Advances in the Evaluation of Gastrointestinal Absorption Considering the Mucus Layer
Abstract
:1. Introduction
2. Gastrointestinal Mucus
3. Components of the Mucus Layer
3.1. Mucin
3.2. Glycans
3.3. Lipids
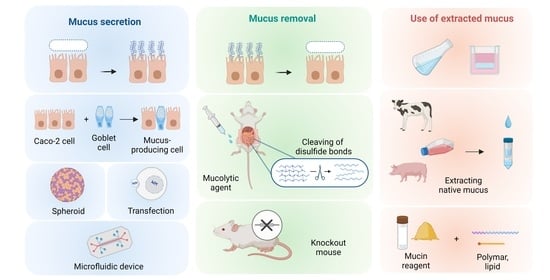
4. In Vivo Experimental Methods
4.1. Mucolytic Agent
4.2. Mucin Knockout/Mucin-Deficient Mice
5. In Vitro Experimental Methods
5.1. Mucus-Secreting Cells
5.2. Extracted and Mimicked Mucus
5.3. Microphysiological Systems (MPS)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Hens, B.; Corsetti, M.; Spiller, R.; Marciani, L.; Vanuytsel, T.; Tack, J.; Talattof, A.; Amidon, G.L.; Koziolek, M.; Weitschies, W.; et al. Exploring gastrointestinal variables affecting drug and formulation behavior: Methodologies, challenges and opportunities. Int. J. Pharm. 2017, 519, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J. Pharm. Pharmacol. 1997, 49, 627–638. [Google Scholar] [CrossRef]
- Terao, T.; Hisanaga, E.; Sai, Y.; Tamai, I.; Tsuji, A. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharmacol. 1996, 48, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Theissig, F.; Engelhardt, H.; Bengmark, S.; Koch, S.; Lochs, H.; Dörffel, Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 2007, 56, 343–350. [Google Scholar] [CrossRef]
- Zanin, M.; Baviskar, P.; Webster, R.; Webby, R. The interaction between respiratory pathogens and mucus. Cell Host Microbe 2016, 19, 159–168. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. Poorly Soluble Drugs 2013, 453, 56–64. [Google Scholar] [CrossRef]
- Lieleg, O.; Ribbeck, K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef]
- Zhou, T.; Arya, V.; Zhang, L. Comparing various in vitro prediction methods to assess the potential of a drug to inhibit P-glycoprotein (P-gp) transporter in vivo. J. Clin. Pharmacol. 2019, 59, 1049–1060. [Google Scholar] [CrossRef]
- Vaidyanathan, J.; Yoshida, K.; Arya, V.; Zhang, L. Comparing various in vitro prediction criteria to assess the potential of a new molecular entity to inhibit organic anion transporting polypeptide 1B1. J. Clin. Pharmacol. 2016, 56, S59–S72. [Google Scholar] [CrossRef]
- Lee, S.-C.; Arya, V.; Yang, X.; Volpe, D.A.; Zhang, L. Evaluation of transporters in drug development: Current status and contemporary issues. Adv. Drug Deliv. Rev. 2017, 116, 100–118. [Google Scholar] [CrossRef]
- Wang, C.K.; Craik, D.J. Cyclic peptide oral bioavailability: Lessons from the past. Pept. Sci. 2016, 106, 901–909. [Google Scholar] [CrossRef]
- Naylor, M.R.; Bockus, A.T.; Blanco, M.-J.; Lokey, R.S. Cyclic peptide natural products chart the frontier of oral bioavailability in the pursuit of undruggable targets. Curr. Opin. Chem. Biol. 2017, 38, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.R.; Jones, L.H. Clinical translation of targeted protein degraders. Clin. Pharmacol. Ther. 2023, 114, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, K.T.G.; Crews, C.M. Targeted protein degradation: A promise for undruggable proteins. Cell Chem. Biol. 2021, 28, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, H.; Ridley, C.; Thornton, D.J. The lipophilic cyclic peptide cyclosporin A induces aggregation of gel-forming mucins. Sci. Rep. 2022, 12, 6153. [Google Scholar] [CrossRef]
- Lee, D.R.; Ho, M.J.; Choi, Y.W.; Kang, M.J. A polyvinylpyrrolidone-based supersaturable self-emulsifying drug delivery system for enhanced dissolution of cyclosporine A. Polymers 2017, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Runge, S.; Ravelli, V.; Mehnert, W.; Thünemann, A.F.; Souto, E.B. Oral bioavailability of cyclosporine: Solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int. J. Pharm. 2006, 317, 82–89. [Google Scholar] [CrossRef]
- Rathod, D.; Fu, Y.; Patel, K. BRD4 PROTAC as a novel therapeutic approach for the treatment of vemurafenib resistant melanoma: Preformulation studies, formulation development and in vitro evaluation. Eur. J. Pharm. Sci. 2019, 138, 105039. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Sarwar, H.S.; Sohail, M.F.; Imran, M.; Qureshi, O.S.; Raza, A.; Ahmad, N.M.; Iqbal, A.; Shahnaz, G. Synthesis and characterization of pre-activated thiolated chitosan nanoparticles for oral delivery of octreotide. J. Drug Deliv. Sci. Technol. 2020, 58, 101807. [Google Scholar] [CrossRef]
- Larhed, A.W.; Artursson, P.; Björk, E. The influence of intestinal mucus components on the diffusion of drugs. Pharm. Res. 1998, 15, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C.; Johansson, M.E. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 2010, 1, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Ermund, A.; Johansson, M.E.V.; Schütte, A.; Hansson, G.C.; Sjövall, H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G430–G438. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, E.K.; Noaksson, K.A.; Phillipson, M.; Johansson, M.E.V.; Hinojosa-Kurtzberg, M.; Holm, L.; Gendler, S.J.; Hansson, G.C. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G203–G210. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.E.; Liu, S.; Lwin, T.M.; Hoffman, R.M.; Batra, S.K.; Bouvet, M. The mucin family of proteins: Candidates as potential biomarkers for colon cancer. Cancers 2023, 15, 1491. [Google Scholar] [CrossRef] [PubMed]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef]
- Bafna, S.; Kaur, S.; Batra, S. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010, 29, 2893–2904. [Google Scholar] [CrossRef]
- Miyazaki, K.; Kishimoto, H.; Kobayashi, H.; Suzuki, A.; Higuchi, K.; Shirasaka, Y.; Inoue, K. The glycosylated N-terminal domain of MUC1 is involved in chemoresistance by modulating drug permeation across the plasma membrane. Mol. Pharmacol. 2022, 103, 166–175. [Google Scholar] [CrossRef]
- Demouveaux, B.; Gouyer, V.; Robbe-Masselot, C.; Gottrand, F.; Narita, T.; Desseyn, J.-L. Mucin CYS domain stiffens the mucus gel hindering bacteria and spermatozoa. Sci. Rep. 2019, 9, 16993. [Google Scholar] [CrossRef]
- Thornton, D.J.; Sheehan, J.K. From mucins to mucus: Toward a more coherent understanding of this essential barrier. Proc. Am. Thorac. Soc. 2004, 1, 54–61. [Google Scholar] [CrossRef]
- Dawson, M.; Wirtz, D.; Hanes, J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J. Biol. Chem. 2003, 278, 50393–50401. [Google Scholar] [CrossRef]
- Davies, J.R.; Svitacheva, N.; Lannefors, L.; Kornfält, R.; Carlstedt, I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem. J. 1999, 344, 321–330. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Sakura, R.; Nagai, K.; Yagi, Y.; Takahashi, Y.; Ide, Y.; Yagi, Y.; Yamamoto, D.; Mizuno, M.; Sato, T. In vitro synthesis of mucin-type O-glycans using saccharide primers comprising GalNAc-Ser and GalNAc-Thr residues. Carbohydr. Res. 2022, 511, 108495. [Google Scholar] [CrossRef]
- Holmén Larsson, J.M.; Thomsson, K.A.; Rodríguez-Piñeiro, A.M.; Karlsson, H.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G357–G363. [Google Scholar] [CrossRef]
- Karlsson, N.G.; Herrmann, A.; Karlsson, H.; Johansson, M.E.V.; Carlstedt, I.; Hansson, G.C. The glycosylation of rat intestinal Muc2 mucin varies between rat strains and the small and large intestine: A study of O-linked oligosaccharides by a mass spectrometric approach. J. Biol. Chem. 1997, 272, 27025–27034. [Google Scholar] [CrossRef] [PubMed]
- Robbe, C.; Capon, C.; Coddeville, B.; Michalski, J.-C. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 2004, 384, 307–316. [Google Scholar] [CrossRef]
- Kalra, A.V.; Campbell, R.B. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. Eur. J. Cancer 2009, 45, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.V.; Campbell, R.B. Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: Overcoming cellular barriers for therapeutic gain. Br. J. Cancer 2007, 97, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S. Mammalian lipids: Structure, synthesis and function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
- Nadziejko, C.E.; Slomiany, B.L.; Slomiany, A. Most of the lipid in purulent sputum is bound to mucus glycoprotein. Exp. Lung Res. 1993, 19, 671–684. [Google Scholar] [CrossRef]
- Murty, V.L.; Sarosiek, J.; Slomiany, A.; Slomiany, B.L. Effect of lipids and proteins on the viscosity of gastric mucus glycoprotein. Biochem. Biophys. Res. Commun. 1984, 121, 521–529. [Google Scholar] [CrossRef]
- Gwozdzinski, K.; Slomiany, A.; Nishikawa, H.; Okazaki, K.; Slomiany, B.L. Gastric mucin hydrophobicity: Effects of associated and covalently bound lipids, proteolysis, and reduction. Biochem. Int. 1988, 17, 907–917. [Google Scholar] [PubMed]
- Yildiz, H.M.; Speciner, L.; Ozdemir, C.; Cohen, D.E.; Carrier, R.L. Food-associated stimuli enhance barrier properties of gastrointestinal mucus. Biomaterials 2015, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Kerss, S.; Allen, A.; Garner, A. A simple method for measuring thickness of the mucus gel layer adherent to rat, frog and human gastric mucosa: Influence of feeding, prostaglandin, N-acetylcysteine and other agents. Clin. Sci. 1982, 63, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, S.; Kitazawa, T.; Morita, T.; Horikiri, Y.; Yoshino, H. Enhancement of intestinal absorption of poorly absorbed hydrophilic compounds by simultaneous use of mucolytic agent and non-ionic surfactant. Eur. J. Pharm. Biopharm. 2006, 62, 52–58. [Google Scholar] [CrossRef]
- Masaoka, Y.; Tanaka, Y.; Kataoka, M.; Sakuma, S.; Yamashita, S. Site of drug absorption after oral administration: Assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur. J. Pharm. Sci. 2006, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Kishimoto, H.; Muratani, M.; Kobayashi, H.; Shirasaka, Y.; Inoue, K. Mucins are involved in the intestinal permeation of lipophilic drugs in the proximal region of rat small intestine. Pharm. Res. 2019, 36, 162. [Google Scholar] [CrossRef]
- Wang, H.H.; Afdhal, N.H.; Gendler, S.J.; Wang, D.Q.-H. Lack of the intestinal Muc1 mucin impairs cholesterol uptake and absorption but not fatty acid uptake in Muc1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G547–G554. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.A.; Achasova, K.M.; Morozova, K.N.; Andreyeva, E.N.; Litvinova, E.A.; Ogienko, A.A.; Morozova, M.V.; Berkaeva, M.B.; Kiseleva, E.; Kozhevnikova, E.N. Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium. Sci. Rep. 2020, 10, 21135. [Google Scholar] [CrossRef]
- Phillipson, M.; Johansson, M.E.V.; Henriksnäs, J.; Petersson, J.; Gendler, S.J.; Sandler, S.; Persson, A.E.G.; Hansson, G.C.; Holm, L. The gastric mucus layers: Constituents and regulation of accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G806–G812. [Google Scholar] [CrossRef]
- Pothuraju, R.; Pai, P.; Chaudhary, S.; Siddiqui, J.A.; Cox, J.L.; Kaur, S.; Rachagani, S.; Roy, H.K.; Bouvet, M.; Batra, S.K. Depletion of transmembrane mucin 4 (Muc4) alters intestinal homeostasis in a genetically engineered mouse model of colorectal cancer. Aging 2022, 14, 2025–2046. [Google Scholar] [CrossRef]
- Sheng, Y.H.; Lourie, R.; Lindén, S.K.; Jeffery, P.L.; Roche, D.; Tran, T.V.; Png, C.W.; Waterhouse, N.; Sutton, P.; Florin, T.H.J.; et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 2011, 60, 1661–1670. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Olli, K.E.; Rapp, C.; O’Connell, L.; Collins, C.B.; McNamee, E.N.; Jensen, O.; Jedlicka, P.; Allison, K.C.; Goldberg, M.S.; Gerich, M.E.; et al. Muc5ac expression protects the colonic barrier in experimental colitis. Inflamm. Bowel Dis. 2020, 26, 1353–1367. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Karlsson, J.; Wikman, A.; Artursson, P. The mucus layer as a barrier to drug absorption in monolayers of human intestinal epithelial HT29-H goblet cells. Int. J. Pharm. 1993, 99, 209–218. [Google Scholar] [CrossRef]
- Wikman, A.; Karlsson, J.; Carlstedt, I.; Artursson, P. A drug absorption model based on the mucus layer producing human intestinal goblet cell line HT29-H. Pharm. Res. 1993, 10, 843–852. [Google Scholar] [CrossRef]
- Pontier, C.; Pachot, J.; Botham, R.; Lenfant, B.; Arnaud, P. HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: Role of the mucus layer. J. Pharm. Sci. 2001, 90, 1608–1619. [Google Scholar] [CrossRef]
- Wikman-Larhed, A.; Artursson, P. Co-cultures of human intestinal goblet (HT29-H) and absorptive (Caco-2) cells for studies of drug and peptide absorption. Eur. J. Pharm. Sci. 1995, 3, 171–183. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- McCright, J.; Sinha, A.; Maisel, K. Generating an in vitro gut model with physiologically relevant biophysical mucus properties. Cell. Mol. Bioeng. 2022, 15, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Lesuffleur, T.; Porchet, N.; Aubert, J.P.; Swallow, D.; Gum, J.R.; Kim, Y.S.; Real, F.X.; Zweibaum, A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993, 106, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Mahlert, L.; Anderski, J.; Mulac, D.; Langer, K. The impact of gastrointestinal mucus on nanoparticle penetration–in vitro evaluation of mucus-penetrating nanoparticles for photodynamic therapy. Eur. J. Pharm. Sci. 2019, 133, 28–39. [Google Scholar] [CrossRef]
- Kodama, N.; Iwao, T.; Katano, T.; Ohta, K.; Yuasa, H.; Matsunaga, T. Characteristic analysis of intestinal transport in enterocyte-like cells differentiated from human induced pluripotent stem cells. Drug Metab. Dispos. 2016, 44, 1662–1667. [Google Scholar] [CrossRef]
- Kauffman, A.L.; Gyurdieva, A.V.; Mabus, J.R.; Ferguson, C.; Yan, Z.; Hornby, P.J. Alternative functional in vitro models of human intestinal epithelia. Front. Pharmacol. 2013, 4, 79. [Google Scholar] [CrossRef]
- Ozawa, T.; Takayama, K.; Okamoto, R.; Negoro, R.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Mizuguchi, H. Generation of enterocyte-like cells from human induced pluripotent stem cells for drug absorption and metabolism studies in human small intestine. Sci. Rep. 2015, 5, 16479. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, T.; Mima, S.; Imakura, Y.; Miyashita, T.; Ogura, I.; Yamada, T.; Yasujima, T.; Yuasa, H.; Iwao, T.; Matsunaga, T. Pharmacokinetic functions of human induced pluripotent stem cell-derived small intestinal epithelial cells. Drug Metab. Pharmacokinet. 2020, 35, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Mizuno, S.; Hashita, T.; Iwao, T.; Matsunaga, T. Using human iPS cell-derived enterocytes as novel in vitro model for the evaluation of human intestinal mucosal damage. Inflamm. Res. 2018, 67, 975–984. [Google Scholar] [CrossRef]
- Múnera, J.O.; Sundaram, N.; Rankin, S.A.; Hill, D.; Watson, C.; Mahe, M.; Vallance, J.E.; Shroyer, N.F.; Sinagoga, K.L.; Zarzoso-Lacoste, A.; et al. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell. 2017, 21, 51–64.e6. [Google Scholar] [CrossRef]
- Onozato, D.; Yamashita, M.; Nakanishi, A.; Akagawa, T.; Kida, Y.; Ogawa, I.; Hashita, T.; Iwao, T.; Matsunaga, T. Generation of intestinal organoids suitable for pharmacokinetic studies from human induced pluripotent stem cells. Drug Metab. Dispos. 2018, 46, 1572–1580. [Google Scholar] [CrossRef]
- King, M. Is cystic fibrosis mucus abnormal? Pediatr. Res. 1981, 15, 120–122. [Google Scholar] [CrossRef]
- Thornton, D.J.; Gray, T.; Nettesheim, P.; Howard, M.; Koo, J.S.; Sheehan, J.K. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L1118–L1128. [Google Scholar] [CrossRef]
- Larhed, A.W.; Artursson, P.; Gråsjö, J.; Björk, E. Diffusion of drugs in native and purified gastrointestinal mucus. J. Pharm. Sci. 1997, 86, 660–665. [Google Scholar] [CrossRef]
- Park, M.-S.; Chung, J.-W.; Kim, Y.-K.; Chung, S.-C.; Kho, H.-S. Viscosity and wettability of animal mucin solutions and human saliva. Oral Dis. 2007, 13, 181–186. [Google Scholar] [CrossRef]
- B Boegh, M.; Baldursdóttir, S.G.; Müllertz, A.; Nielsen, H.M. Property profiling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption. Eur. J. Pharm. Biopharm. 2014, 87, 227–235. [Google Scholar] [CrossRef]
- Kocevar-Nared, J.; Kristl, J.; Smid-Korbar, J. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials 1997, 18, 677–681. [Google Scholar] [CrossRef]
- Schömig, V.J.; Käsdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef]
- Svensson, O.; Arnebrant, T. Mucin layers and multilayers—Physicochemical properties and applications. Curr. Opin. Colloid Interface Sci. 2010, 15, 395–405. [Google Scholar] [CrossRef]
- Boegh, M.; García-Díaz, M.; Müllertz, A.; Nielsen, H.M. Steric and interactive barrier properties of intestinal mucus elucidated by particle diffusion and peptide permeation. Eur. J. Pharm. Biopharm. 2015, 95, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.; Butnarasu, C.; Briatico Vangosa, F.; Pastorino, L.; Visai, L.; Visentin, S.; Petrini, P. Disassembling the complexity of mucus barriers to develop a fast screening tool for early drug discovery. J. Mater. Chem. 2019, 7, 4940–4952. [Google Scholar] [CrossRef] [PubMed]
- Butnarasu, C.; Caron, G.; Pacheco, D.P.; Petrini, P.; Visentin, S. Cystic fibrosis mucus model to design more efficient drug therapies. Mol. Pharm. 2022, 19, 520–531. [Google Scholar] [CrossRef]
- Friedl, H.; Dünnhaupt, S.; Hintzen, F.; Waldner, C.; Parikh, S.; Pearson, J.P.; Wilcox, M.D.; Bernkop-Schnürch, A. Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 2013, 102, 4406–4413. [Google Scholar] [CrossRef]
- Banh, L.; Cheung, K.K.; Chan, M.W.Y.; Young, E.W.K.; Viswanathan, S. Advances in organ-on-a-chip systems for modelling joint tissue and osteoarthritic diseases. Osteoarthr. Cartil. 2022, 30, 1050–1061. [Google Scholar] [CrossRef]
- Franzen, N.; van Harten, W.H.; Retèl, V.P.; Loskill, P.; van den Eijnden-van Raaij, J.; IJzerman, M. Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov. Today 2019, 24, 1720–1724. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Izadifar, Z.; Sontheimer-Phelps, A.; Lubamba, B.A.; Bai, H.; Fadel, C.; Stejskalova, A.; Ozkan, A.; Dasgupta, Q.; Bein, A.; Junaid, A.; et al. Modeling mucus physiology and pathophysiology in human organs-on-chips. Adv. Drug Deliv. Rev. 2022, 191, 114542. [Google Scholar] [CrossRef]
- Kimura, H.; Yamamoto, T.; Sakai, H.; Sakai, Y.; Fujii, T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab A Chip 2008, 8, 741–746. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.; Choi, N.; Kim, H.N.; Kim, B.; Sung, J.H. Development of gut-mucus chip for intestinal absorption study. BioChip J. 2023, 17, 230–243. [Google Scholar] [CrossRef]
- Park, J.-K. Drug permeability assay using microhole-trapped cells in a microfluidic device. Anal. Chem. 2009, 81, 1944–1951. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab A Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Trier, S.; Rahbek, U.L.; Dufva, M.; Kutter, J.P.; Andresen, T.L. A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS ONE 2018, 13, e0197101. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Chou, D.B.; Tovaglieri, A.; Ferrante, T.C.; Duckworth, T.; Fadel, C.; Frismantas, V.; Sutherland, A.D.; Jalili-Firoozinezhad, S.; Kasendra, M.; et al. Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 507–526. [Google Scholar] [CrossRef]
- Kasendra, M.; Luc, R.; Yin, J.; Manatakis, D.V.; Kulkarni, G.; Lucchesi, C.; Sliz, J.; Apostolou, A.; Sunuwar, L.; Obrigewitch, J.; et al. Duodenum intestine-chip for preclinical drug assessment in a human relevant model. eLife 2020, 9, e50135. [Google Scholar] [CrossRef]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 2018, 8, 2871. [Google Scholar] [CrossRef]
- Jia, Z.; Guo, Z.; Yang, C.-T.; Prestidge, C.; Thierry, B. “Mucus-on-Chip”: A new tool to study the dynamic penetration of nanoparticulate drug carriers into mucus. Int. J. Pharm. 2021, 598, 120391. [Google Scholar] [CrossRef]
- Wright, L.; Wignall, A.; Jõemetsa, S.; Joyce, P.; Prestidge, C.A. A membrane-free microfluidic approach to mucus permeation for efficient differentiation of mucoadhesive and mucopermeating nanoparticulate systems. Drug Deliv. Transl. Res. 2023, 13, 1088–1101. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, K.; Sasaki, A.; Mizuuchi, H. Advances in the Evaluation of Gastrointestinal Absorption Considering the Mucus Layer. Pharmaceutics 2023, 15, 2714. https://doi.org/10.3390/pharmaceutics15122714
Miyazaki K, Sasaki A, Mizuuchi H. Advances in the Evaluation of Gastrointestinal Absorption Considering the Mucus Layer. Pharmaceutics. 2023; 15(12):2714. https://doi.org/10.3390/pharmaceutics15122714
Chicago/Turabian StyleMiyazaki, Kaori, Akira Sasaki, and Hiroshi Mizuuchi. 2023. "Advances in the Evaluation of Gastrointestinal Absorption Considering the Mucus Layer" Pharmaceutics 15, no. 12: 2714. https://doi.org/10.3390/pharmaceutics15122714
APA StyleMiyazaki, K., Sasaki, A., & Mizuuchi, H. (2023). Advances in the Evaluation of Gastrointestinal Absorption Considering the Mucus Layer. Pharmaceutics, 15(12), 2714. https://doi.org/10.3390/pharmaceutics15122714