Cocrystallization of Gefitinib Potentiate Single-Dose Oral Administration for Lung Tumor Eradication via Unbalancing the DNA Damage/Repair
Abstract
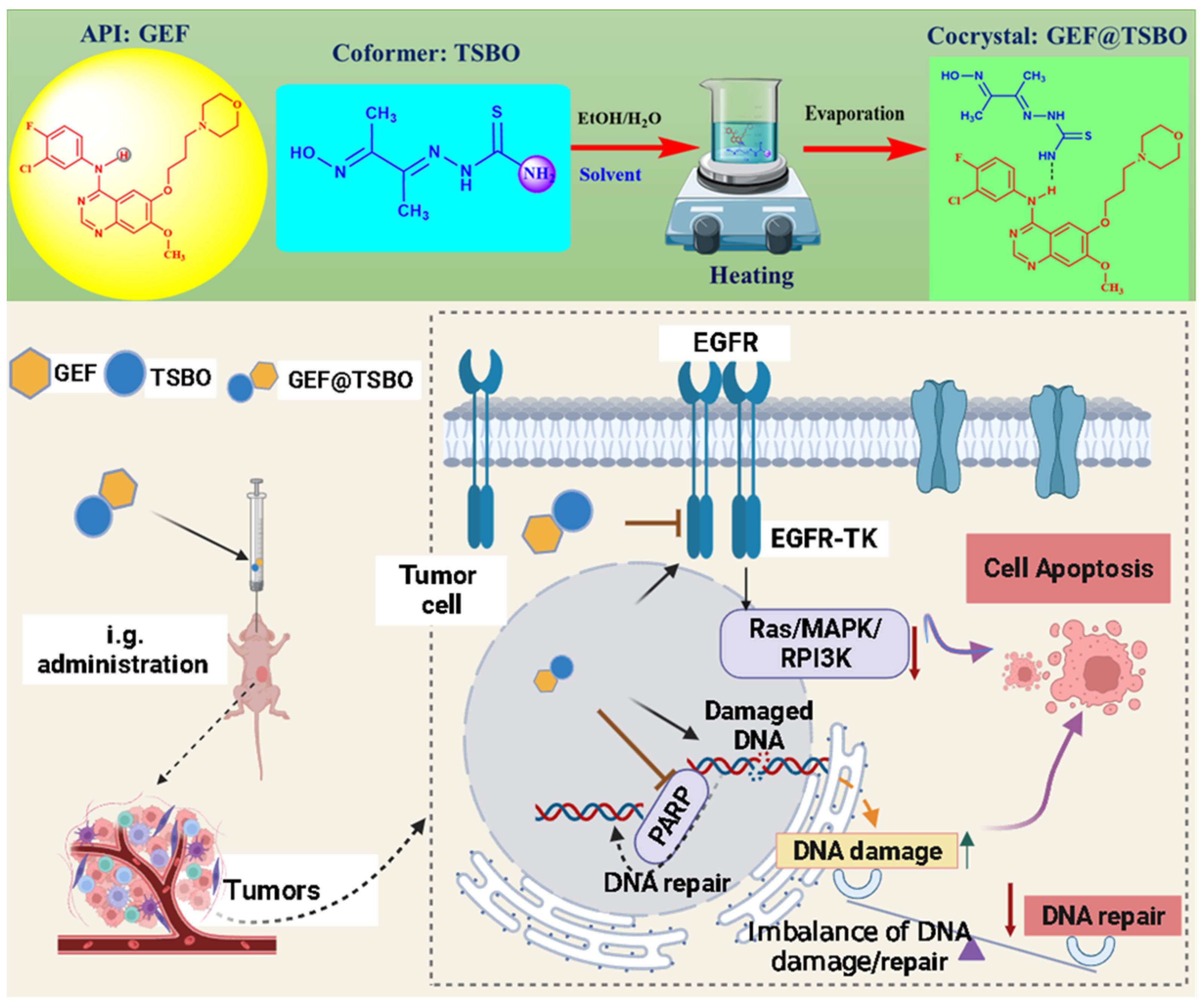
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of TSBO
2.3. Fabrication of Cocrystals
2.4. General Characterizations
2.5. Physicochemical Evaluation
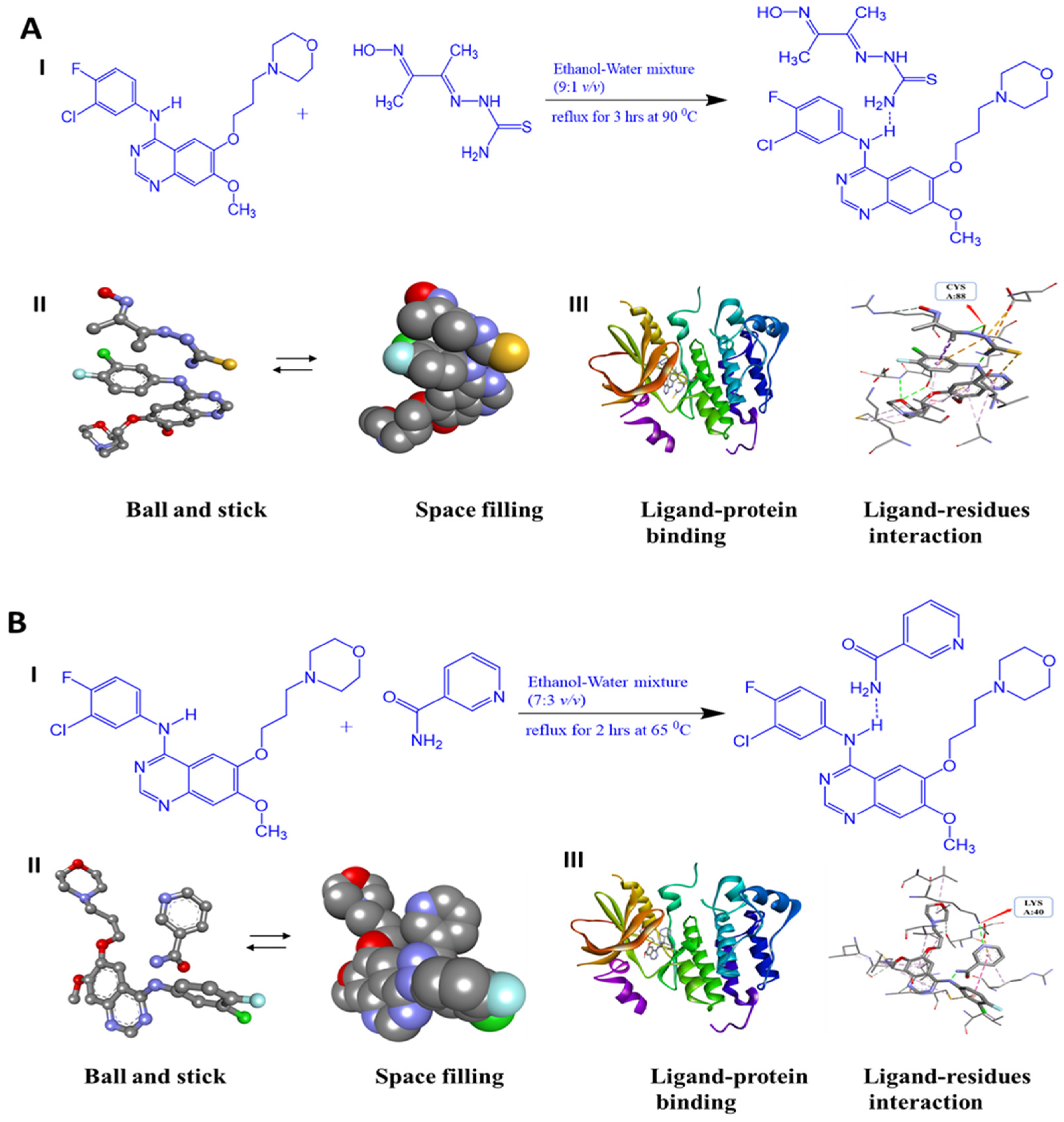
2.6. Molecular Docking
2.7. In Vitro Cytotoxicity
- A(dosing): Absorbance of wells with cells, CCK-8 solution, and drug treatments.
- A(blank): Absorbance of wells with medium and CCK-8 solution without cells.
- A(control): Absorbance of wells with cells, CCK-8 solution without drug treatments.
2.8. Cellular Uptake
2.9. Cell Apoptosis Assay
2.10. Cell Clonogenic Assay
2.11. Western Blot
2.12. Antitumor Effect In Vivo
2.13. Statistical Analysis
3. Results and Discussion
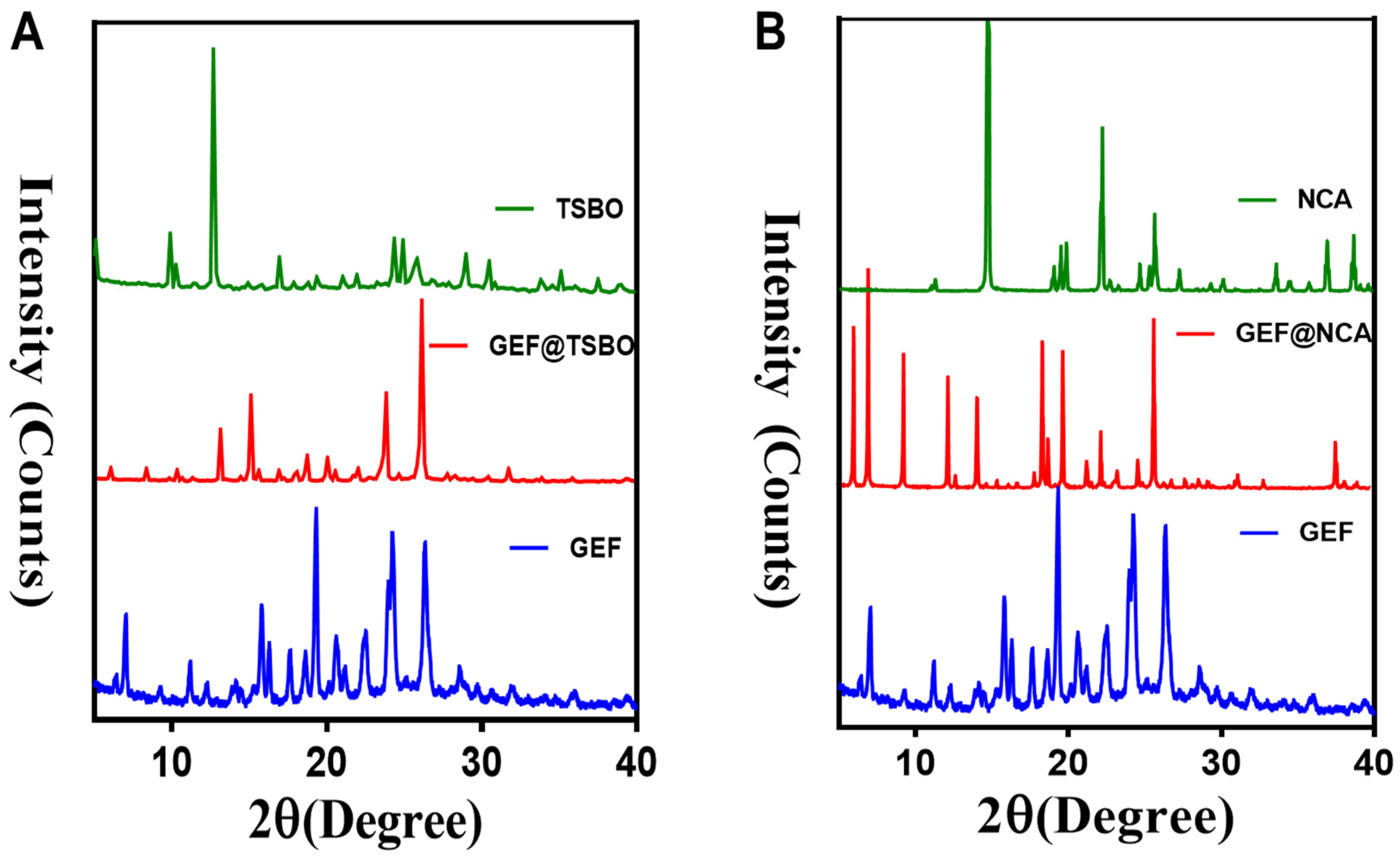
3.1. Synthesis and Characterization
3.2. Solubility and Dissolution Rate
3.3. Molecular Docking Study
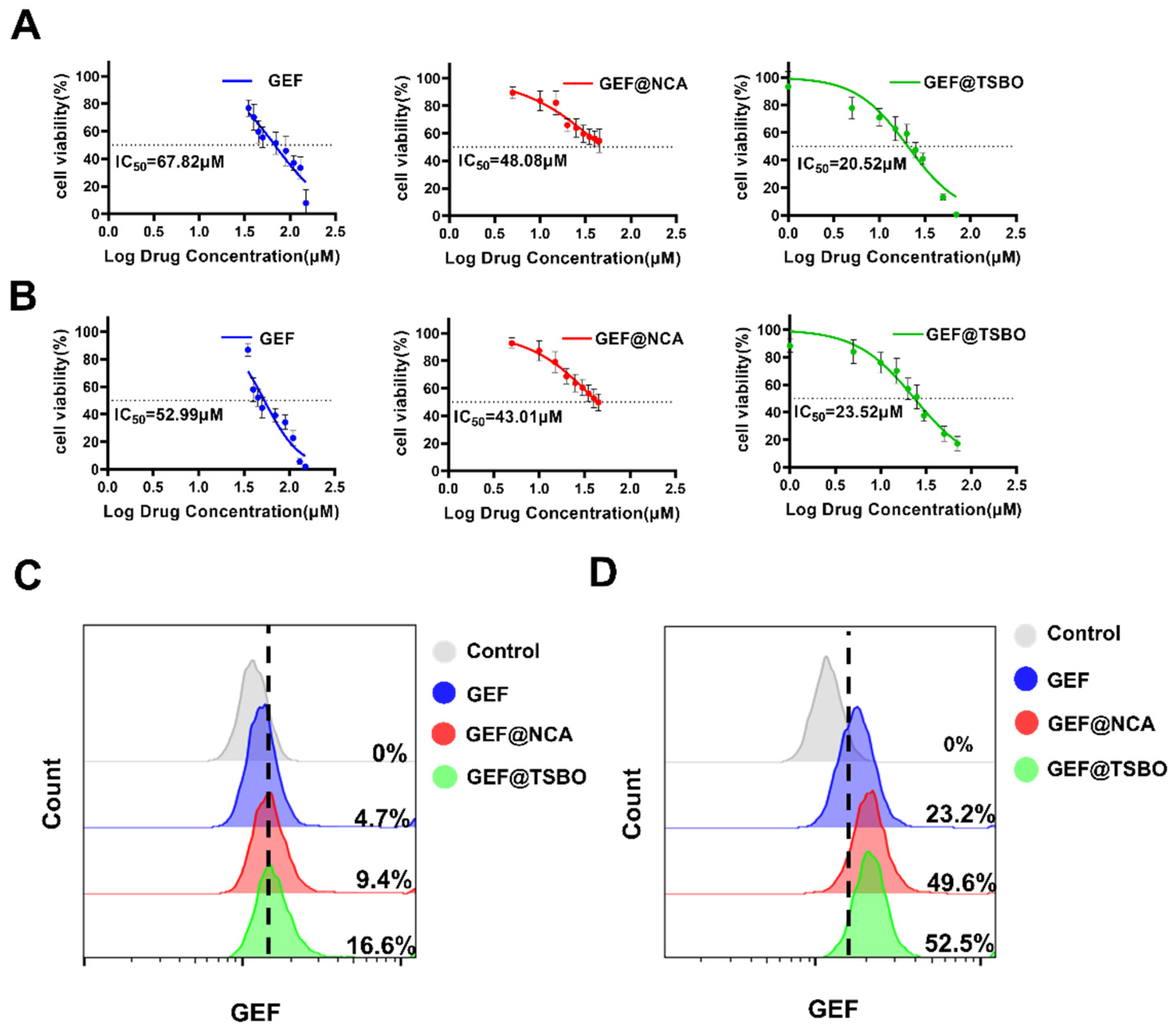
3.4. In Vitro Cytotoxic Effect
3.5. Cellular Uptake of Cocrystals
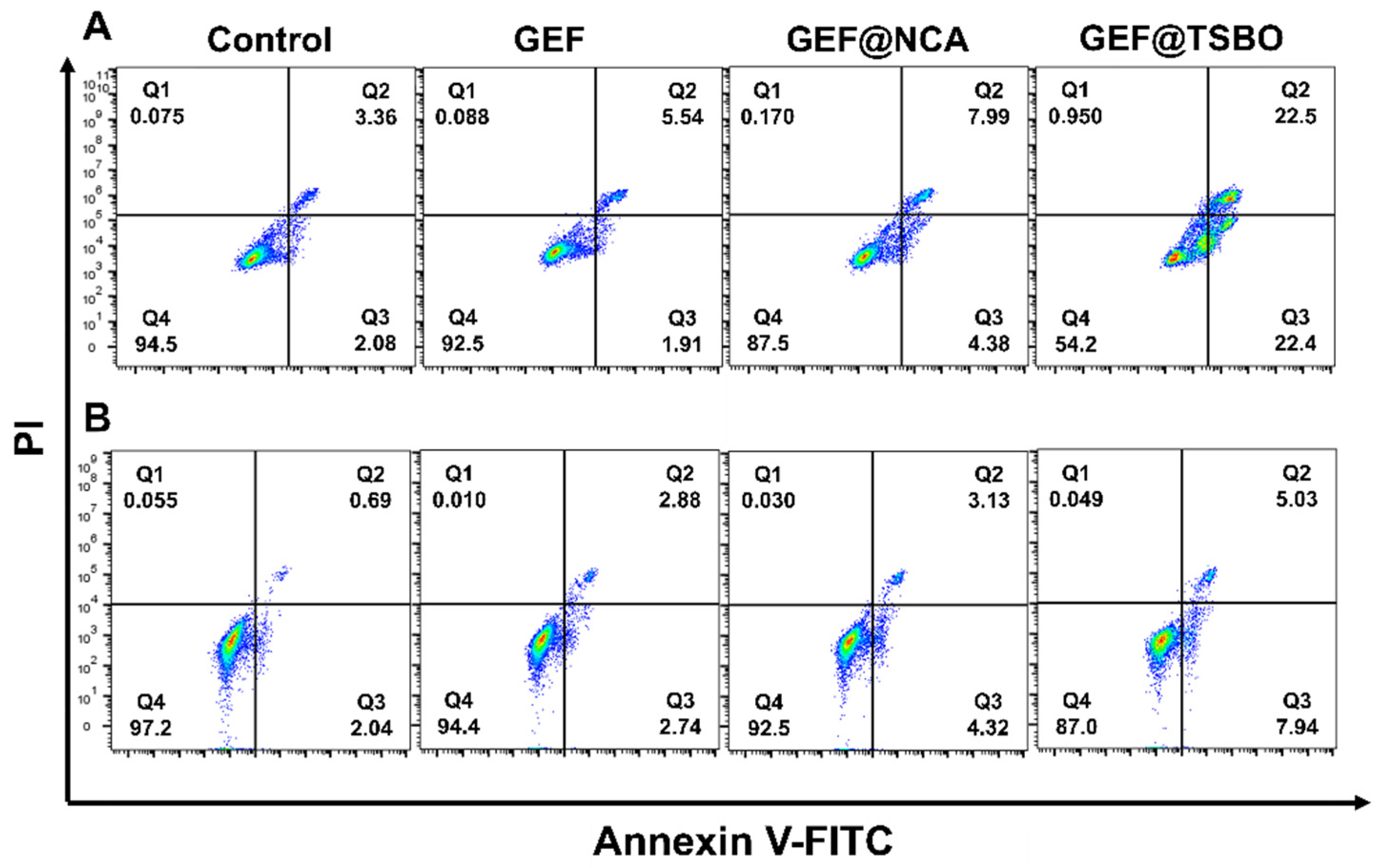
3.6. Cell Apoptosis Assay
3.7. Cell Clonogenic Assay
3.8. Western Blotting Analysis
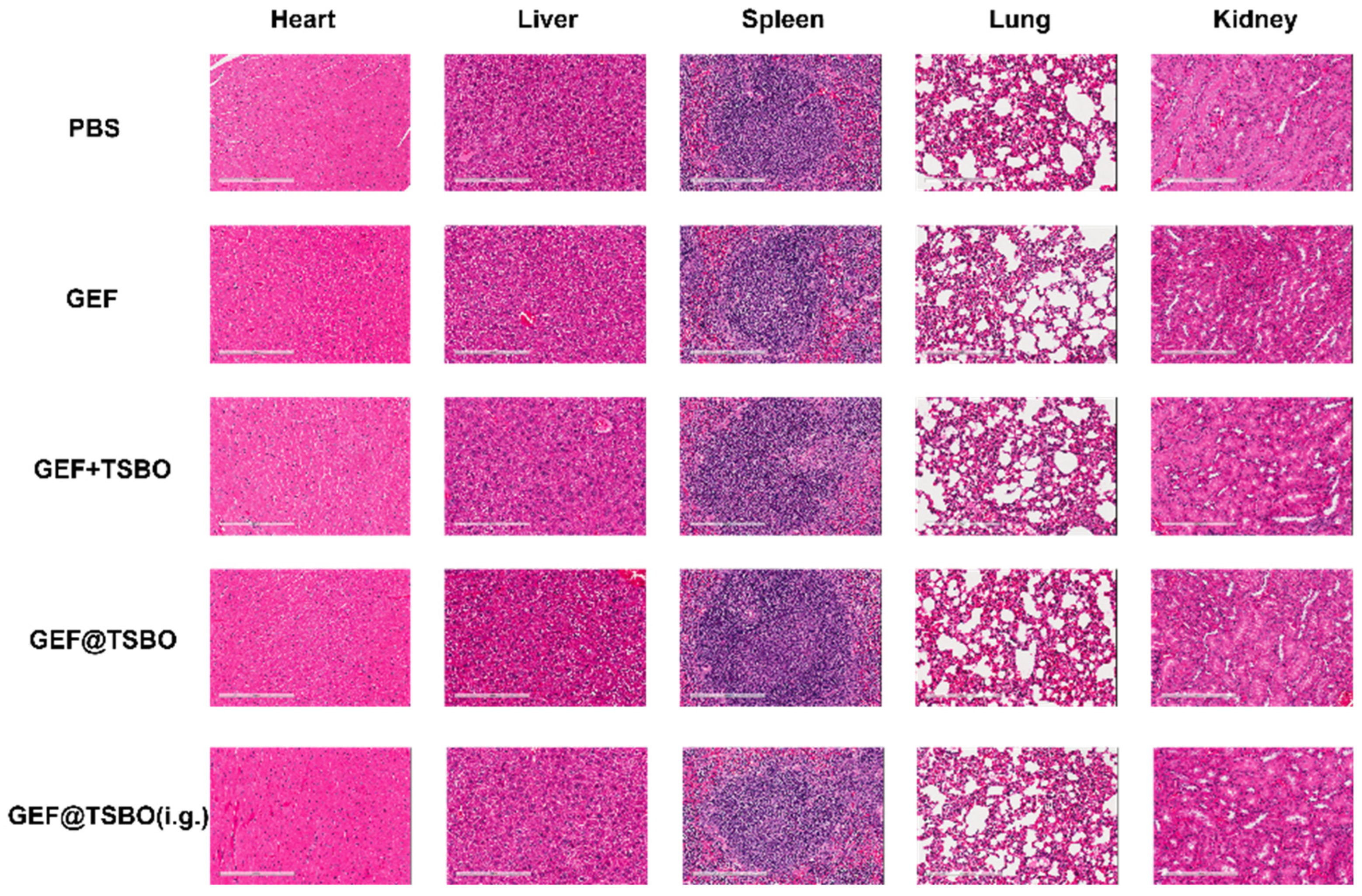
3.9. Antitumor Effect In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kofink, C.; Trainor, N.; Mair, B.; Wöhrle, S.; Wurm, M.; Mischerikow, N.; Roy, M.J.; Bader, G.; Greb, P.; Garavel, G.; et al. A Selective and Orally Bioavailable VHL-Recruiting PROTAC Achieves SMARCA2 Degradation in Vivo. Nat. Commun. 2022, 13, 5969. [Google Scholar] [CrossRef]
- Saraswat, A.L.; Vartak, R.; Hegazy, R.; Patel, A.; Patel, K. Drug Delivery Challenges and Formulation Aspects of Proteolysis Targeting Chimera (PROTACs). Drug Discov. Today 2023, 28, 103387. [Google Scholar] [CrossRef]
- Cerreia Vioglio, P.; Chierotti, M.R.; Gobetto, R. Pharmaceutical Aspects of Salt and Cocrystal Forms of APIs and Characterization Challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef]
- Benowitz, A.B.; Scott-Stevens, P.T.; Harling, J.D. Challenges and Opportunities for in Vivo PROTAC Delivery. Future Med. Chem. 2022, 14, 119–121. [Google Scholar]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Inam, M.; Phan, C.U.; Wang, J.-W.; Jamshed, M.; Hu, X.; Tang, G. Encapsulation of Vortioxetine with Cyclodextrins via Host–Guest Inclusion Complex: Synthesis, Characterization, Solubility, and in Vitro Dissolution Studies. J. Chin. Chem. Soc. 2023, 70, 956–966. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Wu, B.; Chen, F. Improving Solubility of Poorly Water-Soluble Drugs by Protein-Based Strategy: A Review. Int. J. Pharm. 2023, 634, 122704. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of Particle Size on Solubility, Dissolution Rate, and Oral Bioavailability: Evaluation Using Coenzyme Q10 as Naked Nanocrystals. Int. J. Nanomed. 2012, 7, 5733–5744. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Inam, M.; Liu, L.; Wang, J.-W.; Yu, K.-X.; Phan, C.-U.; Shen, J.; Zhang, W.-H.; Tang, G.; Hu, X. Enhancing the Physiochemical Properties of Puerarin via L-Proline Co-Crystallization: Synthesis, Characterization, and Dissolution Studies of Two Phases of Pharmaceutical Co-Crystals. Int. J. Mol. Sci. 2021, 22, 928. [Google Scholar]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical Cocrystals: A Review of Preparations, Physicochemical Properties and Applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Food and Drug Administration. Regulatory Classification of Pharmaceutical Co-Crystals; Guidance for Industry; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; pp. 1–4. [Google Scholar]
- Seki, T.; Hoshino, N.; Suzuki, Y.; Hayashi, S. Functional Flexible Molecular Crystals: Intrinsic and Mechanoresponsive Properties. CrystEngComm 2021, 23, 5686–5696. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; Bahia de Oliveira da Rocha, A.; Gonçalves Cardoso, S.; Rodríguez-Hornedo, N. Cocrystals to Facilitate Delivery of Poorly Soluble Compounds Beyond-Rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef]
- Wong, S.N.; Chen, Y.C.S.; Xuan, B.; Sun, C.C.; Chow, S.F. Cocrystal Engineering of Pharmaceutical Solids: Therapeutic Potential and Challenges. CrystEngComm 2021, 23, 7005–7038. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Phan, C.U.; Chen, Q.; Hu, X.; Shao, G.; Zhou, J.; Lai, L.; Tang, G. Cyclodextrin-Based Host-Guest Complexes Loaded with Regorafenib for Colorectal Cancer Treatment. Nat. Commun. 2021, 12, 759. [Google Scholar] [CrossRef]
- Thorat, S.H.; Sahu, S.K.; Patwadkar, M.V.; Badiger, M.V.; Gonnade, R.G. Drug–Drug Molecular Salt Hydrate of an Anticancer Drug Gefitinib and a Loop Diuretic Drug Furosemide: An Alternative for Multidrug Treatment. J. Pharm. Sci. 2015, 104, 4207–4216. [Google Scholar] [CrossRef]
- Fukuhara, T.; Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; et al. Factors Associated with a Poor Response to Gefitinib in the NEJ002 Study: Smoking and the L858R Mutation. Lung Cancer 2015, 88, 181–186. [Google Scholar] [CrossRef]
- Sharma, M.J.; Kumar, M.S.; Murahari, M.; Mayur, Y.C. Synthesis of Novel Gefitinib-Based Derivatives and Their Anticancer Activity. Arch. Pharm. 2019, 352, 1800381. [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Yuan, W.; He, K.; Keegan, P.; Pazdur, R. FDA Approval of Gefitinib for the Treatment of Patients with Metastatic EGFR Mutation–Positive Non–Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 1307–1312. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Q.; Huang, Y.; Guan, G.; Jiang, Y. Tailoring the Particle Microstructures of Gefitinib by Supercritical CO2 Anti-Solvent Process. J. CO2 Util. 2017, 20, 43–51. [Google Scholar] [CrossRef]
- Saha, R.; Sengupta, S.; Dey, S.K.; Steele, I.M.; Bhattacharyya, A.; Biswas, S.; Kumar, S. A Pharmaceutical Cocrystal with Potential Anticancer Activity. RSC Adv. 2014, 4, 49070–49078. [Google Scholar] [CrossRef]
- Inam, M.; Wu, J.; Shen, J.; Phan, C.U.; Tang, G.; Hu, X. Preparation and Characterization of Novel Pharmaceutical Co-Crystals: Ticagrelor with Nicotinamide. Crystals 2018, 8, 336. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Sugandha, K.; Kaity, S.; Mukherjee, S.; Isaac, J.; Ghosh, A. Solubility Enhancement of Ezetimibe by a Cocrystal Engineering Technique. Cryst. Growth Des. 2014, 14, 4475–4486. [Google Scholar]
- Roy, P.; Pandey, N.; Ghosh, A. Molecular Docking of Pharmaceutical Cocrystal As a Ligand: Do We Need to Rethink? Cryst. Growth Des. 2023, 23, 1–5. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced Methodologies for Cocrystal Synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef]
- Erxleben, A. Cocrystal Applications in Drug Delivery. Pharmaceutics 2020, 12, 834. [Google Scholar] [CrossRef]
- Newman, A.W.; Byrn, S.R. Solid-State Analysis of the Active Pharmaceutical Ingredient in Drug Products. Drug Discov. Today 2003, 8, 898–905. [Google Scholar] [CrossRef]
- Du, Y.; Fang, H.X.; Zhang, Q.; Zhang, H.L.; Hong, Z. Spectroscopic Investigation on Cocrystal Formation between Adenine and Fumaric Acid Based on Infrared and Raman Techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 580–585. [Google Scholar] [CrossRef]
- Peterson, R.D.; Theimer, C.A.; Wu, H.; Feigon, J. New Applications of 2D Filtered/Edited NOESY for Assignment and Structure Elucidation of RNA and RNA-Protein Complexes. J. Biomol. NMR 2004, 28, 59–67. [Google Scholar] [CrossRef]
- Ramesh, K.; Shekar, D.B.; Khadgapathi, P.; Bhikshapathi, D.; Renuka, K. Development, Characterization and in Vivo Evaluation of Tolvaptan Solid Dispersions via Solvent Evaporation Technique. Int. J. Drug Deliv. 2015, 7, 32–43. [Google Scholar]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Dhibar, M.; Chakraborty, S.; Basak, S.; Pattanayak, P.; Chatterjee, T.; Ghosh, B.; Raafat, M.; Abourehab, M.A.S. Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment. Pharmaceuticals 2023, 16, 284. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Garg, U.; Azim, Y.; Alam, M.; Gupta, A.K.; Pradeep, C.P.; Azum, N.; Asiri, A.M. Spectroscopic, Structural, DFT and Molecular Docking Studies on Novel Cocrystal Salt Hydrate of Chromotropic Acid and Its Antibiofilm Activity. Arab. J. Sci. Eng. 2020, 46, 353–364. [Google Scholar]
- Li, L.; Pang, Z.; Ma, K.; Gao, Y.; Zheng, D.; Wei, Y.; Zhang, J.; Qian, S. Effect of Coformer Selection on In Vitro and In Vivo Performance of Adefovir Dipivoxil Cocrystals. Pharm. Res. 2021, 38, 1777–1791. [Google Scholar] [CrossRef]
- Noubissi, F.K.; McBride, A.A.; Leppert, H.G.; Millet, L.J.; Wang, X.; Davern, S.M. Detection and Quantification of γ-H2AX Using a Dissociation Enhanced Lanthanide Fluorescence Immunoassay. Sci. Rep. 2021, 11, 8945. [Google Scholar] [CrossRef]
- Wang, X.; Weaver, D.T. The Ups and Downs of DNA Repair Biomarkers for PARP Inhibitor Therapies. Am. J. Cancer Res. 2011, 1, 301–327. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, M.; Yang, Y.; Hu, J.; Zheng, J.; Deng, W.; Zhou, Y.; Qi, J.; Xu, C.; Chai, G.; Dang, Y.; et al. Cocrystallization of Gefitinib Potentiate Single-Dose Oral Administration for Lung Tumor Eradication via Unbalancing the DNA Damage/Repair. Pharmaceutics 2023, 15, 2713. https://doi.org/10.3390/pharmaceutics15122713
Inam M, Yang Y, Hu J, Zheng J, Deng W, Zhou Y, Qi J, Xu C, Chai G, Dang Y, et al. Cocrystallization of Gefitinib Potentiate Single-Dose Oral Administration for Lung Tumor Eradication via Unbalancing the DNA Damage/Repair. Pharmaceutics. 2023; 15(12):2713. https://doi.org/10.3390/pharmaceutics15122713
Chicago/Turabian StyleInam, Muhammad, Yi Yang, Jialin Hu, Jiena Zheng, Wenxia Deng, You Zhou, Jialong Qi, Chuanshan Xu, Guihong Chai, Yuanye Dang, and et al. 2023. "Cocrystallization of Gefitinib Potentiate Single-Dose Oral Administration for Lung Tumor Eradication via Unbalancing the DNA Damage/Repair" Pharmaceutics 15, no. 12: 2713. https://doi.org/10.3390/pharmaceutics15122713
APA StyleInam, M., Yang, Y., Hu, J., Zheng, J., Deng, W., Zhou, Y., Qi, J., Xu, C., Chai, G., Dang, Y., & Chen, W. (2023). Cocrystallization of Gefitinib Potentiate Single-Dose Oral Administration for Lung Tumor Eradication via Unbalancing the DNA Damage/Repair. Pharmaceutics, 15(12), 2713. https://doi.org/10.3390/pharmaceutics15122713





