Formation Kinetics and Antimicrobial Activity of Silver Nanoparticle Dispersions Based on N-Reacetylated Oligochitosan Solutions for Biomedical Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
3. Results and Discussion
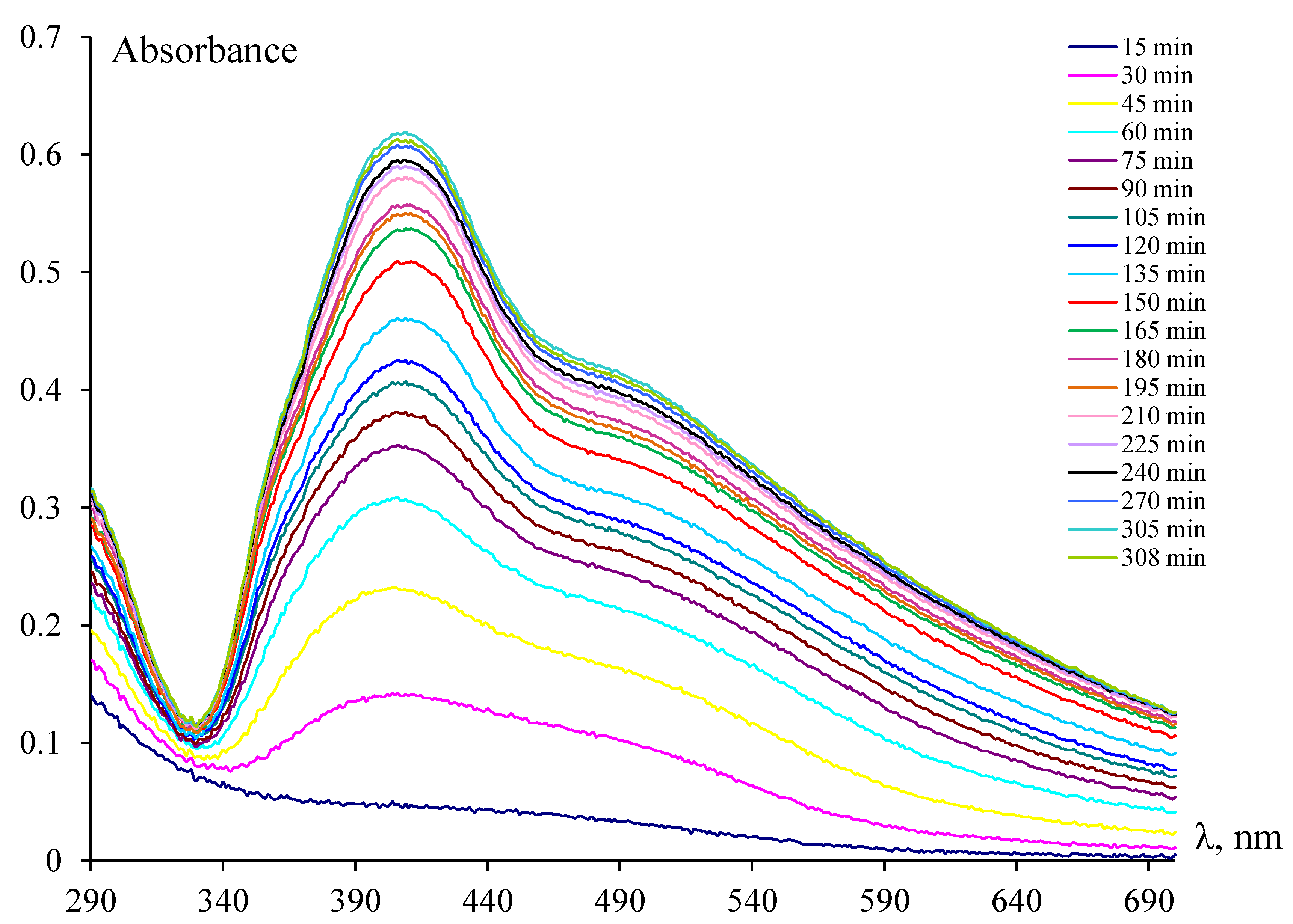
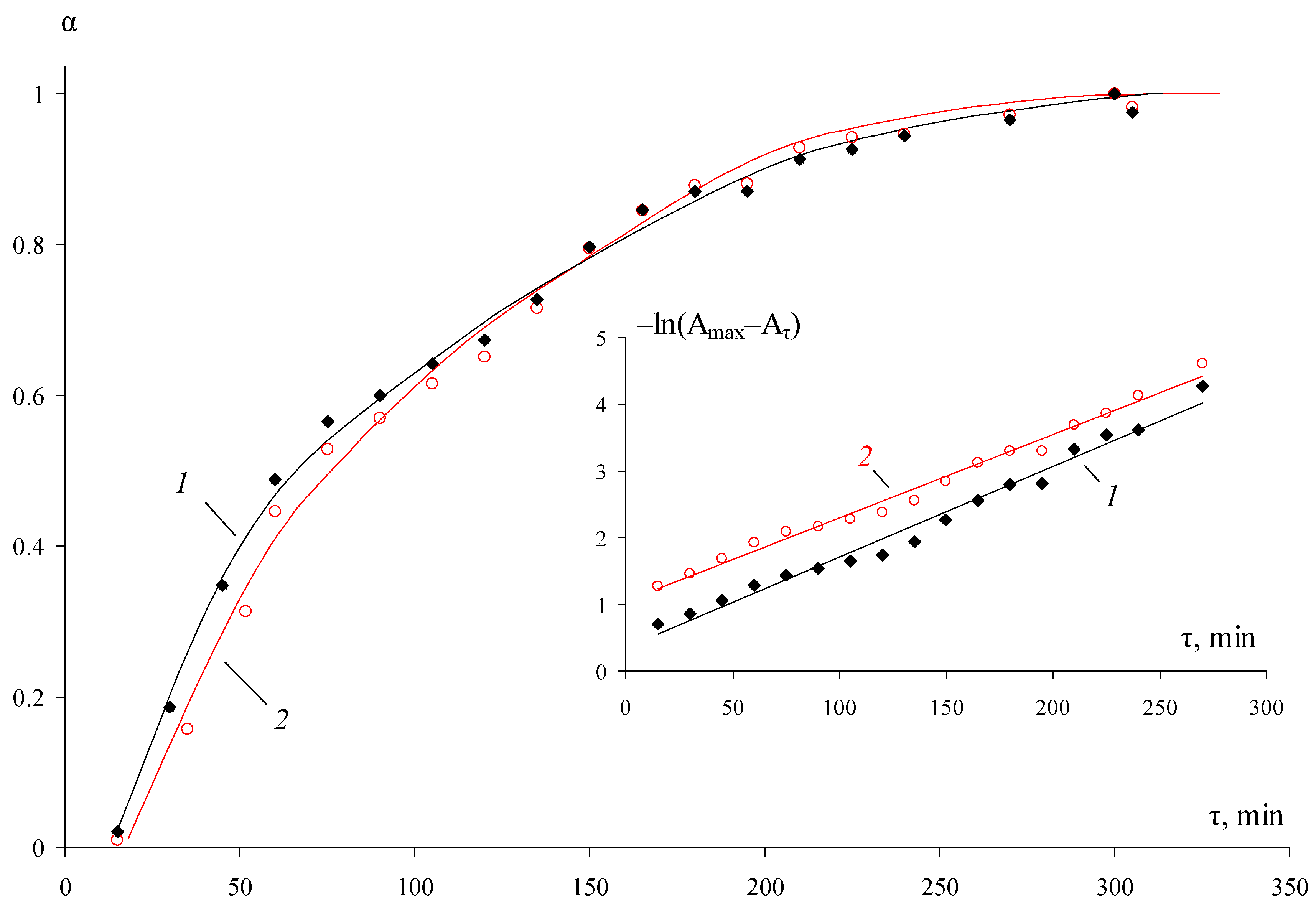
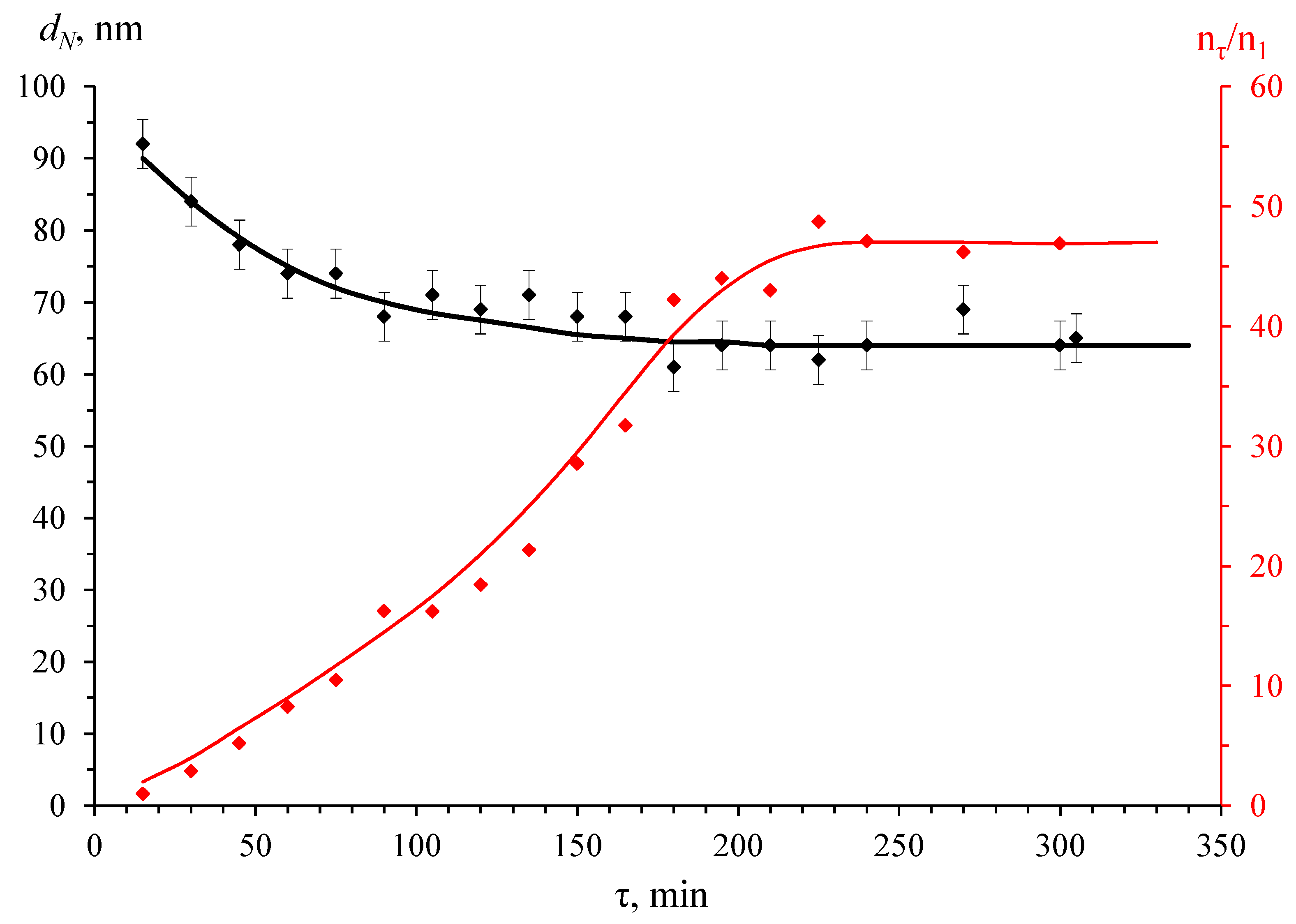
3.1. Synthesis and Characteristics
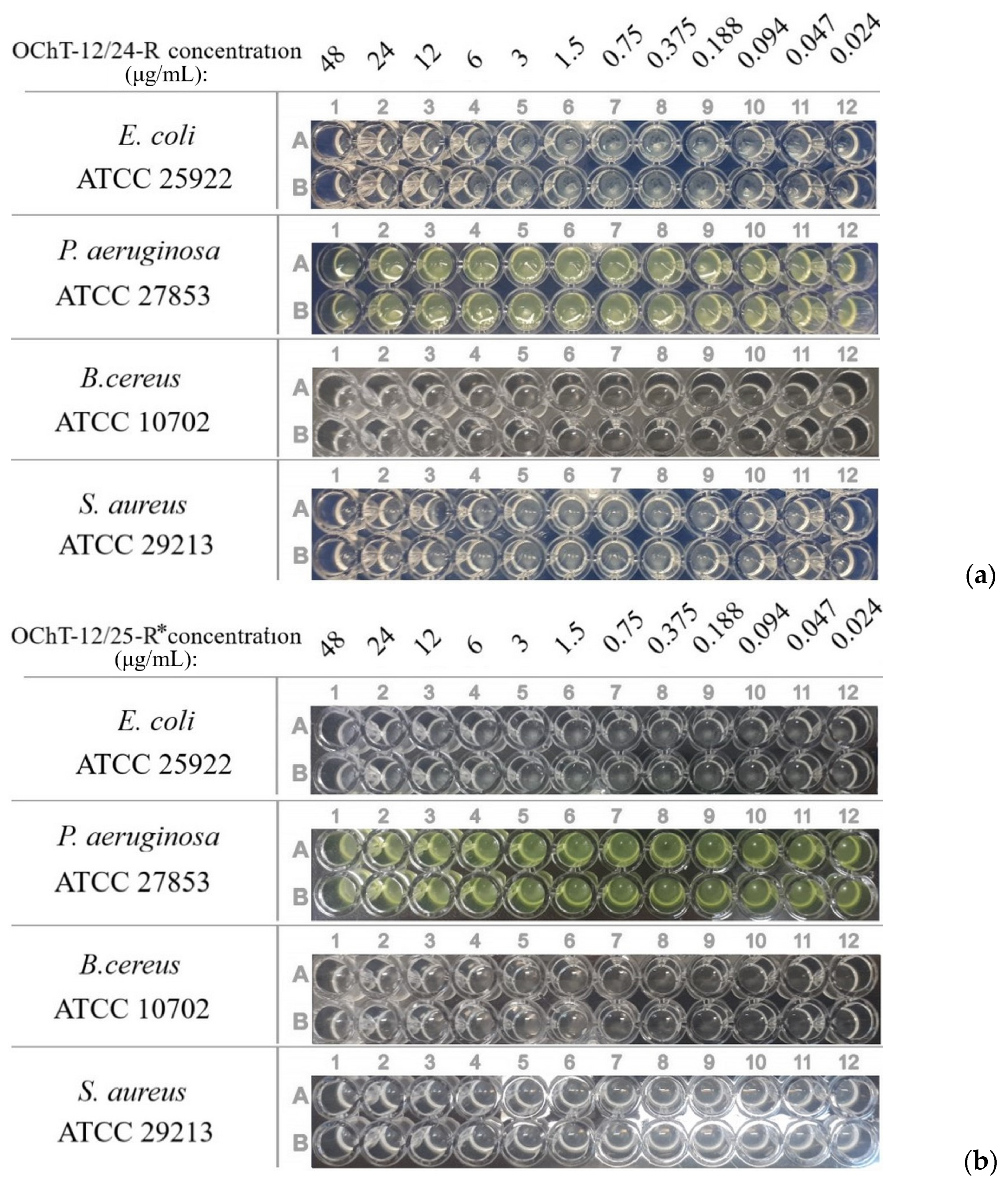
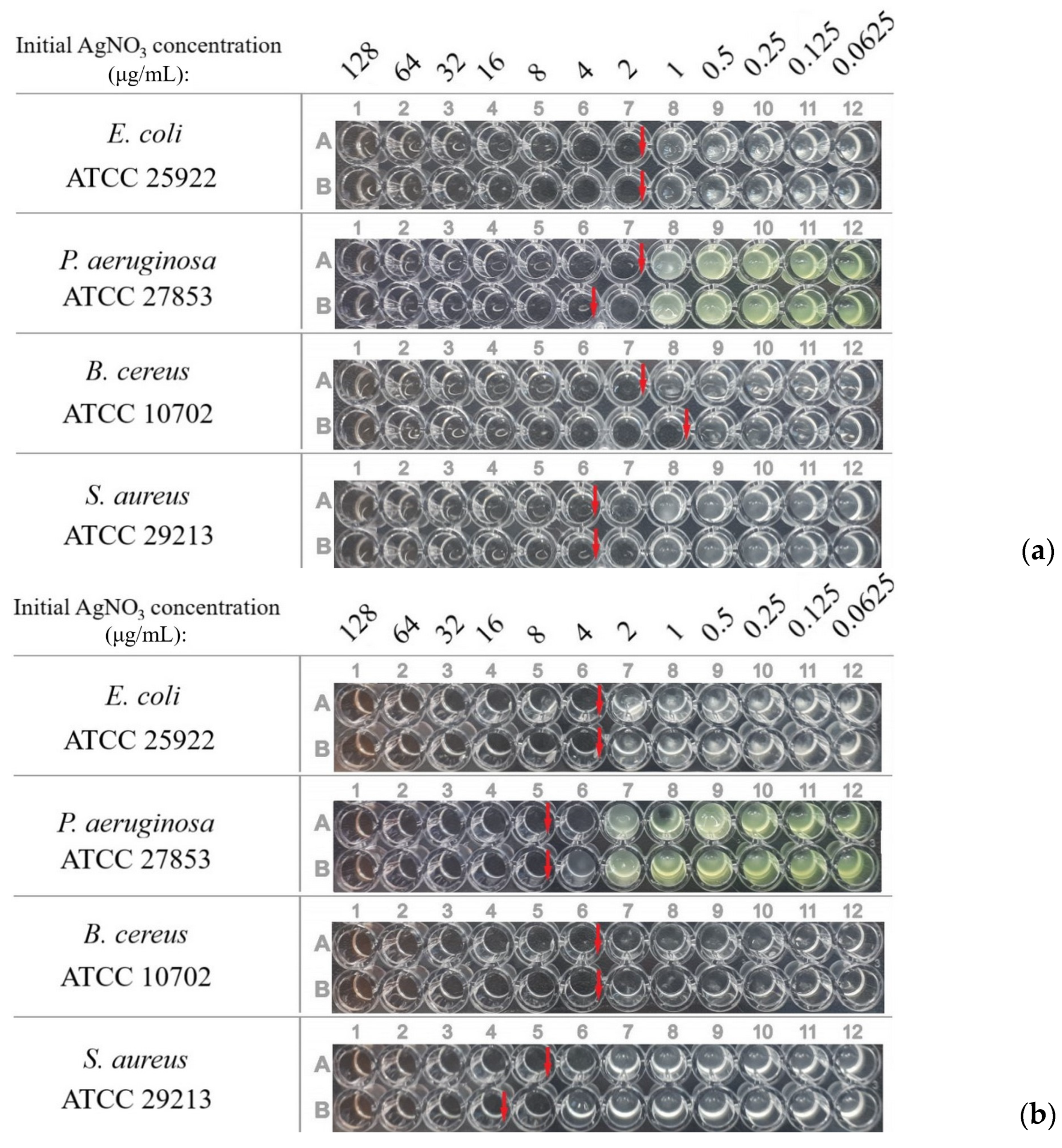
3.2. Antibacterial Activity of Dispersions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 27 September 2023).
- Coates, A.R.M. Antibiotic Resistance; Springer: New York, NY, USA, 2012; 192p. [Google Scholar]
- Manaia, C.M.; Donner, E.; Vaz-Mareira, I.; Hong, P. Antibiotic Resistance in the Environment: A Worldwide Overview; Springer: New York, NY, USA, 2020; 356p. [Google Scholar]
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.L.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. The 2012 Garrod lecture: Discovery of antibacterial drugs in the 21st century. J. Antimicrob. Chemother. 2013, 68, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Mohr, K.I. History of antibiotics research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Mir, S.A.; Shrotriya, V.; Al-Muhimeed, T.I.; Hossain, M.A.; Zaman, M.B. Metal and metal oxide nanostructures applied as alternatives of antibiotics. Inorg. Chem. Comm. 2023, 150, 110503. [Google Scholar] [CrossRef]
- Susanti, D.; Haris, M.S.; Taher, M.; Khotib, J. Natural products-based metallic nanoparticles as antimicrobial agents. Front. Pharmacol. 2022, 13, 895616. [Google Scholar] [CrossRef]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and metal oxide nanoparticles as a novel antibiotic carrier for the direct delivery of antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Maddela, N.R.; Chakraborty, S.; Prasad, R. Nanotechnology for Advances in Medical Microbiology; Springer: New York, NY, USA, 2021; 443p. [Google Scholar]
- Klebowski, B.; Depciuch, J.; Parlinska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Dos Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Fernandez, S.; Lozano-Iturbe, V.; Garcia, B.; Andres, L.J.; Menendez, M.F.; Rodriquez, D.; Varquez, F.; Martin, C.; Quiros, L.M. Antibacterial effect of silver nanorings. BMC Microbiol. 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ahmed, G. Silver nanoparticles damage yeast cell wall. Int. Res. J. Biotechnol. 2012, 3, 37–39. [Google Scholar]
- Rajawat, S.; Malik, M.M. Silver Nanoparticles: Properties, Synthesis Techniques, Characterizations, Antibacterial and Anticancer Studies; ASME Press: New York, NY, USA, 2018; 184p. [Google Scholar]
- Alheety, M.A.; Mahmood, A.R.; Karadag, A. Silver Nanoparticles: Kinetic Factors, Anticancer and Antimicrobial; LAP LAMBERT Academic Publishing: Saarbrucken, Germany, 2019; 168p. [Google Scholar]
- Cao, H. Silver Nanoparticles for Antibacterial Devices: Biocompatibility and Toxicity; CRC Press: Boca Raton, FL, USA, 2017; 773p. [Google Scholar]
- Garipov, I.T.; Khaydarov, R.R.; Gapurova, O.U.; Khaydarov, R.A.; Firdaus, M.L.; Efimova, I.L.; Evgrafova, S.Y. Silver nanoparticles as a new generation of antimicrobial prophylaxis. J. Sib. Fed. Univ. Biol. 2019, 12, 266–276. [Google Scholar] [CrossRef]
- Prahbu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar]
- Rzheussky, S.E. Silver nanoparticles in medicine. Vestn. VGMU 2022, 21, 15–24. [Google Scholar] [CrossRef]
- Jena, P.; Mohanty, S.; Mallick, R.; Jacob, B.; Sonawane, A. Toxicity and antibacterial assessment of chitosan-coated silver nanoparticles on human pathogens and macrophage cells. Int. J. Nanomed. 2012, 7, 1805–1812. [Google Scholar] [CrossRef]
- Krutyakov, A.Y.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcantara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Polinarski, M.A.; Beal, A.L.B.; Silva, F.E.B.; Bernardi-Wenzel, J.; Burin, G.R.M.; de Muniz, G.I.B.; Alves, H.J. New perspectives of using chitosan, silver, and chitosan–silver nanoparticles against multidrug-resistant bacteria. Part. Part. Syst. Charact. 2021, 8, 2100009. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Review of chitosan and its derivatives as antimicrobial agents and their use as textile chemicals. J. Macromol. Sci. C 2003, 43, 223–269. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Rini, N.D.W.; Tsalsabila, A.; Birowosuto, M.D.; Wibowo, A. Chitosan-based smart biomaterials for biomedical applications: Progress and perspectives. ACS Biomater. Sci. Eng. 2023, 9, 3084–3115. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Primozic, M.; Knez, Z.; Leitgeb, M. Chitosan-based (nano)materials for novel biomedical applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed]
- Kankariya, Y.; Chatterjee, B. Biomedical application of chitosan and chitosan derivatives: A comprehencive review. Curr. Pharm. Des. 2023, 29, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/chitosan and its derivatives: Fundamental problems and practical approaches. Biochemistry 2020, 85, 154–176. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Bai, Q.; Zheng, C.; Chen, W.; Sun, N.; Gao, Q.; Liu, J.; Hu, F.; Pimpi, S.; Yan, X.; Zhang, Y.; et al. Current challenges and future applications of antibacterial nanomaterials and chitosan hydrogel in burn wound healing. Mater. Adv. 2022, 3, 6707–6727. [Google Scholar] [CrossRef]
- Sanchez-Machado, D.I.; Lopez-Cervantez, J.; Martinez-Ibarra, D.M.; Escarcega-Galaz, A.A.; Vega-Cazarez, C.A. The use of chitosan as a skin-regeneration agent in burns injuries: A review. e-Polymers 2022, 22, 75–86. [Google Scholar] [CrossRef]
- Zhu, N.; Chatzistavrou, X.; Papagerakis, P.; Ge, L.; Qin, M.; Wang, Y. Silver-doped bioactive glass/chitosan hydrogel with potential application in dental pulp repair. ACS Biomater. Sci. Eng. 2019, 5, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.A.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Meyrinck, A.M.; Bindels, L.B.; De Backer, F.; Pachikian, B.D.; Cani, P.D.; Delzenne, N.M. Dietary supplementation with chitosan derived from mushrooms changes adipocytokine profile in diet-induced obese mice, a phenomenon linked to its lipid-lowering action. Int. Immunopharmacol. 2009, 9, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Modrzejewska, Z.; Zarzycki, R.; Sielski, J. Synthesis of silver nanoparticles in chitosan solution. Prog. Chem. Appl. Chitin Its Deriv. 2010, 15, 63–72. [Google Scholar]
- Alexandrova, V.A.; Shirokova, L.N.; Bondarenko, G.N.; Petrosyan, A.S. Silver-carboxymethyl chitin nanocomposites. Polym. Sci. Ser. A 2013, 55, 107–114. [Google Scholar] [CrossRef]
- Novikov, I.V.; Pigaleva, M.A.; Abramchuk, S.S.; Molchanov, V.S.; Philippova, O.E.; Gallyamov, M.O. Chitosan composites with Ag nanoparticles formed in carbonic acid solutions. Carbohydr. Polym. 2018, 190, 103–112. [Google Scholar] [CrossRef]
- Apryatina, K.V.; Mochalova, A.E.; Gracheva, T.A.; Kuz’micheva, T.A.; Smirnova, L.A.; Smirnova, O.N. Influence of the molecular mass of chitosan on the dimensional characteristics of silver nanoparticles. Polym. Sci. Ser. B 2015, 57, 145–149. [Google Scholar] [CrossRef]
- Chashchin, I.S.; Grigor’ev, T.E.; Abramchuk, S.S.; Bakuleva, N.L. Solvent effect on the structure of composite films obtained from chitosan solutions with a precursor of silver nanoparticles. Dokl. Chem. 2016, 469, 223–226. [Google Scholar] [CrossRef]
- Aleksandrova, V.A.; Shirokova, L.N. Rdiation-chemical reduction of silver ions in polyelectrolyte matrix-carboxymethyl chitin. Polym. Sci. Ser. B 2018, 60, 727–734. [Google Scholar] [CrossRef]
- Shinde, S.; Folliero, V.; Chianese, A.; Zannella, C.; De Filippis, A.; Rosati, L.; Prisco, M.; Falanga, A.; Mali, A.; Galdiero, M.; et al. Synthesis of chitosan-coated silver nanoparticle bioconjugates and their antimicrobial activity against multidrug-resistant bacteria. Appl. Sci. 2021, 11, 9340. [Google Scholar] [CrossRef]
- Kulikouskaya, V.; Hileuskaya, K.; Kraskouski, A.; Kozerozhets, I.; Stepanova, E.; Kuzminski, I.; You, L.; Agabekov, V. Chitosan-capped silver nanoparticles: A comprehensive study of polymer molecular weight effect on the reaction kinetic, physicochemical properties, and synergetic anti-bacterial potential. Polymers 2022, 1, 14. [Google Scholar] [CrossRef]
- Govindan, S.; Nivethaa, E.A.K.; Saravanan, R.; Narayanan, V.; Stephen, A. Synthesis and characterization of chitosan–silver nanocomposite. Appl. Nanosci. 2012, 2, 299–303. [Google Scholar] [CrossRef]
- Mirda, E.; Idroes, R.; Khairan, K.; Tallei, T.E.; Ramli, M.; Earlia, N.; Maulana, A.; Idroes, G.M.; Muslem, M.; Jalil, Z. Synthesis of chitosan-silver nanoparticle composite spheres and their anti-microbial activities. Polymers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Waclawek, S.; Cernik, M.; Padil, V.V.T. Chitosan/gelatin/silver nanoparticles composites films for biodegradable food packaging appli-cations. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkad, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef]
- Aleksandrova, V.A.; Futoryanskaya, A.M.; Klicheva, O.B.; Rashidova, S.S. Nanocomposites of silver and N-carboxymethylchitosan Bombyx mori. Polym. Sci. Ser. A 2020, 62, 515–520. [Google Scholar] [CrossRef]
- Il’ina, A.V.; Varlamov, V.P.; Skryabin, K.G.; Ermakov, Y.A.; Orlov, V.N. Chitosan is a natural polymer for constructing nanoparticles. Dokl. Chem. 2008, 421, 165–167. [Google Scholar] [CrossRef]
- Ur’yash, V.F.; Laruna, V.N.; Kokurina, N.Y.; Kashtanov, E.A.; Bakulin, A.V.; Varlamov, V.P. Dependence of the ordering degree and thermochemical characteristics of chitin and chitosan on their biological origin. Russ. J. Phys. Chem. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Blagodatskikh, I.V.; Kulikov, S.N.; Vyshivannaya, O.V.; Bezrodnykh, E.A.; Tikhonov, V.E. N-reacetylated oligochitosan: pH dependence of self-assembly properties and antibacterial activity. Biomacromolecules. 2017, 18, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Rinudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pirniyazov, K.K.; Tikhonov, V.E.; Rashidova, S.S. Synthesis and properties of oligochitosan ascorbate from Bombyx mori. Bull. Univ. Karaganda Chem. 2021, 101, 91–98. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Balgodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight-activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Dar, T.A.; Khan, M.M.A.; Ahmad, A.; Rinklebe, J.; Chen, Y.; Ahmad, P. Oligochitosan fortifies antioxidative and photosynthetic metabolism and enhances secondary metabolite accumulation in arsenic-stressed peppermint. Front. Plant Sci. 2022, 13, 987746. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Guerrero, U.P.; de la Cabo, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Cherkasova, E.I.; Smirnova, L.A.; Smirnov, V.F. Measurement of molecular mass of chitosan oligomers. Polym. Sci. Ser. B 2006, 48, 80–83. [Google Scholar] [CrossRef]
- Blagodatskikh, I.V.; Bezrodnykhm, E.A.; Abramchuk, S.S.; Muranov, A.V.; Sinitsyna, O.V.; Khokhlov, A.R.; Tikhonov, V.E. Short chain chitosan solutions: Self-assembly and aggregates disruption effects. J. Polym. Res. 2013, 20, 73. [Google Scholar] [CrossRef]
- Kulikov, S.; Tikhonov, V.; Blagodatskikh, I.; Bezrodnykh, E.; Lopatin, S.; Khairullin, R.; Philippova, Y.; Abramchuk, S. Molecular weight and pH aspects of the efficacy of oligochitosan against methicillin-resistant Staphylococcus aureus (MRSA). Carbohydr. Polym. 2012, 87, 545–550. [Google Scholar] [CrossRef]
- Markushin, S.G.; Akopova, I.I.; Blagodatskikh, I.V.; Bezrodnykh, E.A.; Muranov, A.V.; Yamskov, I.A.; Tikhonov, V.E.; Kulikov, S.N. Effect of molecular weight and degree of acetylation on adjunctive properties of chitosan derivatives. Appl. Biochem. Microbiol. 2018, 54, 512–517. [Google Scholar] [CrossRef]
- Hirano, S.; Yamaguchi, R. N-acetylchitosan gel: A polyhydrate of chitin. Biopolymers 1976, 15, 1685–1691. [Google Scholar] [CrossRef]
- Uryupina, O.Y.; Urodkova, E.K.; Tikhonov, V.E.; Zhavoronok, E.S.; Senchikhin, I.N. Formation of silver nanoparticles in aqueous oligochitosan solutions. Colloid J. 2021, 83, 142–150. [Google Scholar] [CrossRef]
- Urodkova, E.K.; Uryupina, O.Y.; Zhavoronok, E.S.; Grammatikova, N.E.; Kharitonova, T.V.; Senchikhin, I.N. Antibacterial activity of silver nanodispersions in solutions of different molecular weight chitosans. ChemistrySelect 2023, 8, e202203609. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. In (CLSI Supplement M100) in: Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Ottoy, M.H.; Varum, K.M.; Smidsrod, O. Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohydr. Polym. 1996, 29, 17–24. [Google Scholar] [CrossRef]
- Kerker, M. The optics of colloidal silver: Something old and something new. J. Colloid Interface Sci. 1985, 105, 297–313. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Rozenberg, A.S.; Uflyand, U.E. Nanoscale Metal Particles in Polymers; Khimiya: Moscow, Russia, 2000; 672p. (In Russian) [Google Scholar]
- Vysotskii, V.V.; Uryupina, O.Y.; Gusel’nikova, A.V.; Roldugin, V.I. On the feasibility of determining nanoparticle concentration by the dynamic light scattering method. Colloid J. 2009, 71, 739–744. [Google Scholar] [CrossRef]
- Dement’eva, O.V.; Rudoy, V.M. Colloidal synthesis of new silver-based nanostructures with tailored localized surface plasmon resonance. Colloid J. 2011, 73, 724–742. [Google Scholar] [CrossRef]
- Roldughin, V.I.; Rudoy, V.M. Absorption of Electromagnetic Radiation by a Nanoparticle in a Nanocomposite: Going beyond the Maxwell-Garnett Approximation. Colloid J. 2017, 79, 809–814. [Google Scholar] [CrossRef]
- Dement’eva, O.V.; Matsur, V.A.; Zaikin, A.S.; Salavatov, N.A.; Staltsov, M.S.; Rudoy, V.M. Octadecyltrimethylammonium bromide micelles as a template in the seedless synthesis of gold nanorods. Colloid J. 2022, 84, 689–695. [Google Scholar] [CrossRef]
- Salavatov, N.A.; Dement’eva, O.V.; Rudoy, V.M. Nanorods gold with organosilica shells as a platform for creating multifunctional nanostructures. Colloid J. 2020, 82, 713–718. [Google Scholar] [CrossRef]
- Ershov, V.; Tarasova, N.; Ershov, B. Evolution of electronic state and properties of silver nanoparticles during their formation in aqueous solution. J. Mol. Sci. 2021, 22, 10673. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef] [PubMed]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Kalenskii, A.V.; Zvekov, A.A.; Nikitin, A.P.; Anan’eva, M.V.; Aduev, B.P. Specific features of plasmon resonance in nanoparticles of different metals. Opt. Spectrosc. 2015, 188, 978–987. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Sayed, S.R.M.; Solkamy, E.N.; Khan, M.; Shaik, M.R.; Warthan, A.A.; Adil, S.F. Evaluation of biological activities of chemically synthesized silver nanoparticles. J. Nanometer. 2015, 7, 789178. [Google Scholar] [CrossRef]
- Shabbeer, H.; Khan, A.; Shah, A.; Rehman, Z.-U.; Shah, S.M.; Khan, A.; Shah, S.S. Effect of acidic and basic conditions on the plasmon band of colloidal silver. Walailak J. Sci. Tech. 2012, 9, 229–237. [Google Scholar] [CrossRef]
- Vysotsky, V.V.; Uryupina, O.Y.; Roldugin, V.I.; Plachev, Y.A. Formation of silver nanoparticles in aqueous carboxymethyl cellulose solutions and the evolution of their sizes. Colloid J. 2009, 71, 156–162. [Google Scholar] [CrossRef]
- Available online: https://dornshuld.chemistry.msstate.edu/books/chemistry/solubility-product-ksp.html (accessed on 27 September 2023).
- Available online: https://chem-net.blogspot.com/2017/03/solubility-product-constants.html (accessed on 27 September 2023).
- Rancoule, C.; Magne, N.; Vallard, A.; Guy, J.-B.; Rodrigues-Lafrasse, C.; Deutsch, E.; Chargari, C. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Lett. 2016, 375, 256–262. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P. Synthesis of biogenic silver nanoparticles (AgCl-NPs) using a Pulicaria vulgaris Gaertn. Aerial part extract and their application as antibacterial, antifungal and antioxidant agents. Nanomaterials 2020, 10, 638. [Google Scholar] [CrossRef]
- Kang, U.O.; Jung, J.-U.; Cho, D.; Kwon, O.H.; Cheon, J.Y.; Park, W.H. Antimicrobial silver chloride nanoparticles stabilized with chitosan oligomer for the healing of burns. Materials 2016, 9, 215. [Google Scholar] [CrossRef]
- Loza, K.; Sengstock, C.; Chernousova, S.; Koller, M.; Epple, M. The predominant species of ionic silver in biological media is colloidally dispersed nanoparticulate silver chloride. RSC Adv. 2014, 4, 35290–35297. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Antibacterial effect of silver nanoparticles is stronger if the production host and the targeted pathogen are closely related. Biomedicine 2022, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrugresistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, S.; Chinnasamy, G.; Bhatnagar, S. Sustainable phyto-fabrication of silver nanoparticles using Gmelina arborea exhibit antimicrobial and biofilm inhibition activity. Sci. Rep. 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gong, J.; Jiang, Y.; Long, Y.; Lei, W.; Gao, X.; Guo, D. Application of silver nanoparticles in parasite treatment. Pharmaceutics 2023, 15, 1783. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green synthesis of silver nanoparticles using Hypericum perforatum L. Aqueous extract with the evaluation of its antibacterial activity against clinical and food pathogens. Pharmaceutics 2023, 14, 1104. [Google Scholar] [CrossRef]
- Urcan, A.C.; Criste, A.D.; Szanto, K.I.; Stefan, R.; Zahan, M.; Musca, A.S.; Focsan, M.; Burtwscu, R.F.; Olah, N.K. Antimicrobial and antiproliferative activity of green synthesized silver nanoparticles using bee bread extracts. Pharmaceutics 2023, 15, 1797. [Google Scholar] [CrossRef]
- Miranda, M.M.; Liu, W.; Godinez-Leon, J.A.; Amanova, A.; Houel-Renault, L.; Lampre, I.; Remita, H.; Gref, R. Colloidal silver nanoparticles obtained via radiolysis: Synthesis optimization and antibacterial properties. Pharmaceutics 2023, 15, 1787. [Google Scholar] [CrossRef]




| Culture | ||||
|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 | Bacillus cereus ATCC 10702 | Staphylococcus aureus ATCC 29213 | |
| MICs of OChT-12/24-R solution, μg/mL | >48 | >48 | >48 | >48 |
| MICs of OChT-12/25-R* solution, μg/mL | >48 | >48 | >48 | >48 |
| Culture | MICs of Dispersions (α ≈ 45%), μg/mL | |
|---|---|---|
| Based on OChT-12/24-R | Based on OChT-12/25-R* | |
| Escherichia coli ATCC 25922 | 2 | 2 |
| Pseudomonas aeruginosa ATCC 27853 | 2 | 2 |
| Bacillus cereus ATCC 10702 | 1–2 | 1 |
| Staphylococcus aureus ATCC 29213 | 8–16 | 4 |
| Culture | MICs of Dispersions (α ≈ 100%), μg/mL | |
|---|---|---|
| Based on OChT-12/24-R | Based on OChT-12/25-R* | |
| Escherichia coli ATCC 25922 | 2 | 4 |
| Pseudomonas aeruginosa ATCC 27853 | 2–4 | 8 |
| Bacillus cereus ATCC 10702 | 1–2 | 4 |
| Staphylococcus aureus ATCC 29213 | 4 | 8–16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urodkova, E.K.; Uryupina, O.Y.; Tikhonov, V.E.; Grammatikova, N.E.; Bol’shakova, A.V.; Sinelshchikova, A.A.; Zvyagina, A.I.; Khmelenin, D.N.; Zhavoronok, E.S.; Senchikhin, I.N. Formation Kinetics and Antimicrobial Activity of Silver Nanoparticle Dispersions Based on N-Reacetylated Oligochitosan Solutions for Biomedical Applications. Pharmaceutics 2023, 15, 2690. https://doi.org/10.3390/pharmaceutics15122690
Urodkova EK, Uryupina OY, Tikhonov VE, Grammatikova NE, Bol’shakova AV, Sinelshchikova AA, Zvyagina AI, Khmelenin DN, Zhavoronok ES, Senchikhin IN. Formation Kinetics and Antimicrobial Activity of Silver Nanoparticle Dispersions Based on N-Reacetylated Oligochitosan Solutions for Biomedical Applications. Pharmaceutics. 2023; 15(12):2690. https://doi.org/10.3390/pharmaceutics15122690
Chicago/Turabian StyleUrodkova, Ekaterina K., Ol’ga Ya. Uryupina, Vladimir E. Tikhonov, Natalia E. Grammatikova, Anastasia V. Bol’shakova, Anna A. Sinelshchikova, Alexandra I. Zvyagina, Dmitry N. Khmelenin, Elena S. Zhavoronok, and Ivan N. Senchikhin. 2023. "Formation Kinetics and Antimicrobial Activity of Silver Nanoparticle Dispersions Based on N-Reacetylated Oligochitosan Solutions for Biomedical Applications" Pharmaceutics 15, no. 12: 2690. https://doi.org/10.3390/pharmaceutics15122690
APA StyleUrodkova, E. K., Uryupina, O. Y., Tikhonov, V. E., Grammatikova, N. E., Bol’shakova, A. V., Sinelshchikova, A. A., Zvyagina, A. I., Khmelenin, D. N., Zhavoronok, E. S., & Senchikhin, I. N. (2023). Formation Kinetics and Antimicrobial Activity of Silver Nanoparticle Dispersions Based on N-Reacetylated Oligochitosan Solutions for Biomedical Applications. Pharmaceutics, 15(12), 2690. https://doi.org/10.3390/pharmaceutics15122690






