.; Liu, G.; Li, S.; Magnus, T.; Pearl, M.; et al. Hyperosmolar BBB opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance. Nat. Protoc. 2022, 17, 76–94. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted disruption of the BBB with focused ultrasound: Association with cavitation activity. Phys. Med. Biol. 2006, 51, 793. [Google Scholar] [CrossRef] [PubMed]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Liu, H.L.; Hua, M.Y.; Chen, P.Y.; Chu, P.C.; Pan, C.H.; Yang, H.W.; Huang, C.Y.; Wang, J.J.; Yen, T.C.; Wei, K.C. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Carpenter, J.; Mehta, R.; Haut, M.; Ranjan, M.; Najib, U.; Lockman, P.; Wang, P.; D’haese, P.; Rezai, A. Blood-brain barrier opening with MRI-guided focused ultrasound elicits meningeal venous permeability in humans with early Alzheimer disease. Radiology 2021, 298, 654–662. [Google Scholar] [CrossRef]
- Rezai, A.; Ranjan, M.; D’Haese, P.; Haut, M.; Carpenter, J.; Najib, U.; Mehta, R.; Chazen, J.; Zibly, Z.; Yates, J.; et al. Noninvasive hippocampal BBB opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Fernández-Rodríguez, B.; Pineda-Pardo, J.; Rodríguez-Rojas, R.; Obeso, I.; Hernández-Fernández, F.; Del Alamo, M.; Mata, D.; Guida, P.; Ordás-Bandera, C.; et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, K.; Sonoda, H.; Sato, Y. A phase 2/3 trial of pabinafusp alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 671–679. [Google Scholar]
- Cuccurazzu, B.; Leone, L. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp. Neurol. 2014, 261, 328–335. [Google Scholar]
- Hjouj, M.; Last, D. MRI study on reversible and irreversible electroporation induced BBB disruption. PLoS ONE 2012, 7, e42817. [Google Scholar] [CrossRef]
- Cichoń, N.; Bijak, M.; Miller, E.; Saluk, J. The influence of electromagnetic fields on the pharmacokinetics of drugs in the brain: Current state of knowledge and directions for the future. Cent. Eur. J. Immunol. 2017, 42, 407–413. [Google Scholar]
- Simpson, R.; Phillis, J. Adenosine in exercise adaptation. Br. J. Sports Med. 1992, 26, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Contreras, O.; Martinez de Lizarrondo, S.; Bardou, I.; Orset, C.; Pruvost, M.; Anfray, A.; Frigout, Y.; Hommet, Y.; Lebouvier, L.; Montaner, J.; et al. Hyperfibrinolysis increases BBB permeability by a plasmin- and bradykinin-dependent mechanism. Blood 2016, 128, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Q.; Xie, C.; Lu, W.; Peng, C.; Wei, X.; Li, X.; Su, B.; Gao, C.; Liu, M. Retro-inverso bradykinin opens the door of blood-brain tumor barrier for nanocarriers in glioma treatment. Cancer Lett. 2015, 369, 144–151. [Google Scholar] [CrossRef]
- Rodríguez-Masso’, S.; Erickson, M.; Banks, W.; Ulrich, H.; Martins, A. The bradykinin B2 receptor agonist (NG291) causes rapid onset of transient BBB disruption without evidence of early brain injury. Front. Neurosci. 2021, 15, 791709. [Google Scholar] [CrossRef]
- Domer, F.; Boertje, S.; Bing, E.; Reddix, I. Histamine- and acetylcholine-induced changes in the permeability of the BBB of normotensive and spontaneously hypertensive rats. Neuropharmacology 1983, 22, 615–619. [Google Scholar] [CrossRef]
- Schilling, L.; Wahl, M. Opening of the BBB during cortical superfusion with histamine. Brain Res. 1994, 653, 289–296. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science 2019, 366, 6465. [Google Scholar] [CrossRef]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut Microbial Molecules in Behavioural and Neurodegenerative Conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Banks, W.A. The Blood–Brain Barrier as an Endocrine Tissue. Nat. Rev. Endocrinol. 2019, 15, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Sellge, G.; Kufer, T.A. PRR-Signaling Pathways: Learning from Microbial Tactics. Semin. Immunol. 2015, 27, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M. For Lack of Gut Microbes, the Blood-Brain Barrier ‘Leaks’. Lab. Anim. 2015, 44, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, Erratum in Sci. Transl. Med. 2014, 6, 266er7. [Google Scholar]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory Immune Cells in Regulation of Intestinal Inflammatory Response to Microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef]
- Terstappen, G.; Meyer, A.; Bell, R.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Gao, X.; Xu, J.; Yao, T.; Liu, X.; Zhang, H.; Zhan, C. Peptide-decorated nanocarriers penetrating the BBB for imaging and therapy of brain diseases. Adv. Drug Deliv. Rev. 2022, 187, 114362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhai, Y.; Hao, Y.; Wang, Q.; Han, F.; Zheng, W.; Hong, J.; Cui, L.; Jin, W.; Ma, S.; et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell. Vesicles 2022, 11, e12255. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.; Mahajan, S. Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J. NeuroImmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2020, 15, 554–563. [Google Scholar] [CrossRef]
- Ma, F.; Yang, L.; Sun, Z.; Chen, J.; Rui, X.; Glass, Z.; Xu, Q. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 2020, 6, eabb4429. [Google Scholar] [CrossRef] [PubMed]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef]
- McDannold, N.; Maier, S.E. Magnetic resonance acoustic radiation force imaging. Med. Phys. 2015, 42, 4838–4846. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.V. Photoacoustic tomography: Applications and advances. Photons Plus Ultrasound Imaging Sens. 2013, 8581, 85810V. [Google Scholar]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Pardridge, W.M. Receptor mediated peptide transport through the blood-brain barrier. Endocr. Metab. Immune Disord.-Drug Targets 2016, 16, 182–187. [Google Scholar]
- Spencer, B.J.; Verma, I.M. Targeted delivery of proteins across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2007, 104, 7594–7599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Neumann, U. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Couch, J.A.; Yu, Y.J.; Zhang, Y. The blood-brain barrier and beyond: Strategies for advancing brain drug delivery. Drug Discov. Today Technol. 2017, 25, 63–71. [Google Scholar]
- Haney, M.; Klyachko, N.; Zhao, Y.; Gupta, R.; Plotnikova, E.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release Off. J. Control. Release Soc. 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Yu, S.; Wang, Y.; Meng, Q.; Liang, G.; Eckenhoff, M.; Wei, H. Intranasal administration of dantrolene increased brain concentration and duration. PLoS ONE 2020, 15, e0229156. [Google Scholar] [CrossRef]
- Qweider, M.; Gilsbach, J.; Rohde, V. Inadvertent intrathecal vincristine administration: A neurosurgical emergency. Case Rep. J. Neurosurg. Spine 2007, 6, 280–283. [Google Scholar] [CrossRef]
- Nassan, M.; Videnovic, A. Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2022, 18, 7–24. [Google Scholar] [CrossRef]
- Zhang, S.; Yue, Z.; Arnold, D.; Artiushin, G.; Sehgal, A. A circadian clock in the BBB regulates xenobiotic efflux. Cell 2018, 173, 130–139. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Nguyen, J. Rapid initiation of guided focused ultrasound-induced BBB disruption using radiofrequency. Ultrasonics 2009, 49, 566–573. [Google Scholar]
- Dromi, S.; Frenkel, V.; Luk, A.; Traughber, B.; Angstadt, M.; Bur, M.; Wood, B.J. Pulsed-high intensity focused ultrasound and low temperature–sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res. 2007, 13, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; McMahon, D.; Hynynen, K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem. Neurosci. 2017, 8, 16–26. [Google Scholar]
- Cui, B.; Cho, S. Blood-brain barrier-on-a-chip for brain disease modeling and drug testing. BMB Rep. 2022, 55, 213–219. [Google Scholar] [CrossRef]
- Hajal, C.; Le Roi, B.; Kamm, R.; Maoz, B. Biology and Models of the Blood-Brain Barrier. Annu. Rev. Biomed. Eng. 2021, 23, 359–384. [Google Scholar] [CrossRef]
- Peng, B.; Hao, S.; Tong, Z.; Bai, H.; Pan, S.; Lim, K.; Li, L.; Voelcker, N.; Huang, W. Blood-brain barrier (BBB)-on-a-chip: A promising breakthrough in brain disease research. Lab. Chip 2022, 22, 3579–3602. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N. MRI-guided focused ultrasound for brain therapy. Handb. Clin. Neurol. 2019, 161, 323–335. [Google Scholar]
- O’Reilly, M.A.; Hynynen, K. Blood-brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology 2012, 263, 96–106. [Google Scholar] [CrossRef]
- Dammann, P.; Krafft, A.J.; Robertson, V. Overcoming the blood-brain barrier: An overview of intranasal, magnetic, and ultrasound-mediated drug delivery. J. Control. Release 2020, 329, 471–482. [Google Scholar]
- Jordão, J.F.; Thévenot, E.; Hynynen, K. Focused ultrasound-mediated BBB disruption as a strategy for the treatment of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 34, 289–294. [Google Scholar]
- Mesiwala, A.H.; Farrell, L.; Wenzel, H.J. High-intensity focused ultrasound selectively disrupts the BBB in vivo. Appl. Phys. Lett. 2002, 80, 4201–4203. [Google Scholar]
- Ting, C.Y.; Fan, C.H.; Liu, H.L. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2018, 8, 1054. [Google Scholar]
- Yang, F.Y.; Liu, S.H. Enhancing of BBB permeability using ultrasound. World J. Radiol. 2012, 4, 345. [Google Scholar]
- Cho, E.E.; Drazic, J.; Hynynen, K. Biophysical mechanisms of BBB opening using focused ultrasound and microbubbles. APL Bioeng. 2018, 2, 031701. [Google Scholar]
- Frenkel, V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug Deliv. Rev. 2008, 60, 1193–1208. [Google Scholar] [CrossRef]
- Price, R.J.; Fisher, D.G.; Suk, J.S. Targeted drug delivery with focused ultrasound-induced BBB opening using acoustically-activated nanodroplets. J. Control. Release 2020, 293, 210–220. [Google Scholar]
- Staahl, B.T.; Doudna, J.A. CRISPR-Cas9: A tool for qualitative and quantitative genetic assessment. ACS Chem. Biol. 2016, 11, 532–534. [Google Scholar]
- Yang, F.; Li, X. Using artificial intelligence to improve the precision of focused ultrasound therapy. Ultrasound Med. Biol. 2019, 45, 12–25. [Google Scholar]
- Chaplin, V.; Lafon, C. Machine learning-based prediction of therapeutic outcomes in focused ultrasound. Ultrasound Med. Biol. 2020, 46, 427–437. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazi, S.K. Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis. Pharmaceutics 2023, 15, 2599. https://doi.org/10.3390/pharmaceutics15112599
Niazi SK. Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis. Pharmaceutics. 2023; 15(11):2599. https://doi.org/10.3390/pharmaceutics15112599
Chicago/Turabian StyleNiazi, Sarfaraz K. 2023. "Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis" Pharmaceutics 15, no. 11: 2599. https://doi.org/10.3390/pharmaceutics15112599
APA StyleNiazi, S. K. (2023). Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis. Pharmaceutics, 15(11), 2599. https://doi.org/10.3390/pharmaceutics15112599






