Antifungal Activities of Natural Products and Their Hybrid Molecules
Abstract
:1. Introduction
| Natural Products | Mode of Antifungal Activity | Bibliography |
|---|---|---|
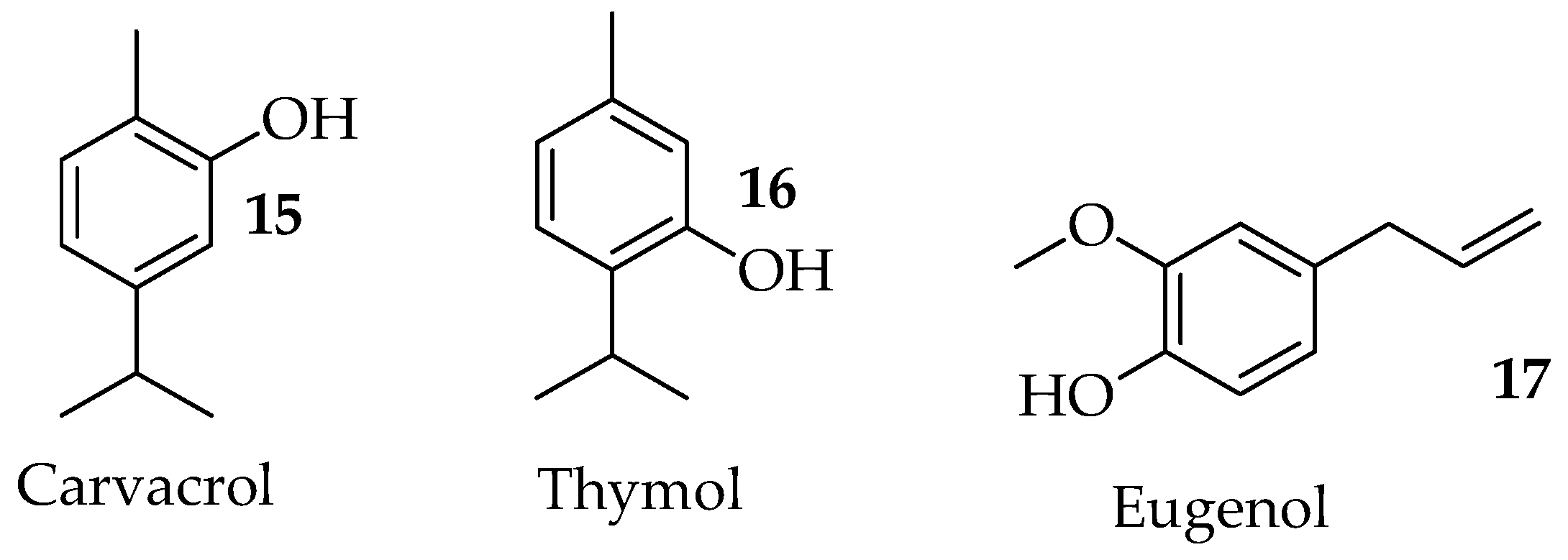
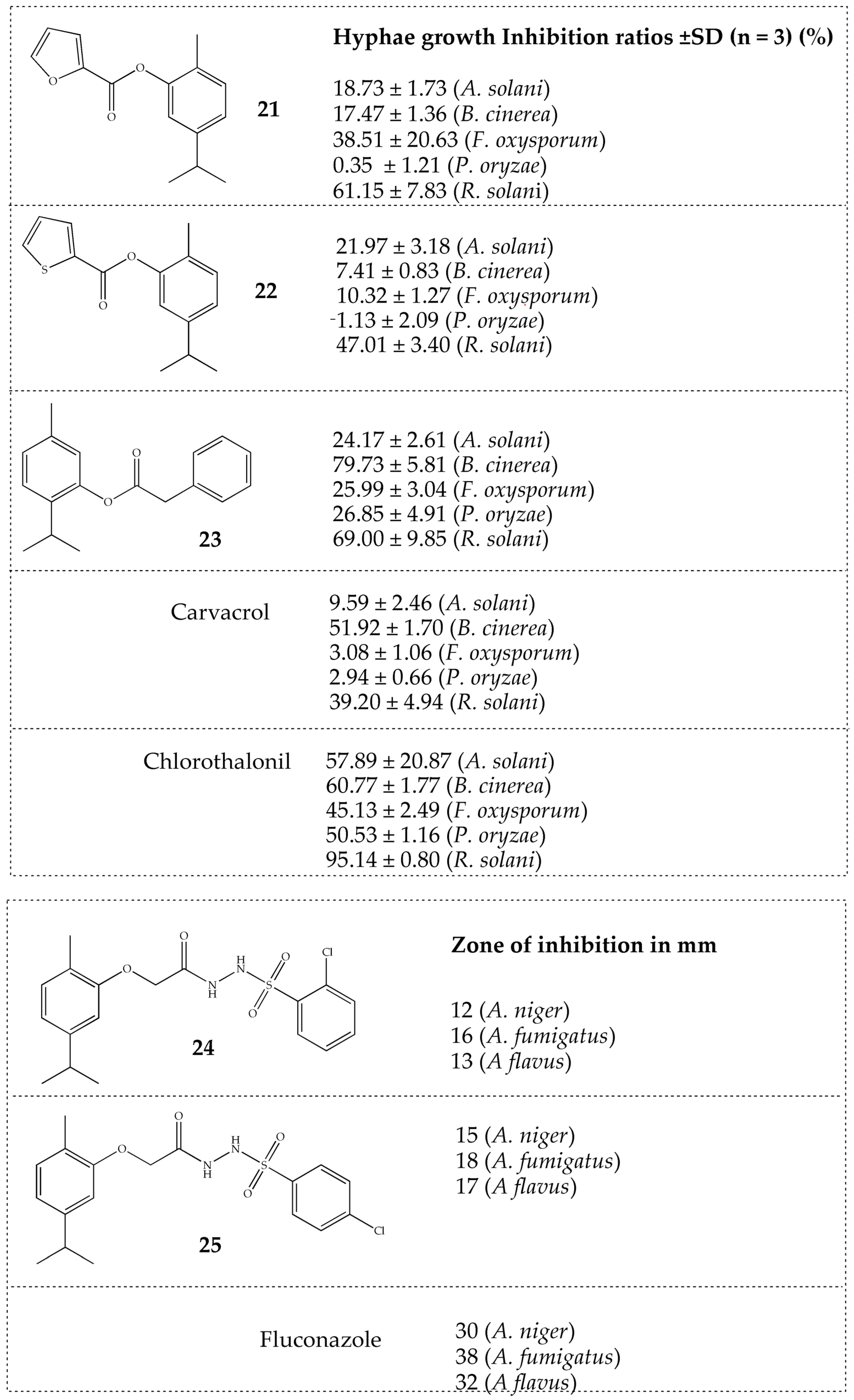
| Carvacrol | Alters the membrane structure of the fungal cell in C. albicans. Disrupts ergosterol biosynthesis and membrane integrity against Candida species. Reduces spore germination, mycelia growth, aflatoxin production, and the ergosterol content of A. flavus. Binds to exogenous ergosterol, promoting interaction with cholesterol in Cryptococcus neoformans. Modulates the expression and activity of antioxidant enzymes in Candida auris. | [32,33,34,35,36] |
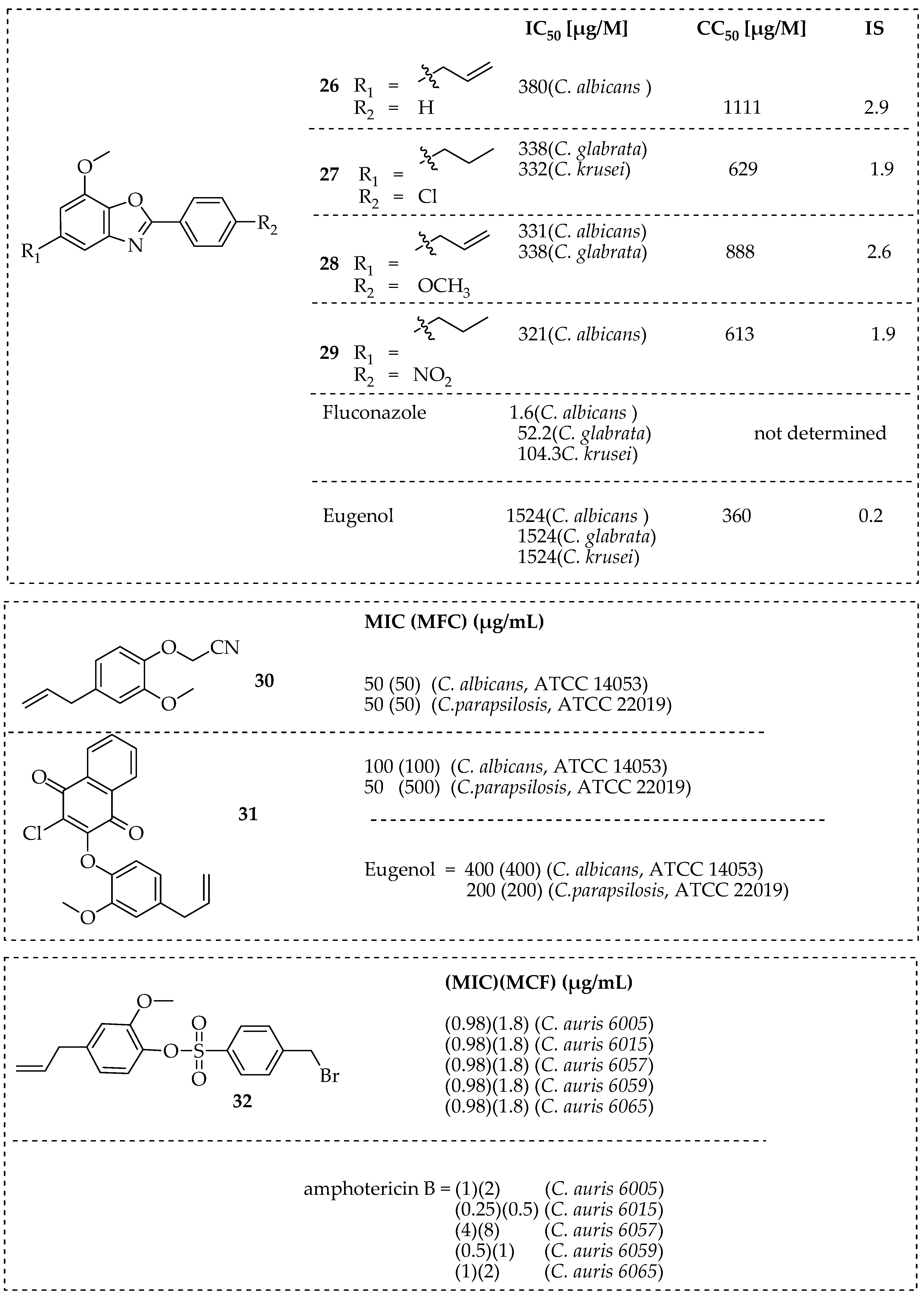
| Eugenol | Exerts antifungal effects on the cell wall and cell membrane of Trichophyton rubrum by inhibiting ergosterol biosynthesis. | [37] |
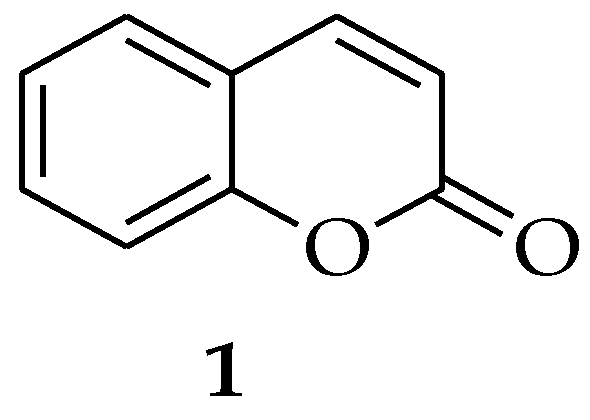
| Coumarin | Induces a series of apoptotic features in C. albicans, including phosphatidylserine externalization, DNA fragmentation, and nuclear condensation. | [14] |
| Cinnamaldehyde | Inhibited germ tube formation, adhesion to epithelial cells, and hydrolytic enzyme secretion of C. albicans. Affects the ergosterol biosynthetic processes and leads to the disruption of the cell membrane integrity of Fusarium sambucinum. Exhibit its antifungal activity against G. citri-aurantii by interfering with the formation of cell walls, resulting in the damage of cell wall permeability and integrity | [38,39] |
| Curcumin | Exerts antifungal activity via disrupting fungal plasma membrane in C. albicans. Alters the membrane-associated properties of ATPase activity, ergosterol biosynthesis, and proteinase secretion in C. albicans and Candida glabrata. Exerts fungal cell membrane disruption and the inhibition of ergosterol synthesis, respiration, succinate dehydrogenase, and NADH oxidase. Induces reactive oxygen species and triggers early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans | [40,41,42,43] |
| Thymol | Affects intracellular calcium homeostasis by suppressing the expression of genes involved in the calcium transporters. Decreases expression levels of genes required for N-glycosylation, thereby reducing protein glycosylation. Decreases ergosterol contents in a HOG pathway-dependent manner in Cryptococcus neoformans. Induces Lipid Peroxidation and disrupts Ergosterol Biosynthesis in Fusarium graminearum. Produces reactive oxygen species (ROS) accumulation and destroys the integrity of the cell wall and cell membrane by inhibiting the genes involved in the cell wall and cell membrane synthesis. | [44,45,46] |
2. Natural Products
2.1. Coumarin
2.2. Essential Oils
2.2.1. Terpenoids
2.2.2. Carvacrol and Thymol
2.2.3. Eugenol
2.3. Cinnamaldehyde
2.4. Curcumin
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.E.; Novak-Frazer, L. Chronic pulmonary aspergillosis—Where are we? And where are we going? J. Fungi 2016, 2, 18. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Spencer, A.C.; Brubaker, K.R.; Garneau-Tsodikova, S. Systemic fungal infections: A pharmacist/researcher perspective. Fungal Biol. Rev. 2023, 44, 100293. [Google Scholar] [CrossRef]
- WHO. WHO Releases First-Ever List of Health-Threatening Fungi; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/news/item/25-10-2022-who-releases-first-ever-list-of-health-threatening-fungi (accessed on 1 September 2023).
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Sakagami, T.; Kawano, T.; Yamashita, K.; Yamada, E.; Fujino, N.; Kaeriyama, M.; Fukuda, Y.; Nomura, N.; Mitsuyama, J.; Suematsu, H.; et al. Antifungal susceptibility trend and analysis of resistance mechanism for Candida species isolated from bloodstream at a Japanese university hospital. J. Infect. Chemother. 2019, 25, 34–40. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef]
- Marak, M.B.; Dhanashree, B. Antifungal susceptibility and biofilm production of Candida spp. Isolated from clinical samples. Int. J. Microbiol. 2018, 2018, 7495218. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mitchell, A.P. Mucosal biofilms of Candida albicans. Curr. Opin Microbiol. 2011, 14, 380–385. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, J.; Yu, L.; Wang, C.; Yang, Y.; Rong, X.; Xu, K.; Chu, M. Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front Cell Infect. Microbiol. 2019, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Rybak, J.M.; Cuomo, C.A.; Rogers, D.P. The molecular and genetic basis of antifungal resistance in the emerging fungal pathogen Candida auris. Curr. Opin. Microbiol. 2022, 70, 102208. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive fungal infections complicating COVID-19: A narrative review. J. Fungi 2021, 7, 921. [Google Scholar] [CrossRef]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants Metabolites: Possibility of Natural Therapeutics Against the COVID-19 Pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef]
- Karagöz, A.Ç.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Dussart, F. Selected fungal natural products with antimicrobial properties. Molecules 2020, 25, 911. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Z.; Zhang, J.; Yang, L. Antifungal Compounds against Candida Infections from Traditional Chinese Medicine. Biomed. Res. Int. 2017, 2017, 4614183. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.F.; et al. New perspectives on how to discover drugs from herbal medicines: CAM’S outstanding contribution to modern therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.V.; Rana, A.; Bhagat, K.; Gulati, H.K.; Kumar, R.; Singh, H.; Sharma, S.; Bedi, P.M.S. Monocarbonyl curcumin based molecular hybrids as potent antibacterial agents. ACS Omega 2019, 4, 11673–11684. [Google Scholar] [CrossRef] [PubMed]
- Koračak, L.; Lupšić, E.; Jovanović, N.T.; Jovanović, M.; Novakovic, M.; Nedialkov, P.; Trendafilova, A.; Zlatovic´, M.; Pešić, M.; Opsenica, I.M. Novel artesunate-pyrimidine-based hybrids with anticancer potential against multidrug-resistant cancer cells. New J. Chem. 2023, 47, 6844–6855. [Google Scholar] [CrossRef]
- Wang, L.; Switalska, M.; Wang, N.; Du, Z.J.; Fukumoto, Y.; Diep, N.K.; Kiguchi, R.; Nokami, J.; JWietrzyk, J.; Inokuchi, T. Design, synthesis, and biological evaluation of artemisinin-indoloquinoline hybrids as potent antiproliferative agents. Molecules 2014, 19, 19021–19035. [Google Scholar] [CrossRef]
- Horwedel, C.; Tsogoeva, S.B.; Wei, S.; Efferth, T. Cytotoxicity of artesunic acid homo- and heterodimer molecules toward sensitive and multidrug-resistant CCRF-CEM leukemia cells. J. Med. Chem. 2010, 53, 4842–4848. [Google Scholar] [CrossRef]
- Reiter, C.; Herrmann, A.; Çapci, A.; Efferth, T.; Tsogoeva, S.B. New artesunic acid homodimers: Potent reversal agents of multidrug resistance in leukemia cells. Bioorg. Med. Chem. 2012, 20, 5637–5641. [Google Scholar] [CrossRef]
- Reiter, C.; Fröhlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtländer, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef]
- Reiter, C.; Çapci, K.A.; Fröhlich, T.; Klein, V.; Zeino, M.; Viertel, K.; Held, J.; Mordmüller, B.; Öztürk, S.E.; Anıl, H.; et al. Synthesis and study of cytotoxic activity of 1,2,4-trioxane- and egonol-derived hybrid molecules against Plasmodium falciparum and multidrug-resistant human leukemia cells. Eur. J. Med. Chem. 2014, 75, 403–4012. [Google Scholar] [CrossRef]
- Lima, I.O.; De Oliveira, P.F.; De Oliveira, W.A.; De Oliveira, L.E.; Menezes, E.A.; Cunha, F.A.; Diniz, M.D.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-Based Untargeted Lipidomics Reveal Mechanism of Antifungal Activity of Carvacrol against Aspergillus flavus. Foods 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, R.D.O.; Teixeira, A.P.D.C.; Oliveira, W.A.D.; Lima, E.D.O.; Lima, I.O. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm. Biol. 2016, 54, 2591–2596. [Google Scholar] [CrossRef]
- Ismail, M.; Srivastava, V.; Marimani, M.; Ahmad, A. Carvacrol modulates the expression and activity of antioxidant enzymes in Candida auris. Res. Microbiol. 2022, 173, 103916. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Pereira, F.; Mendes, J.M.; De Oliveira Lima, E. Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med. Mycol. 2013, 51, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Pootong, A.; Norrapong, B.; Cowawintaweewat, S. Antifungal activity of cinnamaldehyde against Candida albicans. Southeast Asian J. Trop. Med. Public Health 2017, 48, 150–158. [Google Scholar]
- OuYang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. An antifungal mechanism of curcumin lies in membrane-targeted action within Candida albicans. IUBMB Life 2014, 66, 780–785. [Google Scholar] [CrossRef]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Puri, N.; Prasad, R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 2010, 30, 391–404. [Google Scholar] [CrossRef]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Chung, M.S.; Bai, H.W.; Chung, B.Y.; Lee, S. Investigation of antifungal mechanisms of thymol in the human fungal pathogen, Cryptococcus neoformans. Molecules 2021, 26, 3476. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ge, J.; Yu, X. Transcriptome Analysis Reveals the Mechanism of Fungicidal of Thymol Against Fusarium oxysporum f. sp. niveum. Curr. Microbiol. 2018, 75, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Salci, T.P.; Shinobu-Mesquita, C.S.; Capoci, I.R.G.; Svidzinski, T.I.E.; Kioshima, E.S. Early state research on antifungal natural products. Molecules 2014, 19, 2925–2956. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, J.L.; Chu, M.P.; Jia, C. Activity of coumarin against Candida albicans biofilms. J. Mycol. Med. 2019, 29, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Poyraz, S.; Ersatir, M. Recent advances on biologically active coumarin-based hybrid compounds. Med. Chem. Res. 2023, 32, 617–642. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, D.; Wu, Z.; Wei, C.; Wu, S.; Song, B. Coumarin Derivatives Containing Sulfonamide and Dithioacetal Moieties: Design, Synthesis, Antiviral Activity, and Mechanism. J. Agric. Food Chem. 2022, 70, 5773–5783. [Google Scholar] [CrossRef]
- Shen, Y.F.; Liu, L.; Feng, C.Z.; Hu, Y.; Chen, C.; Wang, G.X.; Zhu, B. Synthesis and antiviral activity of a new coumarin derivative against spring viraemia of carp virus. Fish Shellfish Immunol. 2018, 81, 57–66. [Google Scholar] [CrossRef]
- Liang, H.; Shi, Y.; Zeng, K.; Zhao, M.; Tu, P.; Jiang, Y. Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their anti-inflammatory activities. Phytochemistry 2020, 177, 112416. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Peng, T.; Wen, X.; Wang, G.; Liu, S.; Sun, Y.; Shouguo Zhang, S.; Wang, L. Design, synthesis and evaluation of 3-substituted coumarin derivatives as anti-inflammatory agents. Chem. Pharm. Bull. 2020, 68, 443–446. [Google Scholar] [CrossRef]
- Martin, A.L.A.R.; De Menezes, I.R.A.; Sousa, A.K.; Farias, P.A.M.; dos Santos, F.A.V.; Freitas, T.S.; Figueredo, F.G.; Ribeiro-Filho, J.R.; Carvalho, D.T.; Coutinho, H.D.M.; et al. In vitro and in silico antibacterial evaluation of coumarin derivatives against MDR strains of Staphylococcus aureus and Escherichia coli. Microb. Pathog. 2023, 177, 106058. [Google Scholar] [CrossRef]
- Rawat, A.; Vijaya Bhaskar Reddy, A. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Ipek, O.S.; Sucu, B.O.; Gul, S.; Yolacan, C.; Guzel, M. Synthesis of Novel Hybrid Lonidamine-Coumarin Derivatives and Their Anticancer Activities. J. Mol. Struct. 2023, 1281, 135114. [Google Scholar] [CrossRef]
- Kecel-Gunduz, S.; Budama-Kilinc, Y.; Bicak, B.; Gok, B.; Belmen, B.; Aydogan, F.; Yolacan, C. New coumarin derivative with potential antioxidant activity: Synthesis, DNA binding and in silico studies (Docking, MD, ADMET). Arab. J. Chem. 2023, 16, 104440. [Google Scholar] [CrossRef]
- Smyth, T.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 2009, 33, 421–426. [Google Scholar] [CrossRef]
- Khan, M.S.; Agrawal, R.; Ubaidullah, M.; Hassan, M.I.; Tarannum, N. Design, synthesis and validation of anti-microbial coumarin derivatives: An efficient green approach. Heliyon 2019, 5, e02615. [Google Scholar] [CrossRef]
- Vanden, B.A.; McEwen, A.G.; Chebaro, Y.; Potier, N.; Lamour, V. Structural Basis for DNA Gyrase Interaction with Coumermycin A1. J. Med. Chem. 2019, 62, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, A.; Zhu, M.; Qi, X.; Tang, W.; Liu, M.; Lia, D.; Gu, Q. Penicisulfuranol A, a novel C-terminal inhibitor disrupting molecular chaperone function of Hsp90 independent of ATP binding domain. Biochem. Pharmacol. 2019, 163, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, X.; Liang, C.; Zhang, W. Design, Synthesis, and Antifungal Evaluation of Novel Coumarin- Pyrrole Hybrids. J. Heterocycl. Chem. 2021, 58, 450–458. [Google Scholar] [CrossRef]
- Sadgir, N.V.; Adole, V.A.; Dhonnar, S.L.; Jagdale, B.S. Synthesis and biological evaluation of coumarin appended thiazole hybrid heterocycles: Antibacterial and antifungal study. J. Mol. Struct. 2023, 1293, 136229. [Google Scholar] [CrossRef]
- Prachi, T.; Chodvadiya, V.; Shahrukhkhan, S.; Upadhyay, J. Synthesis and antifungal activity of some coumarin based 1, 2, 3-triazole derivatives. J. Adv. Sci. Res. 2020, 11, 132–137. [Google Scholar]
- Yang, G.; Shi, L.; Pan, Z.; Wu, L.; Fan, L.; Wang, C.; Xu, C.; Liang, J. The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities. Arab. J. Chem. 2021, 14, 102880. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Antifungal activities of new coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Ding, P. Physiological production of essential oil in plants—Ontogeny, secretory structures and seasonal variations. Pertanika J. Sch. Res. Rev. 2016, 2, 1–10. [Google Scholar]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.; Donadu, M.G.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J. Infect. Dev. Ctries. 2020, 14, 1054–1064. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R.; Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M. Essential Oil from Coriandrum sativum: A review on Its Phytochemistry and Biological Activity. Molecules 2023, 28, 696. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Irfan, S.; Kamil, M.; Sheikh, S.; Hasan, A.; Ahmad, A.; Lakshmi, V.; Nazir, A.; Mir, S.S. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiology 2016, 85, 436–443. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic–OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia. Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Mohan Karuppayil, S. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 2013, 29, 87–96. [Google Scholar] [CrossRef]
- Ali, T.; Majeed, S.T.; Majeed, R.; Bashir, R.; Mir, S.A.; Jan, I.; Bader, G.N.; Andrabi, K.I. Recent Advances in the Pharmacological Properties and Molecular Mechanisms of Carvacrol. Rev. Bras. Farmacogn. 2023. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Phyther. Res. 2007, 22, 544–549. [Google Scholar]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; et al. Carvacrol Induces Candida albicans Apoptosis Associated With Ca2+/Calcineurin Pathway. Front. Cell Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Jesus, F.P.K.; Ferreiro, L.; Bizzi, K.S.; Loreto, S.; Pilotto, M.B.; Ludwig, A.; Alves, S.H.; Zanette, R.A.; Santurio, J.M. In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. J. Mycol. Med. 2015, 25, 89–93. [Google Scholar] [CrossRef]
- Pete, U.D.; Zade, C.M.; Bhosale, J.D.; Tupe, S.G.; Chaudhary, P.M.; Dikundwar, A.G.; Bendre, R.S. Hybrid molecules of carvacrol and benzoyl urea/thiourea with potential applications in agriculture and medicine. Bioorg. Med. Chem. Lett. 2012, 22, 5550–5554. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Yang, Y.; Fan, L.; Su, F.; Ye, M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019, 33, 1924–1930. [Google Scholar] [CrossRef]
- Bagul, S.D.; Rajput, J.D.; Tadavi, S.K.; Bendre, R.S. Design, synthesis and biological activities of novel 5-isopropyl-2-methylphenolhydrazide-based sulfonamide derivatives. Res. Chem. Intermed. 2017, 43, 2241–2252. [Google Scholar] [CrossRef]
- Licata, M.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Rando, R.; Fede, M.R.; Virga, G.; Raffaele Leone, R.; et al. Study of quantitative and qualitative variations in essential oils of Sicilian oregano biotypes. J. Essent. Oil Res. 2015, 27, 293–306. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; Feo, V.D. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef]
- Sarwar, A.; Latif, Z. GC-MS characterisation and antibacterial activity evaluation of Nigella sativa oil against diverse strains of Salmonella. Nat. Prod. Res. 2015, 29, 447–451. [Google Scholar] [CrossRef]
- Doke, S.K.; Raut, J.S.; Dhawale, S.; Karuppayil, S.M. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J. Gen. Appl. Microbiol. 2014, 60, 163–168. [Google Scholar] [CrossRef]
- de Vasconcelos, L.C.; Sampaio, F.C.; de Jesus dos Reis Albuquerque, A.; de Souza Vasconcelos, L.C. Cell viability of Candida albicans against the antifungal activity of thymol. Braz. Dent. J. 2014, 25, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Sun, L.; Zhang, W. Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immunol. Res. 2016, 64, 1013–1024. [Google Scholar] [CrossRef]
- De Castro, R.D.; de Souza, T.M.P.A.; Bezerra, L.M.D.; Ferreira, G.L.S.; de Brito, C.E.M.M.; Cavalcanti, A.L. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef] [PubMed]
- Bound, D.J.; Murthy, P.S.; Srinivas, P. Synthesis and antibacterial properties of 2,3-dideoxyglucosides of terpene alcohols and phenols. Food Chem. 2015, 185, 192–199. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Mahapatra, M.; Paidesetty, S.K.; Padhy, R.N. Design, molecular docking, and antimicrobial assessment of newly synthesized phytochemical thymol Mannich base derivatives. J. Mol. Struct. 2021, 1244, 130908. [Google Scholar] [CrossRef]
- Natal, C.M.; Fernandes, M.J.G.; Pinto, N.F.S.; Pereira, R.B.; Vieira, T.F.; Rodrigues, A.R.O.; Pereira, D.M.; Sousa, S.F.; Fortes, A.G.; Castanheira, E.M.S.; et al. New carvacrol and thymol derivatives as potential insecticides: Synthesis, biological activity, computational studies and nanoencapsulation. RSC Adv. 2021, 11, 34024–34035. [Google Scholar] [CrossRef]
- Gaba, J.; Sharma, S.; Kaur, P. Preparation and Biological Evaluation of Thymol Functionalized 2-Pyrazoline and Dihydropyrimidinone Hybrids. Org. Prep. Proced. Int. 2022, 54, 312–323. [Google Scholar] [CrossRef]
- Mathela, C.S.; Singh, K.K.; Gupta, V.K. Synthesis and in vitro antibacterial activity of thymol and carvacrol derivatives. Acta Pol. Pharm. Drug Res. 2010, 67, 375–380. [Google Scholar]
- Koshti, S.M.; Sonar, J.P.; Sonawane, A.E.; Pawar, Y.A.; Nagle, P.S.; Mahulikar, P.P.; More, D.H. Synthesis of azo compounds containing thymol moiety. Indian J. Chem.-Sect. B Org. Med. Chem. 2008, 47, 329–331. [Google Scholar]
- Osorio, E.; Arango, G.; Robledo, S.; Muñoz, D.; Jaramillo, L.; Vélez, I. Antileishmanial and cytotoxic activity of synthetic aromatic monoterpens. Acta Farm. Bonaer. 2006, 25, 405–413. [Google Scholar]
- Desai, J.M.; Shah, V.H. Synthesis and biological activity of cyanopyridine, isoxazole and pyrazoline derivatives having thymol moiety. Indian J. Chem.-Sect. B Org. Med. Chem. 2003, 42, 382–385. [Google Scholar] [CrossRef]
- Bendre, S.R.; Rajput, D.J. Outlooks on Medicinal Properties of Eugenol and its Synthetic Derivatives. Nat. Prod. Chem. Res. 2016, 4, 1000212. [Google Scholar] [CrossRef]
- Lone, S.A.; Ahmad, A. Inhibitory effect of novel Eugenol Tosylate Congeners on pathogenicity of Candida albicans. BMC Complement. Med. Ther. 2020, 20, 131. [Google Scholar] [CrossRef]
- Carrasco, H.; Raimondi, M.; Svetaz, L.; Di Liberto, M.; Rodriguez, M.V.; Espinoza, L.; Madrid, A.; Zacchino, S. Antifungal activity of eugenol analogues. Influence of different substituents and studies on mechanism of action. Molecules 2012, 17, 1002–1024. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.I.S.D.; Alvarenga, D.J.; Carmo, L.C.F.D.; Oliveira, L.G.D.; Silva, N.C.; Dias, A.L.T.; Coelho, L.F.L.; Souza, T.B.D.; Dias, D.F.; Carvalho, D.T. Antifungal Activity of New Eugenol-Benzoxazole Hybrids against Candida spp. J. Chem. 2017, 2017, 5207439. [Google Scholar] [CrossRef]
- Dutra, J.A.P.; Maximino, S.C.; Gonçalves, R.d.C.R.; Morais, P.A.B.; de Lima Silva, W.C.; Rodrigues, R.P.; Neto, A.C.; Júnior, V.L.; Borges, W.d.S.; Kitagawa, R.R. Anti-Candida, docking studies, and in vitro metabolism-mediated cytotoxicity evaluation of Eugenol derivatives. Chem. Biol. Drug Des. 2023, 101, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Srivastava, V.; Sekgele, W.; Wani, M.Y.; Al-Bogami, A.S.; Molepo, J.; Ahmad, A. Cellular apoptosis and cell cycle arrest as potential therapeutic targets for eugenol derivatives in Candida auris. PLoS ONE 2023, 18, e0285473. [Google Scholar] [CrossRef]
- Péret, V.A.C.; Reis, R.C.F.M.; Braga, S.F.P.; Benedetti, M.D.; Caldas, I.S.; Carvalho, D.T.; de Andrade Santana, L.F.; Johann, S.; de Souza, T.B. New miconazole-based azoles derived from eugenol show activity against Candida spp. and Cryptococcus gattii by inhibiting the fungal ergosterol biosynthesis. Eur. J. Med. Chem. 2023, 256, 115436. [Google Scholar] [CrossRef]
- Goswami, L.; Gupta, L.; Paul, S.; Vermani, M.; Vijayaraghavan, P.; Bhattacharya, A.K. Design and synthesis of eugenol/isoeugenol glycoconjugates and other analogues as antifungal agents against Aspergillus fumigatus. RSC Med. Chem. 2022, 13, 955–962. [Google Scholar] [CrossRef]
- De Souza, T.B.; Orlandi, M.; Coelho, L.F.L.; Malaquias, L.C.C.; Dias, A.L.T.; De Carvalho, R.R.; Silva, N.C.; Carvalho, D.T. Synthesis and in vitro evaluation of antifungal and cytotoxic activities of eugenol glycosides. Med. Chem. Res. 2014, 23, 496–502. [Google Scholar] [CrossRef]
- Magalhães, L.S.D.; Reis, A.C.C.; Nakao, I.A.; Péret, V.A.C.; Reis, R.C.F.M.; Silva, N.C.; Dias, A.L.T.; Carvalho, D.T.; Dias, D.F.; Brandão, G.C.; et al. Glucosyl-1,2,3-triazoles derived from eugenol and analogues: Synthesis, anti-Candida activity, and molecular modeling studies in CYP-51. Chem. Biol. Drug Des. 2021, 98, 903–913. [Google Scholar] [CrossRef]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, T. Cinnamaldehyde, a Promising Natural Preservative Against Aspergillus flavus. Front. Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.E.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B.E. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 15, 332. [Google Scholar] [CrossRef]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2016, 100, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Homa, M.; Fekete, I.P.; Böszörményi, A.; Singh, Y.R.B.; Selvam, K.P.; Shobana, C.S.; Manikandan, P.; Kredics, L.; Vágvölgyi, C.; Galgóczy, L. Antifungal Effect of Essential Oils against Fusarium Keratitis Isolates. Planta Med. 2015, 81, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Karuppayil, S.M. Phenylpropanoids of plant origin as inhibitors of biofilm formation by Candida albicans. J. Microbiol. Biotechnol. 2014, 24, 1216–1225. [Google Scholar] [CrossRef]
- Shreaz, S.; Shiekh, R.A.; Raja, V.; Wani, W.A.; Behbehani, J.M. Impaired ergosterol biosynthesis mediated fungicidal activity of Co(II) complex with ligand derived from cinnamaldehyde. Chem. Biol. Interact. 2016, 247, 64–74. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Hu, L.; Wang, D.; Liu, L.; Chen, J.; Xue, Y.; Shi, Z. Ca2+ Efflux Is Involved in Cinnamaldehyde-Induced Growth Inhibition of Phytophthora capsici. PLoS ONE 2013, 8, e76264. [Google Scholar] [CrossRef]
- Courchesne, W.E.; Tunc, M.; Liao, S. Amiodarone induces stress responses and calcium flux mediated by the cell wall in Saccharomyces cerevisiae. Can. J. Microbiol. 2009, 55, 288–303. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, H.J.; Molendijk, J.; Berg, M.V.; Westerink, R.H.S. Azole fungicides disturb intracellular Ca2+ in an additive manner in dopaminergic PC12 cells. Toxicol. Sci. 2013, 134, 374–381. [Google Scholar] [CrossRef]
- Wendakoon, C.N.; Morihiko, S. Inhibition of amino acid decarboxylase activity of enterobacter aerogenes by active components in spices. J. Food Prot. 1995, 58, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Bhatia, R.; Khan, N.; Maurya, I.K.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Cinnamic aldehydes affect hydrolytic enzyme secretion and morphogenesis in oral Candida isolates. Microb. Pathog. 2012, 52, 251–258. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Influences of cinnamic aldehydes on H+ extrusion activity and ultrastructure of Candida. J. Med. Microbiol. 2013, 62, 232–240. [Google Scholar] [CrossRef]
- Khan, M.M.I.; Haque, A.M.J.; Kim, K. Electrochemical determination of uric acid in the presence of ascorbic acid on electrochemically reduced graphene oxide modified electrode. J. Electroanal. Chem. 2013, 700, 54–59. [Google Scholar] [CrossRef]
- Rajput, S.B.; Karuppayi, S.M. Small molecules inhibit growth, viability and ergosterol biosynthesis in Candida albicans. Springerplus 2013, 2, 26. [Google Scholar] [CrossRef]
- Lee, H.C.; Cheng, S.S.; Chang, S.T. Antifungal property of the essential oils and their constituents from Cinnamomum osmophloeum leaf against tree pathogenic fungi. J. Sci. Food Agric. 2005, 85, 2047–2053. [Google Scholar] [CrossRef]
- Wani, M.Y.; Ahmad, A.; Aqlan, F.M.; Al-Bogami, A.S. Azole Based Acetohydrazide Derivatives of Cinnamaldehyde Target and Kill Candida albicans by Causing Cellular Apoptosis. ACS Med. Chem. Lett. 2020, 11, 566–574. [Google Scholar] [CrossRef]
- Wani, M.Y.; Ahmad, A.; Aqlan, F.M.; Al-Bogami, A.S. Modulation of key antioxidant enzymes and cell cycle arrest as a possible antifungal mode of action of cinnamaldehyde based azole derivative. Bioorg. Med. Chem. Lett. 2022, 73, 128922. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Legabão, B.C.; Galinari, C.B.; Dos Santos, R.S.; Bruschi, M.L.; Gremião, I.D.; Boechat, J.S.; Pereira, S.A.; Malacarne, L.C.; Caetano, W.; Bonfim-Mendonça, P.S.; et al. In vitro antifungal activity of curcumin mediated by photodynamic therapy on Sporothrix brasiliensis. Photodiagnosis Photodyn. Ther. 2023, 43, 103659. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur. Cardiol. Rev. 2019, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef] [PubMed]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial Activity of Curcumin Against Periodontopathic Bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of Antibacterial Activity of Nanocurcumin with Bulk Curcumin. ACS Omega 2022, 7, 46494–46500. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Bakr, M.M.; Al-karmalawy, A.A.; Moatasim, Y.; El Taweel, A.; Mostafa, A. Scrutinizing the Feasibility of Nonionic Surfactants to Form Isotropic Bicelles of Curcumin: A Potential Antiviral Candidate Against COVID-19. AAPS PharmSciTech 2022, 23, 44. [Google Scholar] [CrossRef]
- Teshima, K.; Tanaka, T.; Zhengmao, Y.; Ikeda, K.; Matsuzaki, T.; Shiroma, T.; Muroya, A.; Hosoda, M.; Yasugi, M.; Komatsu, H. Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Ghobadi, N.; Asoodeh, A. Co-administration of curcumin with other phytochemicals improves anticancer activity by regulating multiple molecular targets. Phyther. Res. 2023, 37, 1688–1702. [Google Scholar] [CrossRef]
- Zorofchian, M.S.; Abdul, K.H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar]
- Cheraghipour, K.; Ezatpour, B.; Masoori, L.; Marzban, A.; Sepahvand, A.; Rouzbahani, A.K.; Moridnia, A.; Khanizadeh, S.; Mahmoudvand, H. Anti-Candida Activity of Curcumin: A Systematic Review. Curr. Drug Discov. Technol. 2020, 16, 379–390. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control Release 2019, 316, 359–3580. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Esmaeelzadeh, M.; Salehi, P.; Bararjanian, M.; Gharaghani, S. Synthesis of new triazole tethered derivatives of curcumin and their antibacterial and antifungal properties. J. Iran Chem. Soc. 2019, 16, 465–477. [Google Scholar] [CrossRef]
- Lal, J.; Gupta, S.K.; Thavaselvam, D.; Agarwal, D.D. Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides. Eur. J. Med. Chem. 2013, 64, 579–588. [Google Scholar] [CrossRef]
- Nagargoje, A.A.; Akolkar, S.V.; Siddiqui, M.M.; Subhedar, D.D.; Sangshetti, J.N.; Khedkar, V.M.; Bapurao, B.S. Quinoline Based Monocarbonyl Curcumin Analogs as Potential Antifungal and Antioxidant Agents: Synthesis, Bioevaluation and Molecular Docking Study. Chem. Biodivers. 2020, 17, e1900624. [Google Scholar] [CrossRef]
- Ahmed, M.; Qadir, M.A.; Shafiq, M.I.; Muddassar, M.; Samra, Z.Q.; Hameed, A. Synthesis, characterization, biological activities and molecular modeling of Schiff bases of benzene sulfonamides bearing curcumin scaffold. Arab. J. Chem. 2019, 12, 41–53. [Google Scholar] [CrossRef]
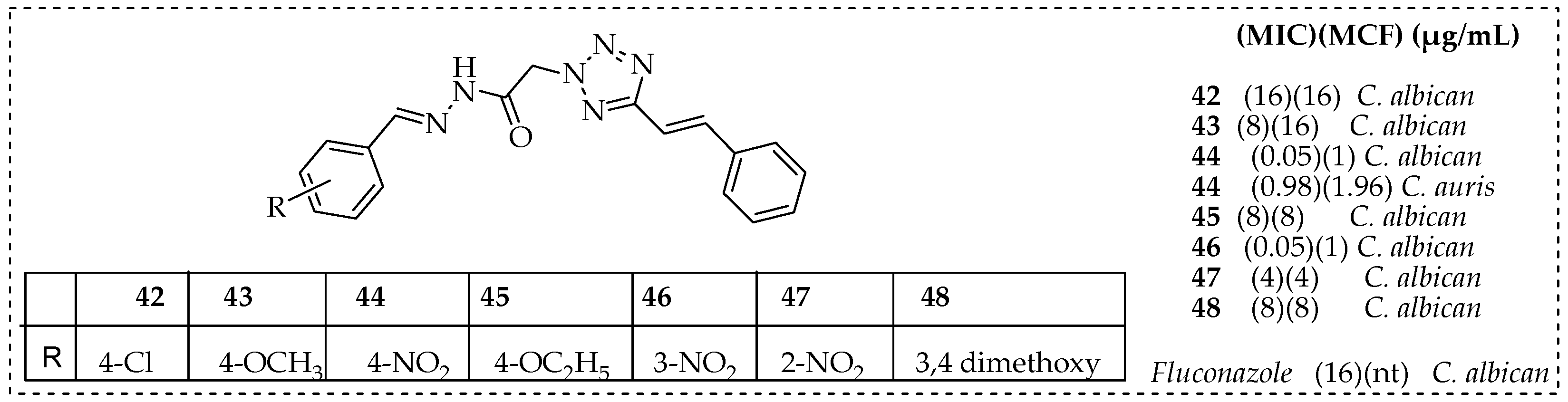
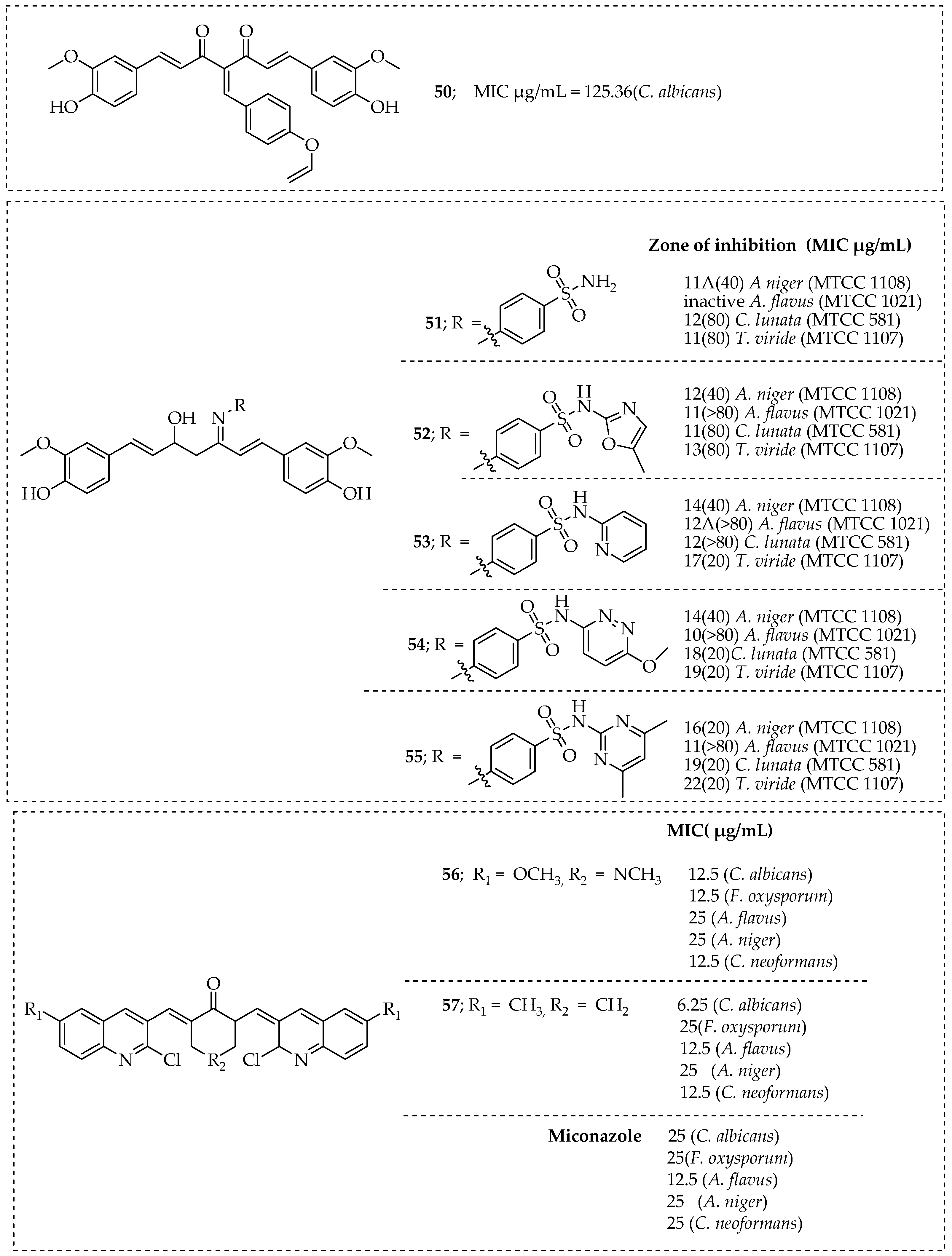
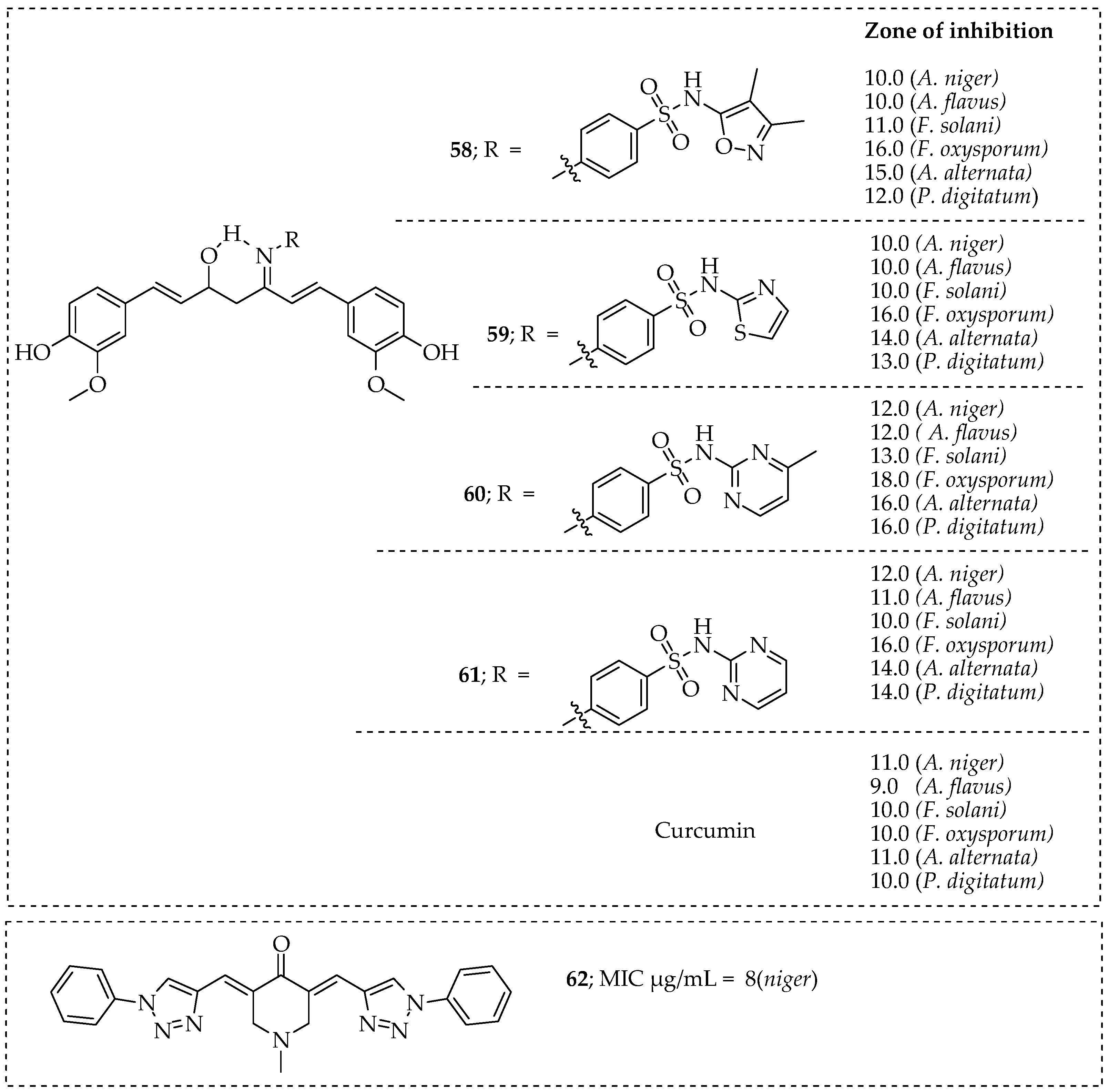
- Deshmukh, T.R.; Khare, S.P.; Krishna, V.S.; Sriram, D.; Jaiprakash, N.; Shingate, B.B. Synthesis of 1,2,3-triazole incorporated monocarbonyl curcumin analogues as potent antitubercular, antifungal and antioxidant agents. Chem. Biol. Interface 2019, 9, 59–70. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khwaza, V.; Aderibigbe, B.A. Antifungal Activities of Natural Products and Their Hybrid Molecules. Pharmaceutics 2023, 15, 2673. https://doi.org/10.3390/pharmaceutics15122673
Khwaza V, Aderibigbe BA. Antifungal Activities of Natural Products and Their Hybrid Molecules. Pharmaceutics. 2023; 15(12):2673. https://doi.org/10.3390/pharmaceutics15122673
Chicago/Turabian StyleKhwaza, Vuyolwethu, and Blessing A. Aderibigbe. 2023. "Antifungal Activities of Natural Products and Their Hybrid Molecules" Pharmaceutics 15, no. 12: 2673. https://doi.org/10.3390/pharmaceutics15122673






