Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease—An Overview
Abstract
:1. Introduction
2. Inflammatory Bowel Disease (IBD)
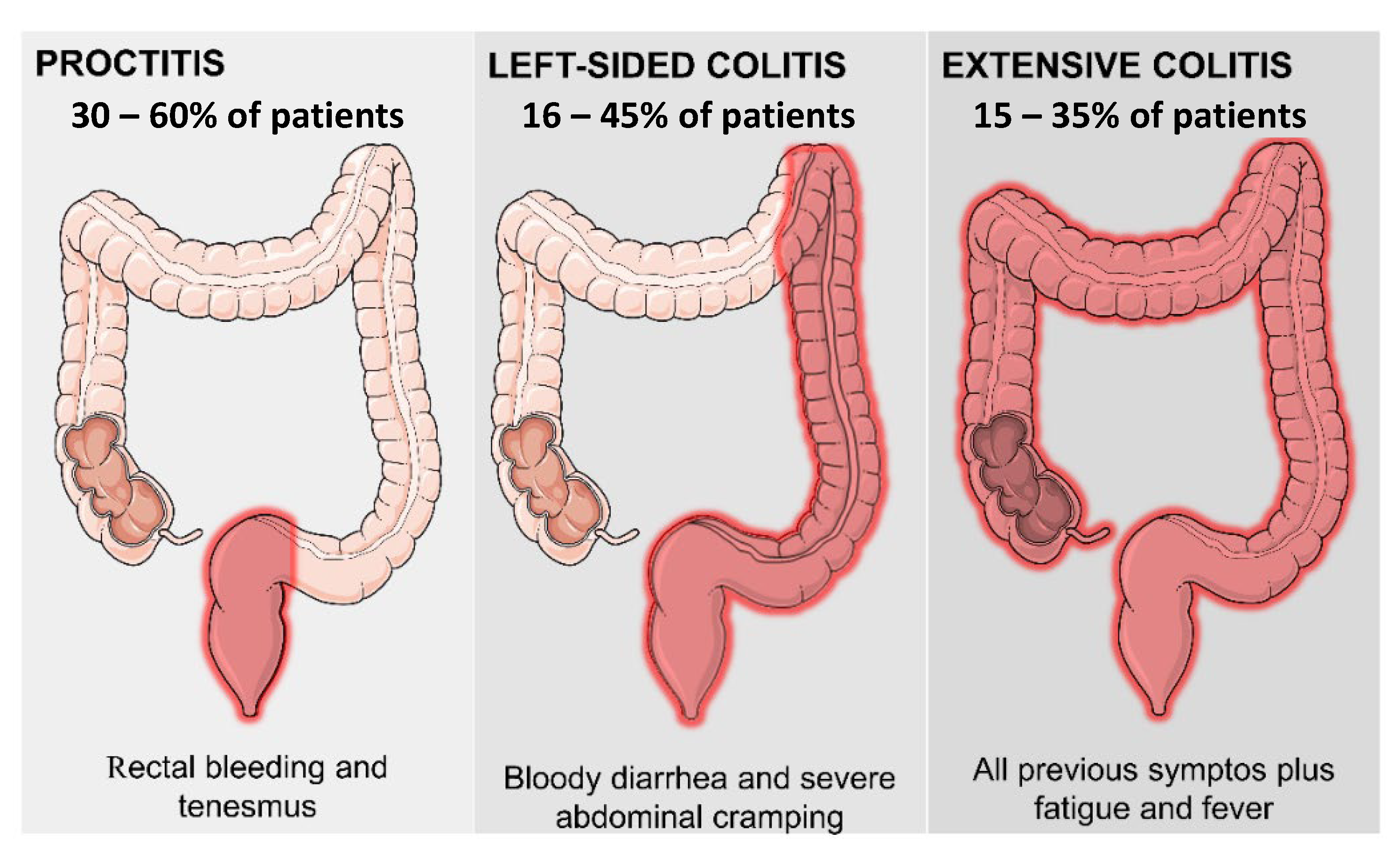
2.1. Ulcerative Colitis
2.2. Crohn’s Disease
2.3. Immunopathogenesis
2.4. Predisposing Factors
3. Conventional Treatment of IBD
4. Gut Microbiota, Dysbiosis, and the Potential Use of Probiotics in IBD Therapy
4.1. The Gut Microbiota
4.2. Dysbiosis
4.3. Potentials of Probiotics
5. Probiotic Delivery Systems
5.1. Microparticle-Based Drug Delivery Systems
5.2. Nanoparticle-Based Drug Delivery Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Oka, A.; Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.C.V.; Peres De, C.L.; Silveira, K.A.F.; Arruda, E.L.; de Almeida Júnior, M.P. Doenças Inflamatórias Intestinais No Brasil: Perfil Das Internações, Entre Os Anos de 2009 a 2019. Rev. Educ. Saúde 2020, 8, 127–135. [Google Scholar] [CrossRef]
- Kulkarni, N.; Jain, P.; Shindikar, A.; Suryawanshi, P.; Thorat, N. Advances in the Colon-Targeted Chitosan Based Multiunit Drug Delivery Systems for the Treatment of Inflammatory Bowel Disease. Carbohydr. Polym. 2022, 288, 119351. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive Drug Delivery Systems in Intestinal Inflammation: State-of-the-Art and Future Perspectives. Adv. Drug Deliv. Rev. 2018, 146, 248–266. [Google Scholar] [CrossRef]
- Lee, J.; Jee, S.R.; Kim, H.W.; Baek, D.H.; Song, G.A.; Moon, W.; Park, S.J.; Kim, H.J.; Lee, J.H.; Park, J.H.; et al. Factors Associated with Low Adherence to Oral 5-Aminosalicylic Acid in Patients with Ulcerative Colitis. PLoS ONE 2019, 14, e0214129. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef]
- Shah, B.M.; Palakurthi, S.S.; Khare, T.; Khare, S.; Palakurthi, S. Natural Proteins and Polysaccharides in the Development of Micro/Nano Delivery Systems for the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2020, 165, 722–737. [Google Scholar] [CrossRef]
- Cottone, M.; Renna, S.; Modesto, I.; Orlando, A. Is 5-ASA Still the Treatment of Choice for Ulcerative Colitis? Curr. Drug Targets 2011, 12, 1396–1405. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Tirapegui, J.; Rogero, M.M. Probiotics for Inflammatory Bowel Diseases: A Promising Adjuvant Treatment. Int. J. Food Sci. Nutr. 2019, 70, 20–29. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Bedani, R.; Dejani, N.N.; Ivo de Medeiros, A.; Sampaio Zuanon, J.A.; Spolidorio, L.C.; Tallarico Adorno, M.A.; Amâncio Varesche, M.B.; Carrilho Galvão, F.; Valentini, S.R.; et al. Effect of a Probiotic Beverage Consumption (Enterococcus Faecium CRL 183 and Bifidobacterium Longum ATCC 15707) in Rats with Chemically Induced Colitis. PLoS ONE 2017, 12, e0175935. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Song, J.-H.; Xu, H.; Li, D.; Goldsmith, J.; Zeng, H.; Parsons-Wingerter, P.A.; Reinecker, H.-C.; Kelly, C.P. Probiotic Yeast Inhibits VEGFR Signaling and Angiogenesis in Intestinal Inflammation. PLoS ONE 2013, 8, e64227. [Google Scholar] [CrossRef]
- Ghasemian, A.; Eslami, M.; Shafiei, M.; Najafipour, S.; Rajabi, A. Probiotics and Their Increasing Importance in Human Health and Infection Control. Rev. Med. Microbiol. 2018, 29, 153–158. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukaš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Wiesmueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia Coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Mardini, H.E.; Grigorian, A.Y. Probiotic Mix VSL#3 Is Effective Adjunctive Therapy for Mild to Moderately Active Ulcerative Colitis. Inflamm. Bowel Dis. 2014, 20, 1562–1567. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus Probiotics: An Alternative to Antibiotics for Livestock Production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Palumbo, V.D.; Romeo, M.; Gammazza, A.M.; Carini, F.; Damiani, P.; Damiano, G.; Buscemi, S.; Lo Monte, A.I.; Gerges-Geagea, A.; Jurjus, A.; et al. The Long-Term Effects of Probiotics in the Therapy of Ulcerative Colitis: A Clinical Study. Biomed. Pap. 2016, 160, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.-X. Sensitive Delivery Systems and Novel Encapsulation Technologies for Live Biotherapeutic Products and Probiotics. Crit. Rev. Microbiol. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Collnot, E.-M.; Ali, H.; Lehr, C.-M. Nano- and Microparticulate Drug Carriers for Targeting of the Inflamed Intestinal Mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef] [PubMed]
- Wilks, S. Morbid Appearances in the Intestine of Miss Bankes. London Med. Gaz. 1859, 2, 264–265. [Google Scholar]
- Fumery, M.; Singh, S.; Dulai, P.S.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Sandborn, W.J. Natural History of Adult Ulcerative Colitis in Population-Based Cohorts: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 343–356.e3. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.M. Surgical Therapy for Ulcerative Colitis and Crohn’s Disease. Gastroenterol. Clin. N. Am. 1999, 28, 371–390. [Google Scholar] [CrossRef]
- Hartwig, O.; Shetab Boushehri, M.A.; Shalaby, K.S.; Loretz, B.; Lamprecht, A.; Lehr, C.-M. Drug Delivery to the Inflamed Intestinal Mucosa—Targeting Technologies and Human Cell Culture Models for Better Therapies of IBD. Adv. Drug Deliv. Rev. 2021, 175, 113828. [Google Scholar] [CrossRef] [PubMed]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Regional ileitis. J. Am. Med. Assoc. 1932, 99, 1323. [Google Scholar] [CrossRef]
- Pinho, M. A Biologia Molecular Das Doenças Inflamatórias Intestinais. Rev. Bras. Coloproctol. 2008, 28, 119–123. [Google Scholar] [CrossRef]
- Gomes, J.P.; Watad, A.; Shoenfeld, Y. Nicotine and Autoimmunity: The Lotus’ Flower in Tobacco. Pharmacol. Res. 2018, 128, 101–109. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Hou, T.; Zeng, H.; Kalambhe, D.; Wang, B.; Shen, X.; Huang, Y. Macrophage-Based Nanotherapeutic Strategies in Ulcerative Colitis. J. Control. Release 2020, 320, 363–380. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut Microbiota in Ulcerative Colitis: Insights on Pathogenesis and Treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Wallace, K.L.; Zheng, L.-B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Kalla, R.; Ho, G.-T. Ulcerative Colitis: Recent Advances in the Understanding of Disease Pathogenesis. F1000Research 2020, 9, 294. [Google Scholar] [CrossRef]
- Rogler, G.; Biedermann, L.; Scharl, M. New Insights into the Pathophysiology of Inflammatory Bowel Disease: Microbiota, Epigenetics and Common Signalling Pathways. Swiss Med. Wkly. 2018, 148, w14599. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Soo, W.; Bryant, R.V.; Jairath, V.; Hart, A.L.; Andrews, J.M. Systematic Review with Meta-Analysis: Faecal Microbiota Transplantation for the Induction of Remission for Active Ulcerative Colitis. Aliment. Pharmacol. Ther. 2017, 46, 213–224. [Google Scholar] [CrossRef]
- Cader, M.Z.; Kaser, A. Recent Advances in Inflammatory Bowel Disease: Mucosal Immune Cells in Intestinal Inflammation. Gut 2013, 62, 1653–1664. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Dvornikova, K.A.; Bystrova, E.Y.; Churilov, L.P.; Lerner, A. Pathogenesis of the Inflammatory Bowel Disease in Context of SARS-COV-2 Infection. Mol. Biol. Rep. 2021, 48, 5745–5758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Law, H.K.W. Deciphering the Role of Autophagy in the Immunopathogenesis of Inflammatory Bowel Disease. Front. Pharmacol. 2022, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, D.; de Voogd, F.A.E.; Pruijt, M.J.; Grootjans, J.; van de Sande, M.G.; D’Haens, G.R. The Role of the Lymphatic System in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 1854. [Google Scholar] [CrossRef]
- De Souza, F.G.; Lo Prete, A.C.; Ribeiro, C.H.M.A. Adesão Ao Tratamento Farmacológico Em Pacientes Com Doenças Inflamatórias Intestinais: Uma Revisão Integrativa Da Literatura. Rev. Eletrônica Acervo Saúde 2021, 13, e4601. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Ahmad, R.; Bakkari, M.A.; Rajput, M.K.S.; Dachineni, R.; Valiveti, C.K.; Kapur, S.; Jayarama Bhat, G.; Singh, A.B.; Tummala, H. Site-Directed Non-Covalent Polymer-Drug Complexes for Inflammatory Bowel Disease (IBD): Formulation Development, Characterization and Pharmacological Evaluation. J. Control. Release 2018, 290, 165–179. [Google Scholar] [CrossRef]
- Shivashankar, R.; Tremaine, W.J.; Harmsen, W.S.; Loftus, E.V. Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clin. Gastroenterol. Hepatol. 2017, 15, 857–863. [Google Scholar] [CrossRef]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Kaplan, G.G. The Global Burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Chhoun, C.; Blair, B.; Joo, L.; Hashir, M.; Dasu, N.; Khalid, Y.; Chiesa, D.; Walters, R.; Suga, H. S22 Outcomes of Patients With Inflammatory Bowel Disease and Alcohol Use From the National Inpatient Sample. Am. J. Gastroenterol. 2022, 117, S6. [Google Scholar] [CrossRef]
- Keefer, L.; Keshavarzian, A.; Mutlu, E. Reconsidering the Methodology of “Stress” Research in Inflammatory Bowel Disease. J. Crohn’s Colitis 2008, 2, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Shinzaki, S.; Yamada, T.; Arimitsu, S.; Komori, M.; Shibukawa, N.; Mukai, A.; Nakajima, S.; Kinoshita, K.; Kitamura, S.; et al. Psychologic Stress and Disease Activity in Patients with Inflammatory Bowel Disease: A Multicenter Cross-Sectional Study. PLoS ONE 2020, 15, e0233365. [Google Scholar] [CrossRef]
- Sajadinejad, M.S.; Asgari, K.; Molavi, H.; Kalantari, M.; Adibi, P. Psychological Issues in Inflammatory Bowel Disease: An Overview. Gastroenterol. Res. Pract. 2012, 2012, 106502. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Singh, S.; Graff, L.A.; Walker, J.R.; Miller, N.; Cheang, M. A Prospective Population-Based Study of Triggers of Symptomatic Flares in IBD. Am. J. Gastroenterol. 2010, 105, 1994–2002. [Google Scholar] [CrossRef]
- Mawdsley, J.E.; Rampton, D.S. Psychological Stress in IBD: New Insights into Pathogenic and Therapeutic Implications. Gut 2005, 54, 1481–1491. [Google Scholar] [CrossRef]
- Agostini, A.; Filippini, N.; Cevolani, D.; Agati, R.; Leoni, C.; Tambasco, R.; Calabrese, C.; Rizzello, F.; Gionchetti, P.; Ercolani, M.; et al. Brain Functional Changes in Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2011, 17, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Tavares Junior, A.G.; De Araújo, J.T.C.; Duarte, J.L.; Silvestre, A.L.P.; Di Filippo, L.D.; Chorilli, M. Polymeric Systems for Colon-Specific Mesalazine Delivery in the Intestinal Bowel Diseases Management. Curr. Med. Chem. 2023, 30, 1351–1367. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Karmeli, F.; Schwartz, L.W.; Simon, P.L. Effect of Aminophenols (5-ASA and 4-ASA) on Colonic Interleukin-1 Generation. Gut 1992, 33, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Corp, D.; Strom, T.B.; Lipman, M.; Peppercorn, M.A.; Moscovitch-lopatin, M.; Strom, T.B.; Hospital, B.I. 5-Aminosalicylic Acid Abrogates T-CeII Proliferation by Blocking Lnterleukin-2 Production in Peripheral Blood Mononuclear Cells. J. Pharmacol. Exp. Ther. 1995, 272, 399–406. [Google Scholar] [PubMed]
- Kaiser, G.C.; Yan, F.; Polk, D.B. Mesalamine Blocks Tumor Necrosis Factor Growth Inhibition and Nuclear Factor ΚB Activation in Mouse Colonocytes. Gastroenterology 1999, 116, 602–609. [Google Scholar] [CrossRef]
- Abdu Allah, H.H.; A El Shorbagi, A.N.; Abdel-Moty, S.G.; El-Awady, R.; Abdel-Alim, A.-A.M. 5-Aminosalyclic Acid (5-ASA): A Unique Anti-Inflammatory Salicylate. Med. Chem. 2016, 6, 306–315. [Google Scholar] [CrossRef]
- Bantel, H. Mesalazine Inhibits Activation of Transcription Factor NF-ΚB in Inflamed Mucosa of Patients with Ulcerative Colitis. Am. J. Gastroenterol. 2000, 95, 3452–3457. [Google Scholar] [CrossRef]
- Pooley, N.; Ghosh, L.; Sharon, P. Up-Regulation of E-Selectin and Intercellular Adhesion Molecule-1 Differs between Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 1995, 40, 219–225. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, S.; Unnikrishnan, M.; Mukherjee, T. Free Radical Scavenging Reactions of Sulfasalazine, 5-Aminosalicylic Acid and Sulfapyridine: Mechanistic Aspects and Antioxidant Activity. Free Radic. Res. 2005, 39, 1163–1172. [Google Scholar] [CrossRef]
- Bucci, C.; Zingone, F.; Tammaro, S.; Iovino, P.; Santonicola, A.; Ciacci, C. Factors Predicting the Adherence to the Therapy of Italian IBD Patients. Gastroenterol. Res. Pract. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Červený, P.; Bortlík, M.; Kuběna, A.; Vlček, J.; Lakatos, P.L.; Lukáš, M. Nonadherence in Inflammatory Bowel Disease: Results of Factor Analysis. Inflamm. Bowel Dis. 2007, 13, 1244–1249. [Google Scholar] [CrossRef]
- Coenen, S.; Weyts, E.; Ballet, V.; Noman, M.; Van Assche, G.; Vermeire, S.; Van Emelen, J.; Ferrante, M. Identifying Predictors of Low Adherence in Patients with Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 503–507. [Google Scholar] [CrossRef]
- Kane, S.; Huo, D.; Aikens, J.; Hanauer, S. Medication Nonadherence and the Outcomes of Patients with Quiescent Ulcerative Colitis. Am. J. Med. 2003, 114, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Shale, M.J.; Riley, S.A. Studies of Compliance with Delayed-Release Mesalazine Therapy in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2003, 18, 191–198. [Google Scholar] [CrossRef]
- Tomar, S.K.; Kedia, S.; Singh, N.; Upadhyay, A.D.; Kamat, N.; Bopanna, S.; Yadav, D.P.; Goyal, S.; Jain, S.; Makharia, G.; et al. Higher Education, Professional Occupation, and Upper Socioeconomic Status Are Associated with Lower Adherence to Medications in Patients with Inflammatory Bowel Disease. JGH Open 2019, 3, 302–309. [Google Scholar] [CrossRef]
- Su, H.-J.; Chiu, Y.-T.; Chiu, C.-T.; Lin, Y.-C.; Wang, C.-Y.; Hsieh, J.-Y.; Wei, S.-C. Inflammatory Bowel Disease and Its Treatment in 2018: Global and Taiwanese Status Updates. J. Formos. Med. Assoc. 2019, 118, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Vierziger, K.; Sturm, A.; Wiedenmann, B.; Dignass, A.U. Mesalamine Promotes Intestinal Epithelial Wound Healing in Vitro through a TGF-Beta-Independent Mechanism. Scand. J. Gastroenterol. 2005, 40, 958–964. [Google Scholar] [CrossRef]
- Ho, G.-T.; Lees, C.; Satsangi, J. Ulcerative Colitis. Medicine 2011, 39, 224–228. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.-L.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Ulcerative Colitis. Mayo Clin. Proc. 2014, 89, 1553–1563. [Google Scholar] [CrossRef]
- Fróes, R.D.S.B. Tratamento Convencional Na Doença Inflamatória Intestinal. Rev. Hosp. Univ. Pedro Ernesto 2012, 11, 27–32. [Google Scholar]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, M.; Hokaria, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent Advances in Colon Drug Delivery Systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Manos, J. The Human Microbiome in Disease and Pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A. Alteration of the Gut Microbiome in Inflammatory Bowel Disease. Digestion 2023, 104, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Hajela, N.; Ramakrishna, B.S.; Nair, G.B.; Abraham, P.; Gopalan, S.; Ganguly, N.K. Gut Microbiome, Gut Function, and Probiotics: Implications for Health. Indian J. Gastroenterol. 2015, 34, 93–107. [Google Scholar] [CrossRef]
- Buttó, L.F.; Schaubeck, M.; Haller, D. Mechanisms of Microbe–Host Interaction in Crohn’s Disease: Dysbiosis vs. Pathobiont Selection. Front. Immunol. 2015, 6, 555. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, N.; Wu, Z.; Song, Y.; Zhang, Y.; Wu, N.; Zhang, F.; Ren, X.; Liu, Y. 5-Aminosalicylic Acid Alters the Gut Bacterial Microbiota in Patients With Ulcerative Colitis. Front. Microbiol. 2018, 9, 1274. [Google Scholar] [CrossRef]
- Fuentes, S.; Rossen, N.G.; van der Spek, M.J.; Hartman, J.H.A.; Huuskonen, L.; Korpela, K.; Salojärvi, J.; Aalvink, S.; de Vos, W.M.; D’Haens, G.R.; et al. Microbial Shifts and Signatures of Long-Term Remission in Ulcerative Colitis after Faecal Microbiota Transplantation. ISME J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.-L.; Barnich, N.; Bringer, M.-A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.-F. High Prevalence of Adherent-Invasive Escherichia Coli Associated with Ileal Mucosa in Crohn’s Disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal T H 17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ Regulatory T-Cell Development by a Commensal Bacterium of the Intestinal Microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Martín, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.-J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermúdez-Humarán, L.G.; Langella, P. The Commensal Bacterium Faecalibacterium Prausnitzii Is Protective in DNBS-Induced Chronic Moderate and Severe Colitis Models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low Counts of Faecalibacterium Prausnitzii in Colitis Microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions Between Commensal Fungi and the C-Type Lectin Receptor Dectin-1 Influence Colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Kaufman, J.; Griffiths, T.A.; Surette, M.G.; Ness, S.; Rioux, K.P. Effects of Mesalamine (5-Aminosalicylic Acid) on Bacterial Gene Expression. Inflamm. Bowel Dis. 2009, 15, 985–996. [Google Scholar] [CrossRef]
- Guarino, M.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Sarao, L.K.; Arora, M. Probiotics, Prebiotics, and Microencapsulation: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Gui, S.-Y.; Gao, X.; Zhang, W.; Fu, Z.-Y.; Tao, L.-M.; Jiang, Z.-X.; Chen, X.; Qian, H.; Wang, X. Research Progress of Natural Product-Based Nanomaterials for the Treatment of Inflammation-Related Diseases. Mater. Des. 2022, 218, 110686. [Google Scholar] [CrossRef]
- Nath, A.; Haktanirlar, G.; Varga, Á.; Molnár, M.A.; Albert, K.; Galambos, I.; Koris, A.; Vatai, G. Biological Activities of Lactose-Derived Prebiotics and Symbiotic with Probiotics on Gastrointestinal System. Medicina 2018, 54, 18. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, Prebiotics and Amelioration of Diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of Immune Cell Function by Short-Chain Fatty Acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in Microencapsulation of Probiotics: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Danese, S. Negative Regulators of Angiogenesis in Inflammatory Bowel Disease: Thrombospondin in the Spotlight. Pathobiology 2008, 75, 22–24. [Google Scholar] [CrossRef]
- Papa, A.; Scaldaferri, F.; Danese, S.; Guglielmo, S.; Roberto, I.; Bonizzi, M.; Mocci, G.; Felice, C.; Ricci, C.; Andrisani, G.; et al. Vascular Involvement in Inflammatory Bowel Disease: Pathogenesis and Clinical Aspects. Dig. Dis. 2008, 26, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, J.H.; Shukla, D.; Grisham, M.B.; Kevil, C.G. Pathogenic Angiogenesis in IBD and Experimental Colitis: New Ideas and Therapeutic Avenues. Am. J. Physiol. Liver Physiol. 2007, 293, G5–G18. [Google Scholar] [CrossRef]
- Kanazawa, S.; Tsunoda, T.; Onuma, E.; Majima, T.; Kagiyama, M.; Kikuchi, K. VEGF, Basic-FGF, and TGF-Beta in Crohn’s Disease and Ulcerative Colitis: A Novel Mechanism of Chronic Intestinal Inflammation. Am. J. Gastroenterol. 2001, 96, 822–828. [Google Scholar] [CrossRef]
- Kapsoritakis, A.; Sfiridaki, A.; Maltezos, E.; Simopoulos, K.; Giatromanolaki, A.; Sivridis, E.; Koukourakis, M.I. Vascular Endothelial Growth Factor in Inflammatory Bowel Disease. Int. J. Colorectal Dis. 2003, 18, 418–422. [Google Scholar] [CrossRef]
- Tsiolakidou, G.; Koutroubakis, I.E.; Tzardi, M.; Kouroumalis, E.A. Increased Expression of VEGF and CD146 in Patients with Inflammatory Bowel Disease. Dig. Liver Dis. 2008, 40, 673–679. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Vetrano, S.; Sans, M.; Arena, V.; Straface, G.; Stigliano, E.; Repici, A.; Sturm, A.; Malesci, A.; Panes, J.; et al. VEGF-A Links Angiogenesis and Inflammation in Inflammatory Bowel Disease Pathogenesis. Gastroenterology 2009, 136, 585–595.e5. [Google Scholar] [CrossRef] [PubMed]
- Zulham, Z.Z.; Wilar, G.; Susilawati, Y.; Subarnas, A.; Chaerunisaa, A.Y. Microparticles of Herbal Extracts with Antioxidant Activity. Pharmacogn. J. 2021, 13, 285–295. [Google Scholar] [CrossRef]
- Da Silva, A.B.; Miniter, M.; Thom, W.; Hewitt, R.E.; Wills, J.; Jugdaohsingh, R.; Powell, J.J. Gastrointestinal Absorption and Toxicity of Nanoparticles and Microparticles: Myth, Reality and Pitfalls Explored through Titanium Dioxide. Curr. Opin. Toxicol. 2020, 19, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Kaur, K.; Mehta, N.; Dhaliwal, S.S.; Kennedy, J.F. Characterization and Optimization of Spray Dried Iron and Zinc Nanoencapsules Based on Potato Starch and Maltodextrin. Carbohydr. Polym. 2022, 282, 119107. [Google Scholar] [CrossRef]
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Recent Advances in Probiotic Encapsulation to Improve Viability under Storage and Gastrointestinal Conditions and Their Impact on Functional Food Formulation. Food Rev. Int. 2023, 39, 992–1013. [Google Scholar] [CrossRef]
- How, Y.; Pui, L. Survivability of Microencapsulated Probiotics in Nondairy Beverages: A Review. J. Food Process. Preserv. 2021, 45, e15641. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Chen, Y.; Xie, Y.; Han, G.; Liu, S.; Song, Y.; Lu, W.; Guo, Y. Living Probiotics—Loaded Hydrogel Microspheres with Gastric Acid Resistance and ROS Triggered Release for Potential Therapy of Inflammatory Bowel Disease. ACS Appl. Polym. Mater. 2023, 5, 957–967. [Google Scholar] [CrossRef]
- Williams, G.T.; Sedgwick, A.C.; Sen, S.; Gwynne, L.; Gardiner, J.E.; Brewster, J.T.; Hiscock, J.R.; James, T.D.; Jenkins, A.T.A.; Sessler, J.L. Boronate Ester Cross-Linked PVA Hydrogels for the Capture and H2O2-Mediated Release of Active Fluorophores. Chem. Commun. 2020, 56, 5516–5519. [Google Scholar] [CrossRef]
- Li, J.; Qiu, H.; Gong, H.; Tong, W. Broad-Spectrum Reactive Oxygen Species Scavenging and Activated Macrophage-Targeting Microparticles Ameliorate Inflammatory Bowel Disease. Biomacromolecules 2021, 22, 3107–3118. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorowska, A.; Romanowski, M.; Walecka-Kapica, E.; Kaczka, A.; Chojnacki, C.; Padysz, M.; Siedlecka, M.; Bierła, J.B.; Steinert, R.E.; Cukrowska, B. Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Protocol of a Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 6587. [Google Scholar] [CrossRef]
- Pinto, L.A.; Corá, L.A.; Rodrigues, G.S.; Prospero, A.G.; Soares, G.A.; de Andreis, U.; de Arruda Miranda, J.R. Pharmacomagnetography to Evaluate the Performance of Magnetic Enteric-Coated Tablets in the Human Gastrointestinal Tract. Eur. J. Pharm. Biopharm. 2021, 161, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; de Mesquita Souza Saraiva, M.; Macedo Junior, W.D.; Márquez Montesinos, J.C.E.; Meneguin, A.B.; Toledo Borges, A.B.; Crusca Junior, E.; Garrido, S.S.; de Almeida, A.M.; Marchetto, R.; et al. Chitosan and HPMCAS Double-Coating as Protective Systems for Alginate Microparticles Loaded with Ctx(Ile21)-Ha Antimicrobial Peptide to Prevent Intestinal Infections. Biomaterials 2023, 293, 121978. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; de Mesquita Souza Saraiva, M.; Monte, D.F.M.; Rodrigues Alves, L.B.; de Almeida, A.M.; Ferreira, T.S.; de Lima, T.S.; Benevides, V.P.; Cabrera, J.M.; Claire, S.; et al. HPMCAS-Coated Alginate Microparticles Loaded with Ctx(Ile 21)-Ha as a Promising Antimicrobial Agent against Salmonella Enteritidis in a Chicken Infection Model. ACS Infect. Dis. 2022, 8, 472–481. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, G.H.; Jun, J.H.; Son, M.; Choi, Y.S.; Choi, M.K.; Kang, M.J. Formulation and in Vivo Evaluation of Probiotics-Encapsulated Pellets with Hydroxypropyl Methylcellulose Acetate Succinate (HPMCAS). Carbohydr. Polym. 2015, 136, 692–699. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.-P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-Encapsulation of Probiotic Lactobacillus Acidophilus and Reishi Medicinal Mushroom (Ganoderma Lingzhi) Extract in Moist Calcium Alginate Beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Thakur, K.; Zhang, W.-W.; Feng, J.-Y.; Zhang, J.-G.; Hu, F.; Liao, C.; Wei, Z.-J. Double-Layer Mucin Microencapsulation Enhances the Stress Tolerance and Oral Delivery of Lactobacillus Plantarum B2. Food Hydrocoll. 2023, 141, 108678. [Google Scholar] [CrossRef]
- Yan, H.; Seignez, C.; Hjorth, M.; Winkeljann, B.; Blakeley, M.; Lieleg, O.; Phillipson, M.; Crouzier, T. Immune-Informed Mucin Hydrogels Evade Fibrotic Foreign Body Response In Vivo. Adv. Funct. Mater. 2019, 29, 1902581. [Google Scholar] [CrossRef]
- Pourjafar, H.; Noori, N.; Gandomi, H.; Basti, A.A.; Ansari, F. Viability of Microencapsulated and Non-Microencapsulated Lactobacilli in a Commercial Beverage. Biotechnol. Rep. 2020, 25, e00432. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Antunes, B.F.; Toledo Borgues, A.B.; Costa de Pontes, J.T.; Meneguin, A.B.; Chorilli, M.; Trovatti, E.; Teixeira, S.R.; Pavan, F.R.; Vicente, E.F. Conjugation of Ctx(Ile21)-Ha Antimicrobial Peptides to Chitosan Ultrathin Films by N -Acetylcysteine Improves Peptide Physicochemical Properties and Enhances Biological Activity. ACS Omega 2022, 7, 28238–28247. [Google Scholar] [CrossRef]
- Shahzadi, I.; Fürst, A.; Akkus-Dagdeviren, Z.B.; Arshad, S.; Kurpiers, M.; Matuszczak, B.; Bernkop-Schnürch, A. Less Reactive Thiol Ligands: Key towards Highly Mucoadhesive Drug Delivery Systems. Polymers 2020, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Fang, H.; Chang, S.; Ren, G.; Cheng, X.; Pan, Y.; Wu, R.; Liu, H.; Wu, J. Construction of Probiotic Double-Layered Multinucleated Microcapsules Based on Sulfhydryl-Modified Carboxymethyl Cellulose Sodium for Increased Intestinal Adhesion of Probiotics and Therapy for Intestinal Inflammation Induced by Escherichia Coli O157:H7. ACS Appl. Mater. Interfaces 2023, 15, 18569–18589. [Google Scholar] [CrossRef]
- Zavaleta, E.B.; Coavichi, L.I.L.; Rodríguez, L.C.V.; Andrade, E.F.; García, H.S.; Rascón Díaz, M.P. Co-Microencapsulation of Lactobacillus Rhamnosus and Krill Oil by Spray-Drying. Food Biosci. 2022, 50, 102133. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Alves, P.; Simões, P.N.; Rúa, M.L.; Fuciños, C.; Gomez-Zavaglia, A. Microencapsulation of Lactobacillus Plantarum in W/O Emulsions of Okara Oil and Block-Copolymers of Poly(Acrylic Acid) and Pluronic Using Microfluidic Devices. Food Res. Int. 2021, 140, 110053. [Google Scholar] [CrossRef]
- Sharifi, E.; Yazdani, Z.; Najafi, M.; Hosseini-khah, Z.; Jafarpour, A.; Rafiei, A. The Combined Effect of Fish Oil Containing Omega-3 Fatty Acids and Lactobacillus Plantarum on Colorectal Cancer. Food Sci. Nutr. 2022, 10, 4411–4418. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Saaby, L.; Knøchel, S.; Nielsen, D.S. Dark Chocolate as a Stable Carrier of Microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol. Lett. 2019, 366, 74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, M.; McClements, D.J.; Liu, X.; Liu, F. Recent Advances in the Design and Fabrication of Probiotic Delivery Systems to Target Intestinal Inflammation. Food Hydrocoll. 2022, 125, 107438. [Google Scholar] [CrossRef]
- Thakur, V.; Singh, B.; Sharma, A.; Kumari, N.; Kumar, I.; Verma, K.; Kumar, A.; Rana, S. Importance of Nanocarriers and Probiotics in the Treatment of Ulcerative Colitis. Int. J. Appl. Pharm. 2021, 13, 77–85. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Nanomaterial-Based Encapsulation for Controlled Gastrointestinal Delivery of Viable Probiotic Bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel Nano-Encapsulated Probiotic Agents: Encapsulate Materials, Delivery, and Encapsulation Systems. J. Control. Release 2022, 349, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.G.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; Mohamed, S.A.M.; Metwally, A.S.; El-Shetry, S.E.; Saleh, A.A.; ElSawy, N.A.; Ibrahim, D. Probiotics-Loaded Nanoparticles Attenuated Colon Inflammation, Oxidative Stress, and Apoptosis in Colitis. Sci. Rep. 2022, 12, 5116. [Google Scholar] [CrossRef]
- Alkushi, A.G.; Elazab, S.T.; Abdelfattah-Hassan, A.; Mahfouz, H.; Salem, G.A.; Sheraiba, N.I.; Mohamed, E.A.A.; Attia, M.S.; El-Shetry, E.S.; Saleh, A.A.; et al. Multi-Strain-Probiotic-Loaded Nanoparticles Reduced Colon Inflammation and Orchestrated the Expressions of Tight Junction, NLRP3 Inflammasome and Caspase-1 Genes in DSS-Induced Colitis Model. Pharmaceutics 2022, 14, 1183. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Khavarpour, M.; Khalili, S. Survival of Lactobacillus Acidophilus as Probiotic Bacteria Using Chitosan Nanoparticles. Int. J. Eng. 2017, 30, 456–463. [Google Scholar] [CrossRef]
- Saadatzadeh, A.; Atyabi, F.; Fazeli, M.R.; Dinarvand, R.; Jamalifar, H.; Abdolghaffari, A.H.; Mahdaviani, P.; Mahbod, M.; Baeeri, M.; Baghaei, A.; et al. Biochemical and Pathological Evidences on the Benefit of a New Biodegradable Nanoparticles of Probiotic Extract in Murine Colitis. Fundam. Clin. Pharmacol. 2012, 26, 589–598. [Google Scholar] [CrossRef]
- Sahu, B.K.; Sharma, S.; Kaur, K.; Chandel, M.; Sood, P.; Singh, M.; Shanmugham, V. Farm Waste-Eggshell Nanoparticles Constitute Gel for Safe Navigation of Probiotic across the Stomach. Mater. Today Commun. 2023, 34, 104876. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Heelan, W.J.; Chen, Y.; Li, Z.; Hu, Q. Mucoadhesive Probiotic Backpacks with ROS Nanoscavengers Enhance the Bacteriotherapy for Inflammatory Bowel Diseases. Sci. Adv. 2022, 8, eabp8798. [Google Scholar] [CrossRef]
- Wei, H.; Geng, W.; Yang, X.-Y.; Kuipers, J.; van der Mei, H.C.; Busscher, H.J. Activation of a Passive, Mesoporous Silica Nanoparticle Layer through Attachment of Bacterially-Derived Carbon-Quantum-Dots for Protection and Functional Enhancement of Probiotics. Mater. Today Bio 2022, 15, 100293. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, J.; Shi, T.; Zhang, Y.; Chen, F.; Yang, C.; Guo, X.; Liu, G.; Shao, D.; Leong, K.W.; et al. Probiotic-Inspired Nanomedicine Restores Intestinal Homeostasis in Colitis by Regulating Redox Balance, Immune Responses, and the Gut Microbiome. Adv. Mater. 2023, 35, 2207890. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, X.; Pang, Y.; Cheng, S.; Liu, J. Biointerfacial Self-Assembly Generates Lipid Membrane Coated Bacteria for Enhanced Oral Delivery and Treatment. Nat. Commun. 2019, 10, 5783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, Q.; Guo, M.; Li, B.; Peng, W.; Wang, D.; Ming, D.; Zheng, B. Nanoarmour-Shielded Single-Cell Factory for Bacteriotherapy of Parkinson’s Disease. J. Control. Release 2021, 338, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Shan, J.; Xing, J.; Liu, X.; Ma, Y.; Qian, H.; Chen, X.; Wang, X.; Wu, L.-M.; et al. Multifunctional Two-Dimensional Bi2Se3 Nanodiscs for Anti-Inflammatory Therapy of Inflammatory Bowel Diseases. Acta Biomater. 2023, 160, 252–264. [Google Scholar] [CrossRef] [PubMed]



| Microorganisms | Benefit | Reference |
|---|---|---|
| Lactobacillus spp. and Bifidobacterium spp. | Inflammation reduction | [14] |
| Lactobacillus and Bifidobacterium plus Streptococcus or E. coli Nissle 1917 | Symptom remission | [15] |
| Lactobacillus spp., Bifidobacterium spp., and Streptococcus thermophilus | Symptom attenuation | [16] |
| Saccharomyces boulardii | Angiogenesis blockage | [13] |
| Bacillus | Survive in acid conditions | [17] |
| Saccharomyces boulardii, Akkermansia muciniphila, and Faecalibacterium prausnitzi | Intestinal barrier reinforcement; colonic inflammation reduction | [19] |
| Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707 | Inflammation and ulceration reduction; Lactobacillus spp. and Bifidobacterium spp. population increase in rat colon | [12] |
| 5-ASA plus L. salivarius, L. acidophilus, and B. bifidum BGN4 | Recovery time reduction; symptom attenuation | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, S.A.; Roque-Borda, C.A.; Duarte, J.L.; Di Filippo, L.D.; Borges Cardoso, V.M.; Pavan, F.R.; Chorilli, M.; Meneguin, A.B. Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease—An Overview. Pharmaceutics 2023, 15, 2600. https://doi.org/10.3390/pharmaceutics15112600
Lopes SA, Roque-Borda CA, Duarte JL, Di Filippo LD, Borges Cardoso VM, Pavan FR, Chorilli M, Meneguin AB. Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease—An Overview. Pharmaceutics. 2023; 15(11):2600. https://doi.org/10.3390/pharmaceutics15112600
Chicago/Turabian StyleLopes, Sílvio André, Cesar Augusto Roque-Borda, Jonatas Lobato Duarte, Leonardo Delello Di Filippo, Vinícius Martinho Borges Cardoso, Fernando Rogério Pavan, Marlus Chorilli, and Andréia Bagliotti Meneguin. 2023. "Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease—An Overview" Pharmaceutics 15, no. 11: 2600. https://doi.org/10.3390/pharmaceutics15112600
APA StyleLopes, S. A., Roque-Borda, C. A., Duarte, J. L., Di Filippo, L. D., Borges Cardoso, V. M., Pavan, F. R., Chorilli, M., & Meneguin, A. B. (2023). Delivery Strategies of Probiotics from Nano- and Microparticles: Trends in the Treatment of Inflammatory Bowel Disease—An Overview. Pharmaceutics, 15(11), 2600. https://doi.org/10.3390/pharmaceutics15112600











