Modified Potato Starch as a Potential Retardant for Prolonged Release of Lidocaine Hydrochloride from Methylcellulose Hydrophilic Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Modified Starches
2.2.2. Preparation of Hydrophilic Formulations with Lidocaine Hydrochloride
2.2.3. Evaluation of the Release Kinetics of LH
Release Study
Kinetic Models
2.2.4. Differential Scanning Calorimetry (DSC) and Fourier-Transform Infrared Spectroscopy (FTIR) of LH Compositions with Modified Starches
2.2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. Determination of the Viscosity of the Formulations
2.2.8. Determination of pH of Starch Samples and Evaluated Formulations
2.2.9. Visualization of the Starch Samples
3. Results
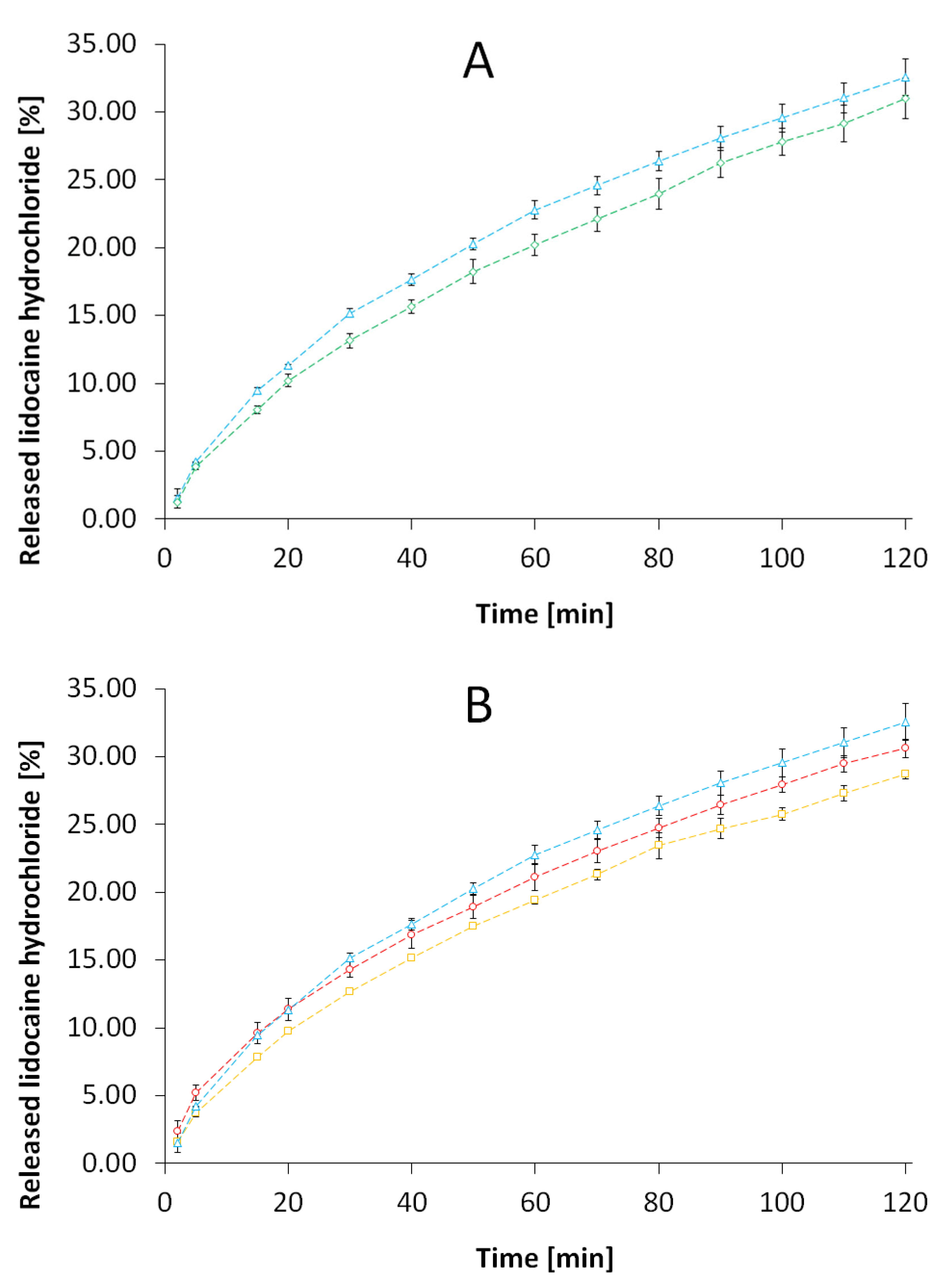
3.1. Evaluation of the Release Kinetics of LH from the Formulations
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. Differential Scanning Calorimetry (DSC)
3.4. Determination of the Viscosity of the Formulations
3.5. Determination of pH of Starch Samples and Evaluated Formulations
3.6. Visualization of the Starch Samples
4. Discussion
4.1. Evaluation of the Release Kinetics of LH from the Formulations
4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
4.3. Differential Scanning Calorimetry (DSC)
4.4. Determination of the Viscosity of the Formulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daraz, Y.M.; Abdelghffar, O.H. Lidocaine Infusion: An Antiarrhythmic with Neurologic Toxicities. Cureus 2022, 2019, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, Z.; Gökçe, Ö. Tribenoside and lidocaine in the local treatment of hemorrhoids: An overview of clinical evidence. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2742–2751. [Google Scholar]
- Finder, R.; Moore, P. Adverse drug reactions to local anesthetic. Dent. Clin. N. Am. 2002, 46, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Mishra, B.P.; Awasthi, D.; Sahoo, A. Articaine as an alternative in lidocaine allergy: Case report of a seventy year old male patient. Int. J. Surg. Case Rep. 2020, 77, 941–943. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Rehan, M.; El-Sawy, S.M.; El-bisi, M.K.; Ahmed-Farid, O.A.; Abdel-Mohdy, F.A. Lidocaine/β-cyclodextrin inclusion complex as drug delivery system. Eur. Polym. J. 2018, 108, 304–310. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Rajabalaya, R.; Chakraborty, S.; Nanda, A. Mathematical modeling of drug release profiles for modified hydrophobic HPMC based gels. Pharmazie 2012, 67, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.B.; Rosen, M.R. The Rheology Modifiers Handbook: Practical Use & Application; William Andrew Pub: Norwich, New York, USA, 2000; ISBN 0815514417. [Google Scholar]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Halal, S.L.M.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreño, N.L.V.; Dias, A.R.G.; da Rosa Zavareze, E. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, T.S.; Felizardo, S.G.; Jane, J.; Franco, C.M.L. Effect of annealing on the semicrystalline structure of normal and waxy corn starches. Food Hydrocoll. 2012, 29, 93–99. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications-A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Sun, H.; Shao, X.; Jiang, R.; Shen, Z.; Ma, Z. Mechanical and barrier properties of corn distarch phosphate-zein bilayer films by thermocompression. Int. J. Biol. Macromol. 2018, 118, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients; Royal Pharmaceutical Society of Great Britain: London, UK, 2009. [Google Scholar]
- Kapelko-Żeberska, M.; Zięba, T.; Pietrzak, W.; Gryszkin, A. Effect of citric acid esterification conditions on the properties of the obtained resistant starch. Int. J. Food Sci. Technol. 2016, 51, 1647–1654. [Google Scholar] [CrossRef]
- Kapelko-Zeberska, M.; Buksa, K.; Szumny, A.; Zieba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT-Food Sci. Technol. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Kobryń, J.; Zięba, T.; Sowa, S.K.; Musiał, W. Influence of acetylated annealed starch on the release of β-escin from the anionic and non-ionic hydrophilic gels. Pharmaceutics 2020, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Zięba, T.; Wilczak, A.; Kobryń, J.; Musiał, W.; Kapelko-Żeberska, M.; Gryszkin, A.; Meisel, M. The annealing of acetylated potato starch with various substitution degrees. Molecules 2021, 26, 2096. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Berenjian, K.; Khazaei, R. Preparation of the potential ocular inserts by electrospinning method to achieve the prolong release profile of triamcinolone acetonide. Adv. Pharm. Bull. 2018, 8, 21–27. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Pooshang Bagheri, K.; Shokrgozar, M.A. Prolongation of bactericidal efficiency of chitosan—Bioactive glass coating by drug controlled release. Prog. Org. Coat. 2020, 139, 105440. [Google Scholar] [CrossRef]
- Pettit, N.N.; Miceli, M.H.; Rivera, C.G.; Narayanan, P.P.; Perissinotti, A.J.; Hsu, M.; Delacruz, J.; Gedrimaite, Z.; Han, Z.; Steinbeck, J.; et al. Multicentre study of posaconazole delayed-release tablet serum level and association with hepatotoxicity and QTc prolongation. J. Antimicrob. Chemother. 2017, 72, 2355–2358. [Google Scholar] [CrossRef] [Green Version]
- Marioane, C.A.; Bunoiu, M.; Mateescu, M.; Sfîrloagă, P.; Vlase, G.; Vlase, T. Preliminary study for the preparation of transmucosal or transdermal patches with acyclovir and lidocaine. Polymers 2021, 13, 3596. [Google Scholar] [CrossRef] [PubMed]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.; Hollmann, M.W.; Stevens, M.F.; Lirk, P.; Brandenburger, T.; Piegeler, T.; Werdehausen, R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: A narrative review. Br. J. Anaesth. 2019, 123, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhu, K.J. The release behavior of brilliant blue from calcium-alginate gel beads coated by chitosan: The preparation method effect. Eur. J. Pharm. Biopharm. 2002, 53, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H.S. Masking the Bitter Taste of Injectable Lidocaine HCl Formulation for Dental Procedures. AAPS PharmSciTech 2014, 16, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, B.; Roy, G.; Ghosh, S. Development of Denticap, a Matrix Based Sustained Release Formulation for Treatment of Toothache, Dental Infection and Other Gum Problem. Curr. Drug Deliv. 2009, 6, 199–207. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Grzybowska, K.; Hawelek, L.; Swiety-Pospiech, A.; Masiewicz, E.; Paluch, M.; Sawicki, W.; Chmielewska, A.; Bujak, P.; Markowski, J. Molecular dynamics studies on the water mixtures of pharmaceutically important ionic liquid lidocaine HCl. Mol. Pharm. 2012, 9, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Habitante, A.M.B.Q.; Sobral, P.J.A.; Carvalho, R.A.; Solorza-Feria, J.; Bergo, P.V.A. Phase transitions of cassava starch dispersions prepared with glycerol solutions. J. Therm. Anal. Calorim. 2008, 93, 599–604. [Google Scholar] [CrossRef]
- Donmez, O.A.; Bozdogan, A.; Kunt, G.; Div, Y. Spectrophotometric Multicomponent Analysis of a Mixture of Chlorhexidine Hydrochloride and Lidocaine Hydrochloride in Pharmaceutical Formulation Using Derivative Spectrophotometry and Partial LeasttSquares Multivariate Calibration 1. J. Anal. Chem. 2010, 65, 30–35. [Google Scholar] [CrossRef]
- Lee, S.L.; Raw, A.S.; Yu, L. Dissolution Testing, in Biopharmaceutics Applications in Drug Development; Springer: New York, NY, USA, 2008; Volume 3, pp. 49–50. [Google Scholar]
- Costa, P.; Sousa Lo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Gohel, M.C.; Panchal, M.K.; Jogani, V.V. Novel mathematical method for quantitative expression of deviation from the Higuchi model. AAPS PharmSciTech 2000, 1, 6. [Google Scholar] [CrossRef]
- Kanwar, N.; Kumar, R.; Sinha, V.R. Preparation and Evaluation of Multi-Particulate System (Pellets) of Prasugrel Hydrochloride. Open Pharm. Sci. J. 2015, 2, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Sjöberg, H.; Karami, K.; Beronius, P.; Sundelöf, L.O. Ionization conditions for iontophoretic drug delivery. A revised pK(a) of lidocaine hydrochloride in aqueous solution at 25 °C established by precision conductometry. Int. J. Pharm. 1996, 141, 63–70. [Google Scholar] [CrossRef]
- Lagan, G.; McLure, H.A. Review of local anaesthetic agents. Curr. Anaesth. Crit. Care 2004, 15, 247–254. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Barrios-Guzmán, A.J.; García-Enriquez, S.; de Jesús Rivera-Prado, J.; Manríquez-González, R. Chemical and Mechanical Evaluation of Bio-composites Based on Thermoplastic Starch and Wood Particles Prepared by Thermal Compression. BioResources 2014, 9, 2960–2974. [Google Scholar] [CrossRef]
- Gala, U.; Chuong, M.C.; Varanasi, R.; Chauhan, H. Characterization and Comparison of Lidocaine-Tetracaine and Lidocaine-Camphor Eutectic Mixtures Based on Their Crystallization and Hydrogen-Bonding Abilities. AAPS PharmSciTech 2015, 16, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Blaine, R.L. Determination of Polyethylene Crystallinity by DSC. TA Instrum. 2002, 2002, 1–3. [Google Scholar]









| Starch Type | CA, dm [%] | NPS, dm [%] | Roasting Temperature [°C] |
|---|---|---|---|
| S1 | 2.5 | 97.5 | 120 |
| S2 | 2.5 | 97.5 | 140 |
| S3 | 5.0 | 95.0 | 120 |
| S4 | 5.0 | 95.0 | 140 |
| C | LH [g] | MC [g] | S1 [g] | S2 [g] | S3 [g] | S4 [g] | Water [g] | |
|---|---|---|---|---|---|---|---|---|
| F | ||||||||
| F0 | 2.0 | 2.0 | 1.0 | 95.0 | ||||
| F1 | 2.0 | 2.5 | 3.0 | 92.5 | ||||
| F2 | 2.0 | 2.5 | 3.0 | 92.5 | ||||
| F3 | 2.0 | 2.5 | 3.0 | 92.5 | ||||
| F4 | 2.0 | 2.5 | 3.0 | 92.5 | ||||
| REF | 2.0 | 2.0 | 96.0 | |||||
| Kinetic Process | Equation | Release Rate |
|---|---|---|
| Zero order | ||
| 1st order | ||
| 2nd order | ||
| Higuchi model |
| C | LH [g] | S1 [g] | S2 [g] | S3 [g] | S4 [g] | Water [g] | |
|---|---|---|---|---|---|---|---|
| F | |||||||
| HS1 | 0.8278 | 1.6556 | 3.0 | ||||
| HS2 | 0.7949 | 1.5899 | 3.0 | ||||
| HS3 | 0.9725 | 1.9449 | 3.0 | ||||
| HS4 | 0.9790 | 1.9581 | 3.0 | ||||
| Formulation | SD | SD | SD | SD | ||||
|---|---|---|---|---|---|---|---|---|
| F0 | 2.37 × 10−1 | 6.80 × 10−3 | 2.89 × 10−3 | 1.05 × 10−4 | 3.54 × 10−5 | 1.57 × 10−6 | 3.11 | 0.08 |
| r2 | 0.9692 | 0.9837 | 0.9933 | 0.9984 | ||||
| F1 | 2.25 × 10−1 | 6.63 × 10−4 | 2.76 × 10−3 | 8.19 × 10−6 | 3.40 × 10−5 | 3.04 × 10−7 | 2.96 | 0.00 |
| r2 | 0.9603 | 0.9767 | 0.9886 | 0.9996 | ||||
| F2 | 4.09 × 10−2 | 4.33 × 10−4 | 9.65 × 10−4 | 3.24 × 10−5 | 2.64 × 10−5 | 1.75 × 10−6 | 1.91 | 0.02 |
| r2 | 0.9376 | 0.9872 | 0.9996 | 0.9832 | ||||
| F3 | 3.85 × 10−2 | 2.12 × 10−4 | 9.35 × 10−4 | 6.74 × 10−6 | 2.68 × 10−5 | 6.77 × 10−7 | 1.87 | 0.01 |
| r2 | 0.9791 | 0.9962 | 0.9957 | 0.9957 | ||||
| F4 | 1.87 × 10−1 | 7.12 × 10−3 | 2.20 × 10−3 | 7.94 × 10−5 | 2.59 × 10−5 | 8.93 × 10−7 | 2.35 | 0.09 |
| r2 | 0.9679 | 0.9787 | 0.9871 | 0.9964 | ||||
| REF | 3.77 × 10−2 | 7.91 × 10−4 | 8.52 × 10−4 | 5.75 × 10−5 | 2.23 × 10−5 | 2.81 × 10−6 | 1.82 | 0.04 |
| r2 | 0.9681 | 0.9908 | 0.9875 | 0.9920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobryń, J.; Raszewski, B.; Zięba, T.; Musiał, W. Modified Potato Starch as a Potential Retardant for Prolonged Release of Lidocaine Hydrochloride from Methylcellulose Hydrophilic Gel. Pharmaceutics 2023, 15, 387. https://doi.org/10.3390/pharmaceutics15020387
Kobryń J, Raszewski B, Zięba T, Musiał W. Modified Potato Starch as a Potential Retardant for Prolonged Release of Lidocaine Hydrochloride from Methylcellulose Hydrophilic Gel. Pharmaceutics. 2023; 15(2):387. https://doi.org/10.3390/pharmaceutics15020387
Chicago/Turabian StyleKobryń, Justyna, Bartosz Raszewski, Tomasz Zięba, and Witold Musiał. 2023. "Modified Potato Starch as a Potential Retardant for Prolonged Release of Lidocaine Hydrochloride from Methylcellulose Hydrophilic Gel" Pharmaceutics 15, no. 2: 387. https://doi.org/10.3390/pharmaceutics15020387
APA StyleKobryń, J., Raszewski, B., Zięba, T., & Musiał, W. (2023). Modified Potato Starch as a Potential Retardant for Prolonged Release of Lidocaine Hydrochloride from Methylcellulose Hydrophilic Gel. Pharmaceutics, 15(2), 387. https://doi.org/10.3390/pharmaceutics15020387






