Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Manufacturing of MPs Containing AmB and ITR (AmB-ITR MPs)
2.2.2. Optimisation of AmB-ITR MPs
Taguchi Design
- Determination of the yield
- Determination of particle size
- Determination of the antifungal in vitro activity
- Determination of the encapsulation efficiency
- Determination of the AmB aggregation state
Box–Behnken DoE
2.2.3. Physicochemical Characterisation of Optimised AmB-ITR MPs
Scanning Electron Microscopy (SEM)
Modulated Temperature Differential Scanning Calorimetry (MT-DSC)
2.2.4. In vitro Lung Deposition
2.2.5. Statistical Analysis
3. Results
3.1. Optimisation of AmB-ITR-MPs
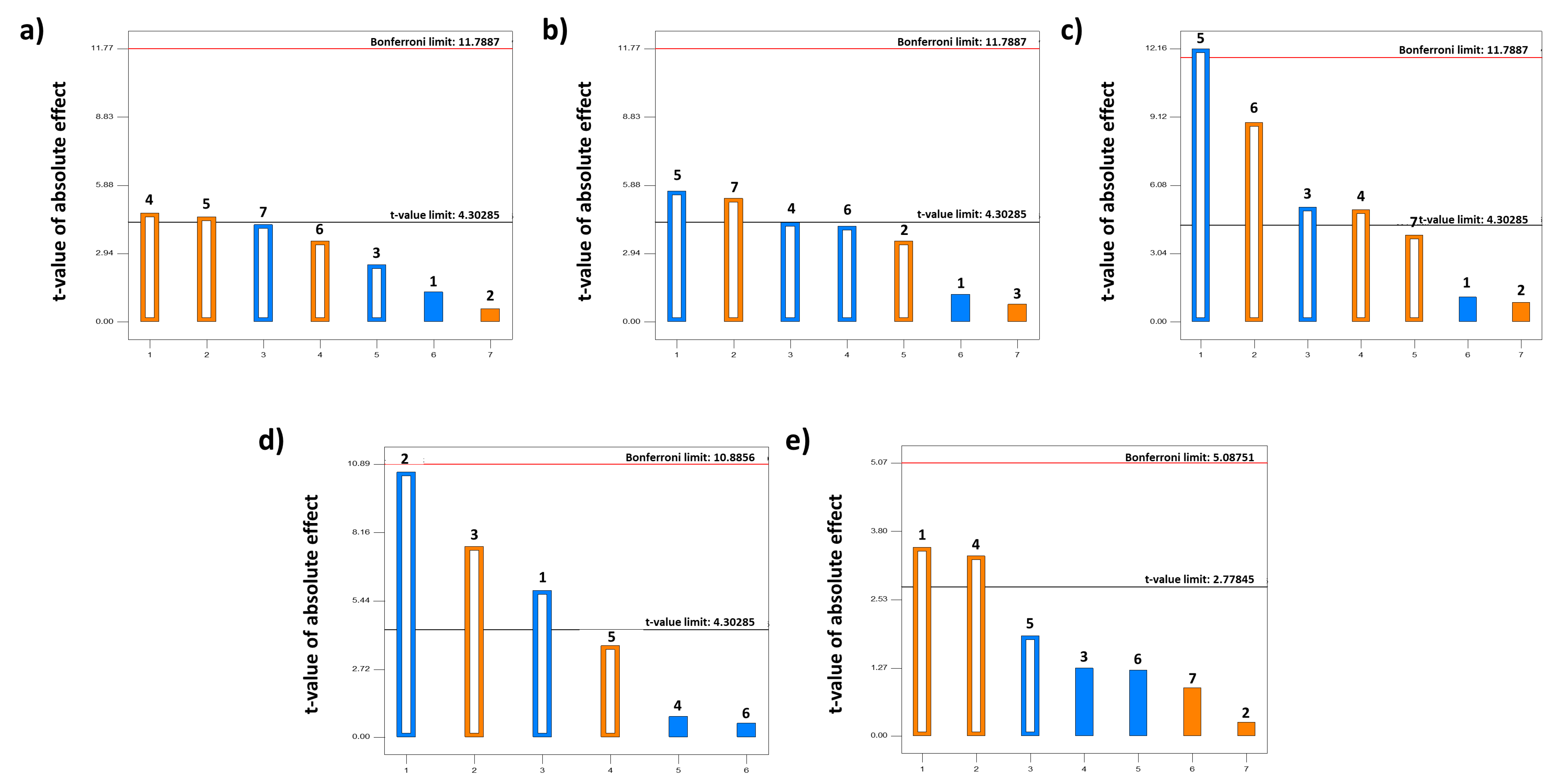
3.1.1. Taguchi Design
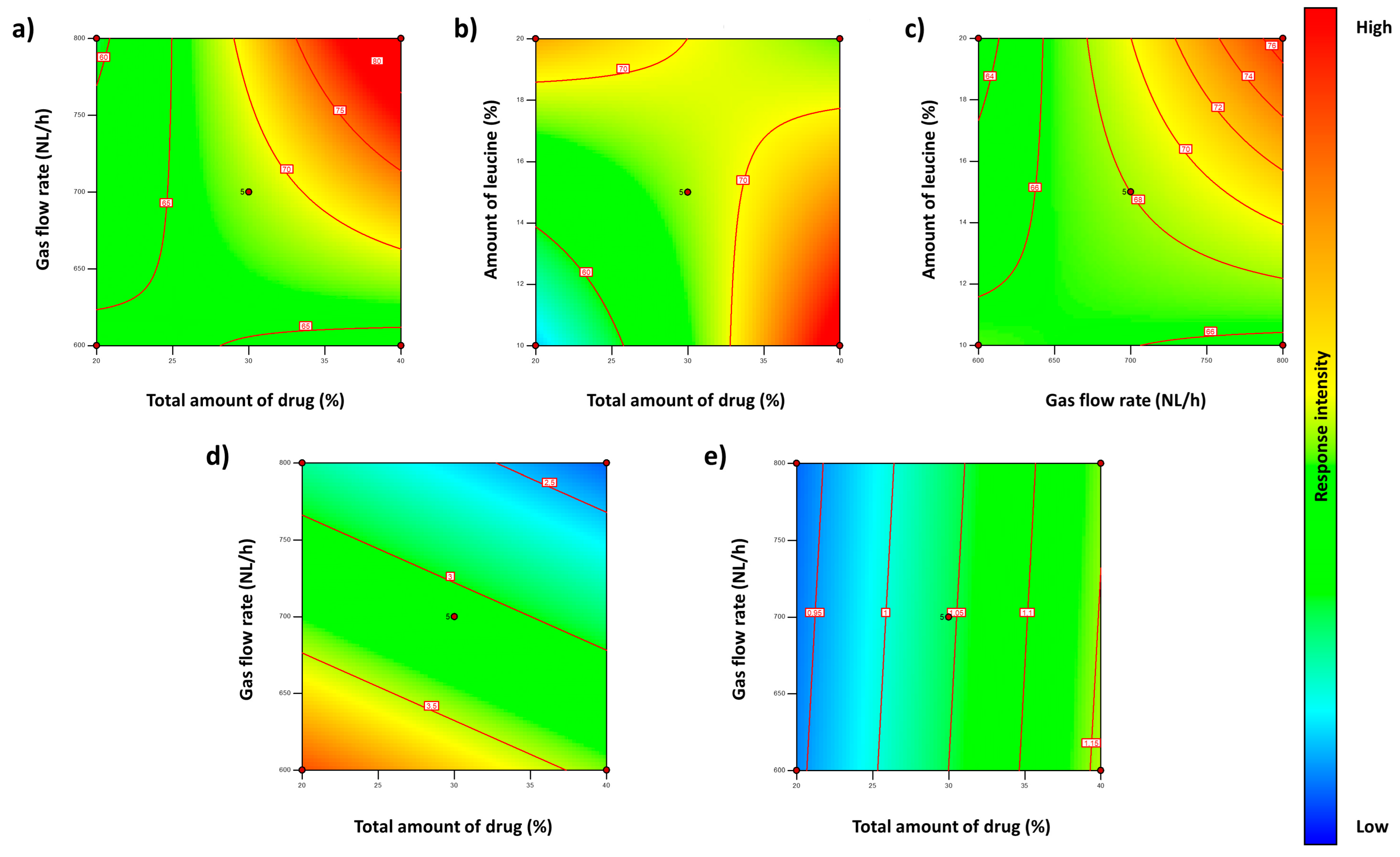
3.1.2. Box–Behnken Design
3.2. Physicochemical Characterisation of AmB-ITR MPs
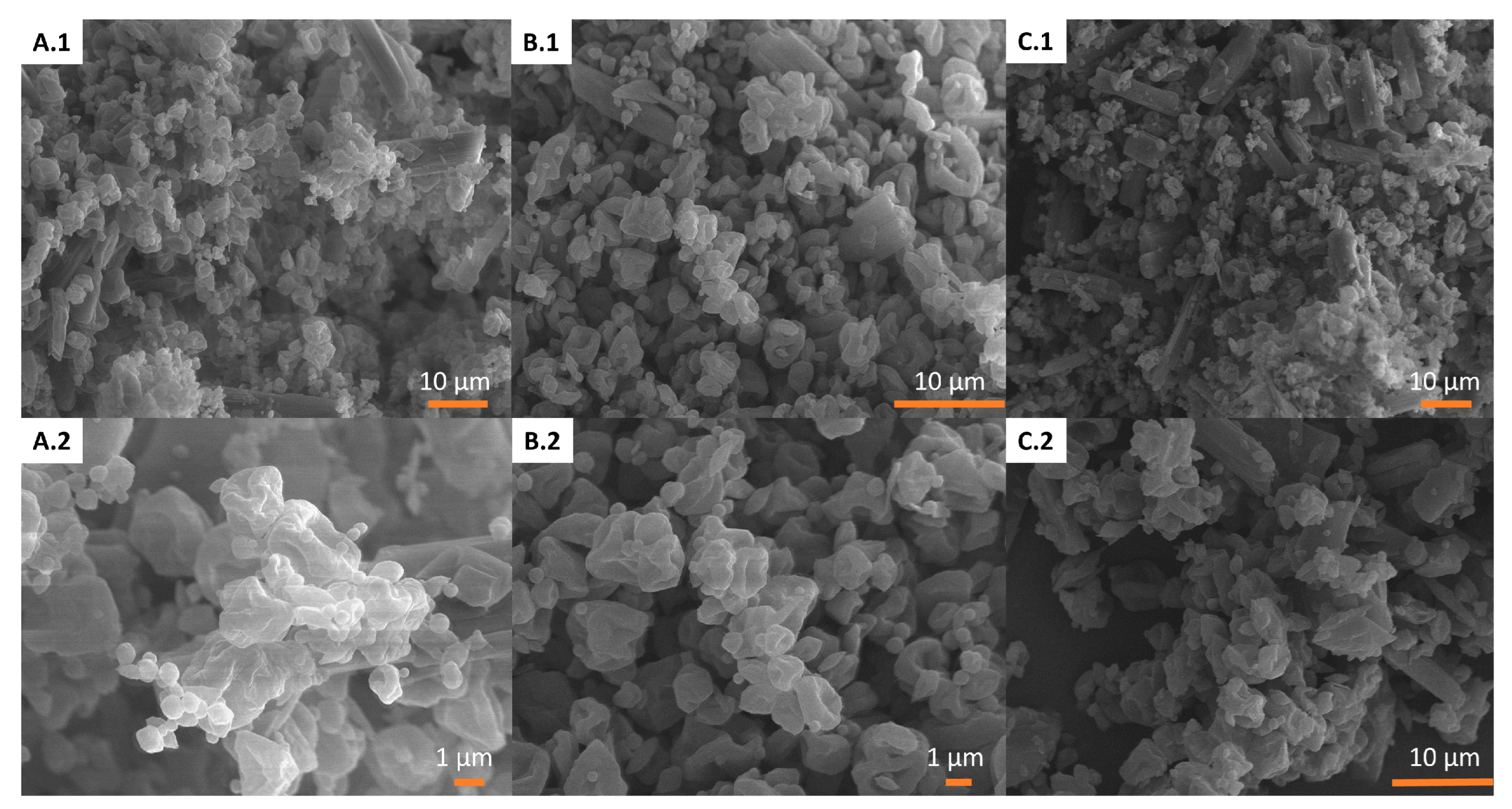
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Modulated Temperature Differential Scanning Calorimetry (MT-DSC)
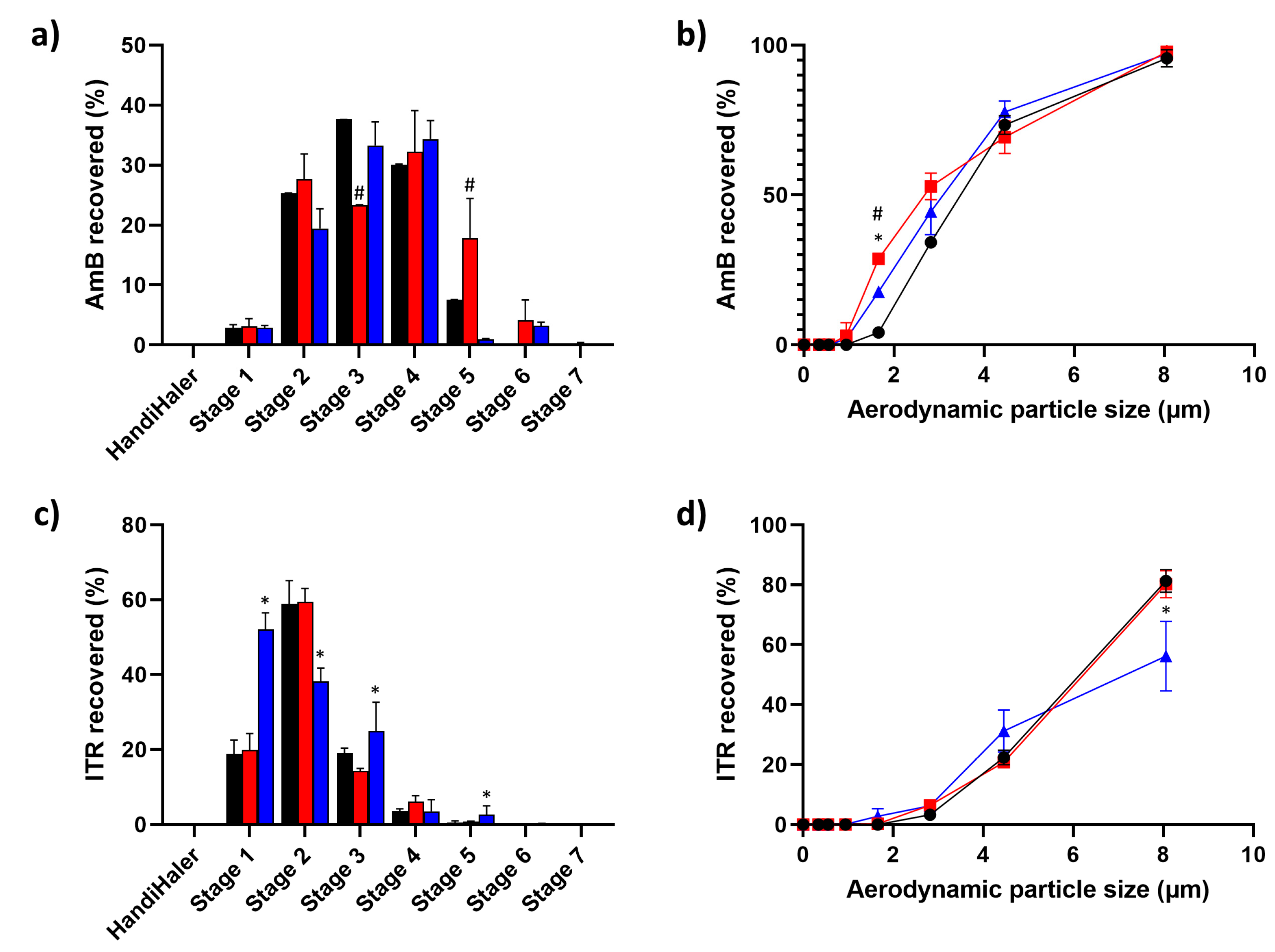
3.3. Mass Median Aerodynamic Diameter (MMAD) and Fine Particle Fraction (FPF)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.A.; Kumar, D.; Bolás, F.; Ballesteros, M.P.; et al. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm. 2023, 635, 122788. [Google Scholar] [CrossRef]
- Farahani, A.; Ghiasvand, F.; Davoudi, S.; Ahmadinejad, Z. Invasive aspergillosis in liver transplant recipients, an infectious complication with low incidence but significant mortality. World J. Transpl. 2023, 13, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Glampedakis, E.; Boillat-Blanco, N.; Oddo, M.; Pagani, J.L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1706–1708. [Google Scholar] [CrossRef]
- Rouze, A.; Lemaitre, E.; Nseir, S. COVID-19-associated invasive pulmonary aspergillosis: High incidence or difficult diagnosis? Intensive Care Med. 2021, 47, 1337–1338. [Google Scholar] [CrossRef] [PubMed]
- Curbelo, J.; Galván, J.M.; Aspa, J. Actualización sobre Aspergillus, Pneumocystis y otras micosis pulmonares oportunistas. Arch. Bronconeumol. 2015, 51, 647–653. [Google Scholar] [CrossRef]
- Henao-Martinez, A.F.; Corbisiero, M.F.; Salter, I.; Chastain, D.B.; Thompson, G.R. Invasive pulmonary aspergillosis real-world outcomes: Clinical features and risk factors associated with increased mortality. Med. Mycol. 2023, 61, myad074. [Google Scholar] [CrossRef]
- Fernández-García, R.; de Pablo, E.; Ballesteros, M.P.; Serrano, D.R. Unmet clinical needs in the treatment of systemic fungal infections: The role of amphotericin B and drug targeting. Int. J. Pharm. 2017, 525, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Witt, M.D.; Beck, C.K. Amphotericin B: Is a lipid-formulation gold standard feasible? Clin. Infect. Dis. 2004, 38, 304–305, author reply 306–307. [Google Scholar] [CrossRef][Green Version]
- Janik, S.; Grela, E.; Staczek, S.; Zdybicka-Barabas, A.; Luchowski, R.; Gruszecki, W.I.; Grudzinski, W. Amphotericin B-Silver Hybrid Nanoparticles Help to Unveil the Mechanism of Biological Activity of the Antibiotic: Disintegration of Cell Membranes. Molecules 2023, 28, 4687. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a new “gold standard”. Clin. Infect. Dis. 2003, 37, 415–425. [Google Scholar] [CrossRef]
- Fakhim, H.; Badali, H.; Dannaoui, E.; Nasirian, M.; Jahangiri, F.; Raei, M.; Vaseghi, N.; Ahmadikia, K.; Vaezi, A. Trends in the Prevalence of Amphotericin B-Resistance (AmBR) among Clinical Isolates of Aspergillus Species. J. Mycol. Med. 2022, 32, 101310. [Google Scholar] [CrossRef]
- Chen, M.M.; Shi, G.H.; Dai, Y.; Fang, W.X.; Wu, Q. Identifying genetic variants associated with amphotericin B (AMB) resistance in Aspergillus fumigatus via k-mer-based GWAS. Front. Genet. 2023, 14, 1133593. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, R.; Prada, M.; Bolas-Fernandez, F.; Ballesteros, M.P.; Serrano, D.R. Oral Fixed-Dose Combination Pharmaceutical Products: Industrial Manufacturing Versus Personalized 3D Printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Duconge, J.; Ruano, G. Fixed-dose combination products and unintended drug interactions: Urgent need for pharmacogenetic evaluation. Pharmacogenomics 2015, 16, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Ferreira, A.; Santos, J.; Lapa, R.; Yliperttula, M.; Urtti, A.; Vale, N. New In Vitro-In Silico Approach for the Prediction of In Vivo Performance of Drug Combinations. Molecules 2021, 26, 4257. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; Walsh, D.; O’Connell, P.; Slowing, K.; Raposo, R.; Paloma Ballesteros, M.; Jiménez-Cebrián, A.; Chamorro-Sancho, M.J.; Bolás-Fernández, F.; Healy, A.M.; et al. Can amphotericin B and itraconazole be co-delivered orally? Tailoring oral fixed-dose combination coated granules for systemic mycoses. Eur. J. Pharm. Biopharm. 2023, 183, 74–91. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.G.; Castanheira, M. Susceptibility patterns of amphotericin B, itraconazole, posaconazole, voriconazole and caspofungin for isolates causing invasive mould infections from the SENTRY Antifungal Surveillance Program (2018–2021) and application of single-site epidemiological cutoff values to evaluate amphotericin B activity. Mycoses 2023, 66, 854–868. [Google Scholar] [CrossRef]
- Karashima, M.; Sano, N.; Yamamoto, S.; Arai, Y.; Yamamoto, K.; Amano, N.; Ikeda, Y. Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations. Eur. J. Pharm. Biopharm. 2017, 115, 65–72. [Google Scholar] [CrossRef]
- Newman, S.P. AEROSOLS. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 58–64. [Google Scholar]
- Smith, C.; Goldman, R.D. Nebulizers versus pressurized metered-dose inhalers in preschool children with wheezing. Can. Fam. Physician 2012, 58, 528–530. [Google Scholar]
- Zhong, Q. Co-Spray Dried Mannitol/Poly(amidoamine)-Doxorubicin Dry-Powder Inhaler Formulations for Lung Adenocarcinoma: Morphology, In Vitro Evaluation, and Aerodynamic Performance. AAPS PharmSciTech 2018, 19, 531–540. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Bhoop, B.S.; Katare, O.P. Alginate coated chitosan microparticles mediated oral delivery of diphtheria toxoid. (Part A). Systematic optimization, development and characterization. Int. J. Pharm. 2015, 495, 220–233. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Varaticeanu, C.; Senila, M.; Roman, C. Optimization of Lipid Extraction from Spirulina spp. by Ultrasound Application and Mechanical Stirring Using the Taguchi Method of Experimental Design. Molecules 2022, 27, 6794. [Google Scholar] [CrossRef]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef]
- Fittler, A.; Kocsis, B.; Gerlinger, I.; Botz, L. Optimization of bioassay method for the quantitative microbiological determination of amphotericin B. Mycoses 2010, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Ballesteros, M.P.; Schätzlein, A.G.; Torrado, J.J.; Uchegbu, I.F. Amphotericin B formulations—The possibility of generic competition. Pharm. Nanotechnol. 2013, 1, 250–258. [Google Scholar] [CrossRef]
- Sipos, B.; Benei, M.; Katona, G.; Csoka, I. Optimization and Characterization of Sodium Alginate Beads Providing Extended Release for Antidiabetic Drugs. Molecules 2023, 28, 6980. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, W.; Chen, G.; Li, J.; Dou, G.; Gan, H.; Han, P.; Du, L.; Gu, R. Cepharanthine Dry Powder Inhaler for the Treatment of Acute Lung Injury. Molecules 2023, 28, 4441. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Li, X.; Vogt, F.G.; Hayes, D.; Zwischenberger, J.B.; Park, E.-S.; Mansour, H.M. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int. J. Pharm. 2013, 455, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Pujol, I.; Pastor, F.J.; dos Santos Lazéra, M.; Guarro, J. Evaluation of the Neo-Sensitabs diffusion method for determining the antifungal susceptibilities of Cryptococcus gattii isolates, using three different agar media. Rev. Iberoam. Micol. 2008, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; Muñoz-García, J.C.; Wallace, M.; Fabian, L.; González-Burgos, E.; Gómez-Serranillos, M.P.; Raposo, R.; Bolás-Fernández, F.; Ballesteros, M.P.; Healy, A.M.; et al. Self-assembling, supramolecular chemistry and pharmacology of amphotericin B: Poly-aggregates, oligomers and monomers. J. Control. Release 2022, 341, 716–732. [Google Scholar] [CrossRef]
- Campbell, H.R.; Alsharif, F.M.; Marsac, P.J.; Lodder, R.A. The Development of a Novel Pharmaceutical Formulation of D-Tagatose for Spray-Drying. J. Pharm. Innov. 2022, 17, 194–206. [Google Scholar] [CrossRef]
- Poozesh, S.; Bilgili, E. Scale-up of pharmaceutical spray drying using scale-up rules: A review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar] [CrossRef]
- Thybo, P.; Hovgaard, L.; Lindelov, J.S.; Brask, A.; Andersen, S.K. Scaling up the spray drying process from pilot to production scale using an atomized droplet size criterion. Pharm. Res. 2008, 25, 1610–1620. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Pan, P.; Huang, Y.; Chen, T.; Yuan, T.; Ma, Y.; Han, G.; Li, J.; Jin, Y.; et al. Pulmonary delivery of resveratrol-beta-cyclodextrin inclusion complexes for the prevention of zinc chloride smoke-induced acute lung injury. Drug Deliv. 2022, 29, 1122–1131. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Griesser, U.; Walzel, P.; Urbanetz, N.A. Spray Drying of Mannitol as a Drug Carrier—The Impact of Process Parameters on Product Properties. Dry. Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Wang, G.; Zhao, Z.; Jiang, Z.; Cui, Y.; Yue, X.; Huang, Z.; Huang, Y.; Pan, X.; et al. Co-spray-dried poly-L-lysine with L-leucine as dry powder inhalations for the treatment of pulmonary infection: Moisture-resistance and desirable aerosolization performance. Int. J. Pharm. 2022, 624, 122011. [Google Scholar] [CrossRef]
- Thiyagarajan, D.; Huck, B.; Nothdurft, B.; Koch, M.; Rudolph, D.; Rutschmann, M.; Feldmann, C.; Hozsa, C.; Furch, M.; Besecke, K.F.W.; et al. Spray-dried lactose-leucine microparticles for pulmonary delivery of antimycobacterial nanopharmaceuticals. Drug Deliv. Transl. Res. 2021, 11, 1766–1778. [Google Scholar] [CrossRef]
- Prota, L.; Santoro, A.; Bifulco, M.; Aquino, R.P.; Mencherini, T.; Russo, P. Leucine enhances aerosol performance of naringin dry powder and its activity on cystic fibrosis airway epithelial cells. Int. J. Pharm. 2011, 412, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Cuoco, A.; Eriksen, J.B.; Luppi, B.; Brandl, M.; Brandl, A.B. When Interactions Between Bile Salts and Cyclodextrin Cause a Negative Food Effect: Dynamic Dissolution/Permeation Studies with Itraconazole (Sporanox®) and Biomimetic Media. J. Pharm. Sci. 2023, 112, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Ordoubadi, M.; Shepard, K.B.; Wang, H.; Wang, Z.; Pluntze, A.M.; Churchman, J.P.; Vehring, R. On the Physical Stability of Leucine-Containing Spray-Dried Powders for Respiratory Drug Delivery. Pharmaceutics 2023, 15, 435. [Google Scholar] [CrossRef]
- Barac, A.; Ong, D.S.Y.; Jovancevic, L.; Peric, A.; Surda, P.; Tomic Spiric, V.; Rubino, S. Fungi-Induced Upper and Lower Respiratory Tract Allergic Diseases: One Entity. Front. Microbiol. 2018, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour Aghdam, M.; Ghanbarzadeh, S.; Javadzadeh, Y.; Hamishehkar, H. Aggregated Nanotransfersomal Dry Powder Inhalation of Itraconazole for Pulmonary Drug Delivery. Adv. Pharm. Bull. 2016, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Elefanti, A.; Mouton, J.W.; Verweij, P.E.; Tsakris, A.; Zerva, L.; Meletiadis, J. Amphotericin B- and voriconazole-echinocandin combinations against Aspergillus spp.: Effect of serum on inhibitory and fungicidal interactions. Antimicrob. Agents Chemother. 2013, 57, 4656–4663. [Google Scholar] [CrossRef]
- Rambach, G.; Striednig, B.; Neurauter, M.; Hermann, M.; Wurzner, R.; Lass-Florl, C.; Speth, C. Indications that the Antimycotic Drug Amphotericin B Enhances the Impact of Platelets on Aspergillus. Antimicrob. Agents Chemother. 2022, 66, e0068122. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wang, X.; Wang, Z. Effect of Amphotericin B on the Thermodynamic Properties and Surface Morphology of the Pulmonary Surfactant Model Monolayer during Respiration. Molecules 2023, 28, 4840. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy-A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef]

| Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 7 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AmB (%) | ITR (%) | γ-CD (%) | Leucine (%) | Inlet Temperature (°C) | Solution Feed Rate (%) | Gas Flow Rate (NL/h) | Yield (%) | Particle Size, D50 (µm) | Inhibition Halo (mm) | AmB Encapsulation Efficiency (%) | Aggregation Ratio (Dimer/Monomer) | |
| 1 | 5 | 0 | 5 | 5 | 120 | 5 | 400 | 26.56 | 4.87 | 25.12 | 76.97 | 0.72 |
| 2 | 10 | 10 | 5 | 15 | 120 | 10 | 400 | 25.46 | 5.76 | 26.62 | 187.28 | 1.33 |
| 3 | 10 | 10 | 5 | 5 | 150 | 5 | 800 | 64.92 | 2.86 | 24.51 | 53.77 | 0.77 |
| 4 | 10 | 0 | 10 | 15 | 120 | 5 | 800 | 49.80 | 2.49 | 26.45 | 77.01 | 1.05 |
| 5 | 5 | 10 | 10 | 5 | 120 | 10 | 800 | 12.96 | 6.33 | 26.60 | 70.73 | 0.62 |
| 6 | 5 | 0 | 5 | 15 | 150 | 10 | 800 | 71.32 | 2.52 | 26.09 | 80.48 | 0.81 |
| 7 | 10 | 0 | 10 | 5 | 150 | 10 | 400 | 8.58 | 4.67 | 22.60 | 86.40 | 0.82 |
| 8 | 5 | 10 | 10 | 15 | 150 | 5 | 400 | 65.00 | 3.34 | 23.14 | 76.61 | 0.76 |
| Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 | Response 6 |
|---|---|---|---|---|---|---|---|---|---|
| Total Amount of Drug (%) | Gas Flow Rate (NL/h) | Amount of Leucine (%) | Yield (%) | Particle Size, D50 (µm) | Efficacy (%) | AmB Encapsulation Efficiency (%) | ITR Encapsulation Efficiency (%) | Aggregation Ratio (Dimer/Monomer) | |
| 1 | 20 | 700 | 10 | 44.56 | 4.08 | 94.19 | 65.18 | 86.39 | 0.892 |
| 2 | 30 | 700 | 15 | 74.72 | 3.29 | 91.81 | 86.97 | 95.94 | 0.939 |
| 3 | 40 | 700 | 10 | 74.48 | 3.16 | 93.75 | 55.95 | 96.31 | 1.161 |
| 4 | 30 | 800 | 20 | 72.68 | 2.21 | 92.38 | 88.62 | 87.01 | 1.120 |
| 5 | 40 | 700 | 20 | 71.22 | 2.44 | 91.61 | 100.27 | 92.01 | 1.183 |
| 6 | 20 | 600 | 15 | 62.22 | 3.63 | 98.75 | 91.86 | 92.01 | 1.131 |
| 7 | 30 | 700 | 15 | 68.14 | 2.40 | 96.89 | 87.95 | 95.15 | 1.088 |
| 8 | 30 | 600 | 20 | 57.70 | 4.01 | 91.09 | 79.01 | 72.52 | 0.994 |
| 9 | 20 | 700 | 20 | 75.82 | 2.99 | 93.06 | 80.65 | 65.12 | 0.907 |
| 10 | 40 | 800 | 15 | 77.32 | 2.56 | 93.30 | 79.63 | 75.63 | 1.120 |
| 11 | 40 | 600 | 15 | 58.84 | 3.60 | 98.46 | 92.06 | 99.68 | 1.270 |
| 12 | 30 | 800 | 10 | 71.34 | 2.15 | 91.13 | 84.16 | 100.63 | 1.136 |
| 13 | 30 | 700 | 15 | 78.46 | 3.35 | 86.09 | 89.88 | 100 | 0.977 |
| 14 | 30 | 600 | 10 | 71.22 | 3.15 | 88.67 | 93.33 | 83.18 | 0.972 |
| 15 | 30 | 700 | 15 | 71.28 | 3.24 | 95.76 | 94.20 | 100 | 1.011 |
| 16 | 30 | 700 | 15 | 71.96 | 3.82 | 88.14 | 94.53 | 93.37 | 0.910 |
| 17 | 20 | 800 | 15 | 54.22 | 3.02 | 90.56 | 88.35 | 71.82 | 0.945 |
| Formulation | Total Amount of Drug (%) | Gas Flow Rate (NL/h) | Amount of Leucine (%) | Amount of γ-CD (%) | Amount of Mannitol (%) | Yield (%) | Particle Size, D50 (µm) | Efficacy (%) | AmB Encapsulation Efficiency (%) | ITR Encapsulation Efficiency (%) | Aggregation Ratio (Dimer/Monomer) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 20 | 800 | 20 | 20 | 40 | 78.54 (57.26–88.34) | 3.05 (1.98–3.05) | 100 (90.89–94.47) | 82.66 (78.25–96.30) | 82.81 (85.49–107.12) | 0.924 (0.87–1.05) |
| F2 | 30 | 768 | 20 | 30 | 20 | 69.56 (65.24–82.84) | 3.19 (2.08–2.91) | 94.89 (90.89–94.47) | 88.14 (81.08–91.63) | 93.06 (90.16–101.38) | 0.909 (0.990–1.120) |
| F3 | 40 | 667 | 10 | 40 | 10 | 69.20 (66.02–88.62) | 3.45 (2.89–3.87) | 90.00 (90.89–94.47) | 87.82 (77.45–106.80) | 83.62 (90.73–103.30) | 0.911 (1.03–1.19) |
| Formulation | Drug | FPF < 5 µm (%) | FPF < 3 µm (%) | MMAD (µm) |
|---|---|---|---|---|
| F1 | AmB | 81.60 ± 0.05 | 43.07 ± 0.04 | 3.39 ± 0.01 |
| ITR | 42.24 ± 11.33 | 12.91 ± 3.01 | 5.80 ± 0.07 | |
| F2 | AmB | 91.17 ± 1.14 | 43.48 ± 4.29 | 3.03 ± 0.07 |
| ITR | 25.78 ± 0.64 | 8.43 ± 0.01 | 5.77 ± 0.31 | |
| F3 | AmB | 67.59 ± 8.12 | 35.71 ± 8.09 | 3.04 ± 0.35 |
| ITR | 38.63 ± 9.53 | 15.15 ± 4.99 | 6.79 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celi, S.S.; Fernández-García, R.; Afonso-Urich, A.I.; Ballesteros, M.P.; Healy, A.M.; Serrano, D.R. Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections. Pharmaceutics 2023, 15, 2601. https://doi.org/10.3390/pharmaceutics15112601
Celi SS, Fernández-García R, Afonso-Urich AI, Ballesteros MP, Healy AM, Serrano DR. Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections. Pharmaceutics. 2023; 15(11):2601. https://doi.org/10.3390/pharmaceutics15112601
Chicago/Turabian StyleCeli, Salomé S., Raquel Fernández-García, Andreina I. Afonso-Urich, M. Paloma Ballesteros, Anne Marie Healy, and Dolores R. Serrano. 2023. "Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections" Pharmaceutics 15, no. 11: 2601. https://doi.org/10.3390/pharmaceutics15112601
APA StyleCeli, S. S., Fernández-García, R., Afonso-Urich, A. I., Ballesteros, M. P., Healy, A. M., & Serrano, D. R. (2023). Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections. Pharmaceutics, 15(11), 2601. https://doi.org/10.3390/pharmaceutics15112601