Preparation and In Vitro Evaluation of a Gadolinium-Containing Vitamin E TPGS Micelle as a Potential Contrast Agent for MR Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
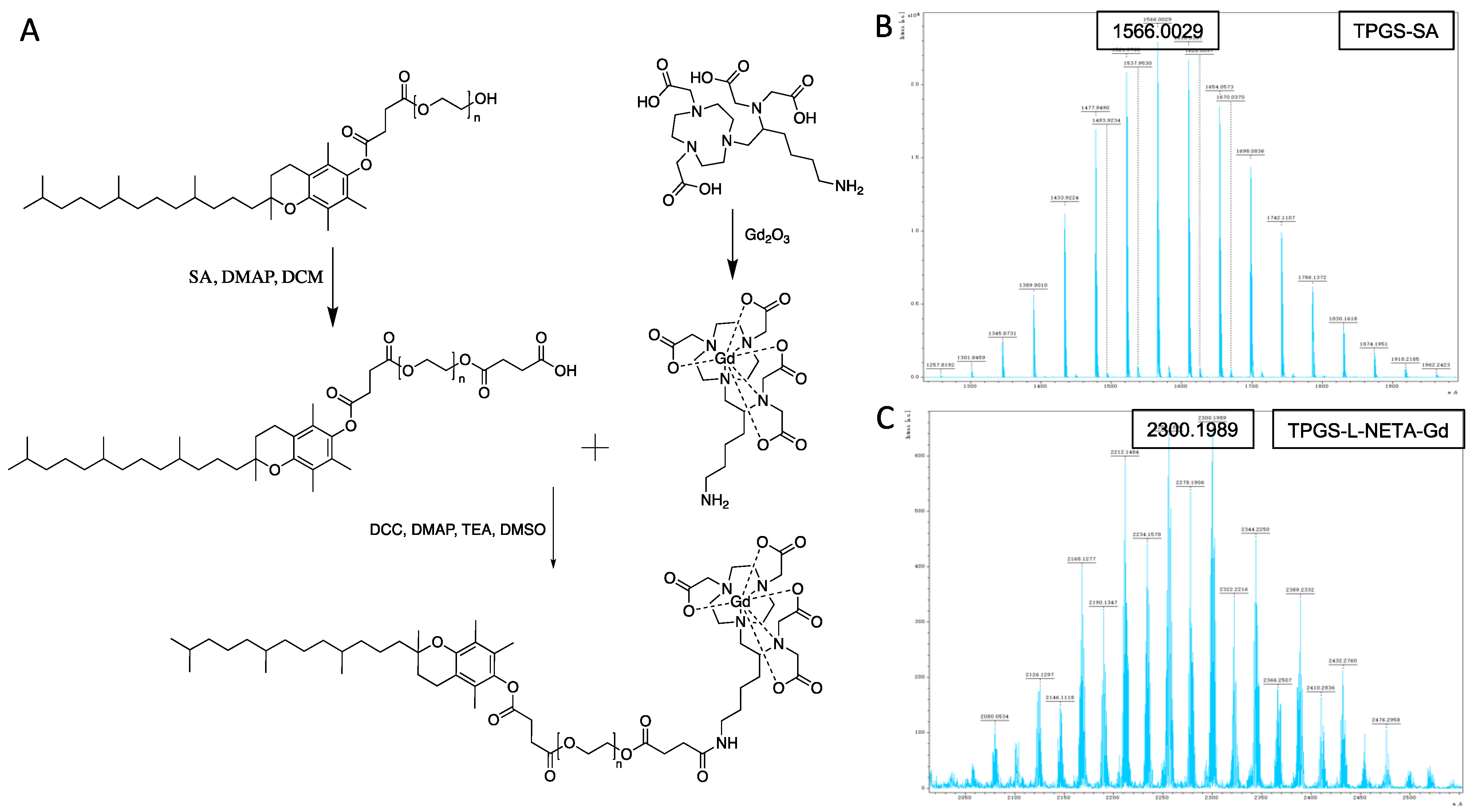
2.2. Synthesis of TPGS-SA
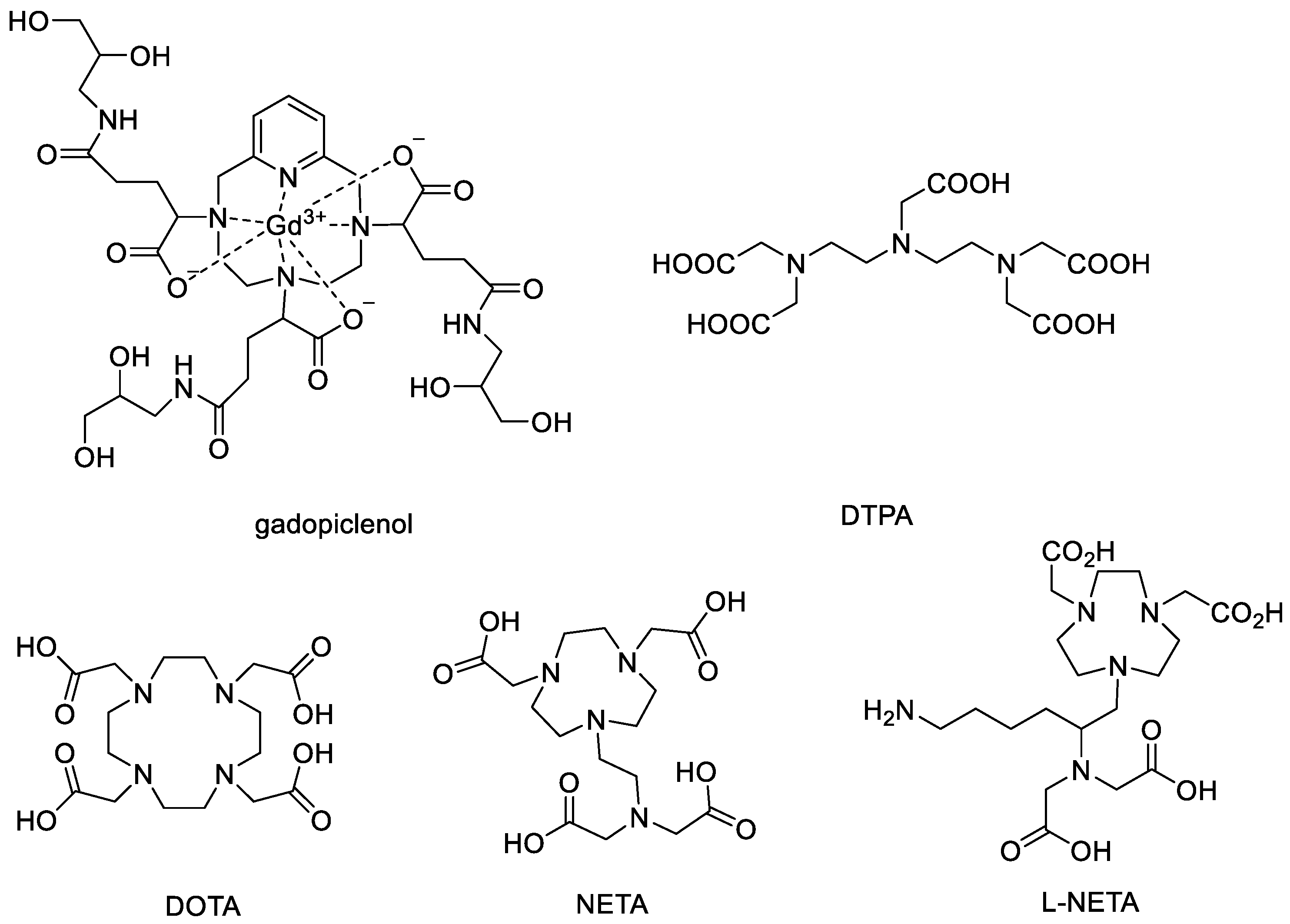
2.3. Synthesis of L-NETA-Gd
2.4. Synthesis of TPGS-L-NETA-Gd
2.5. Preparation of TLNm
2.6. Cell Culture and Animal Tumor Model
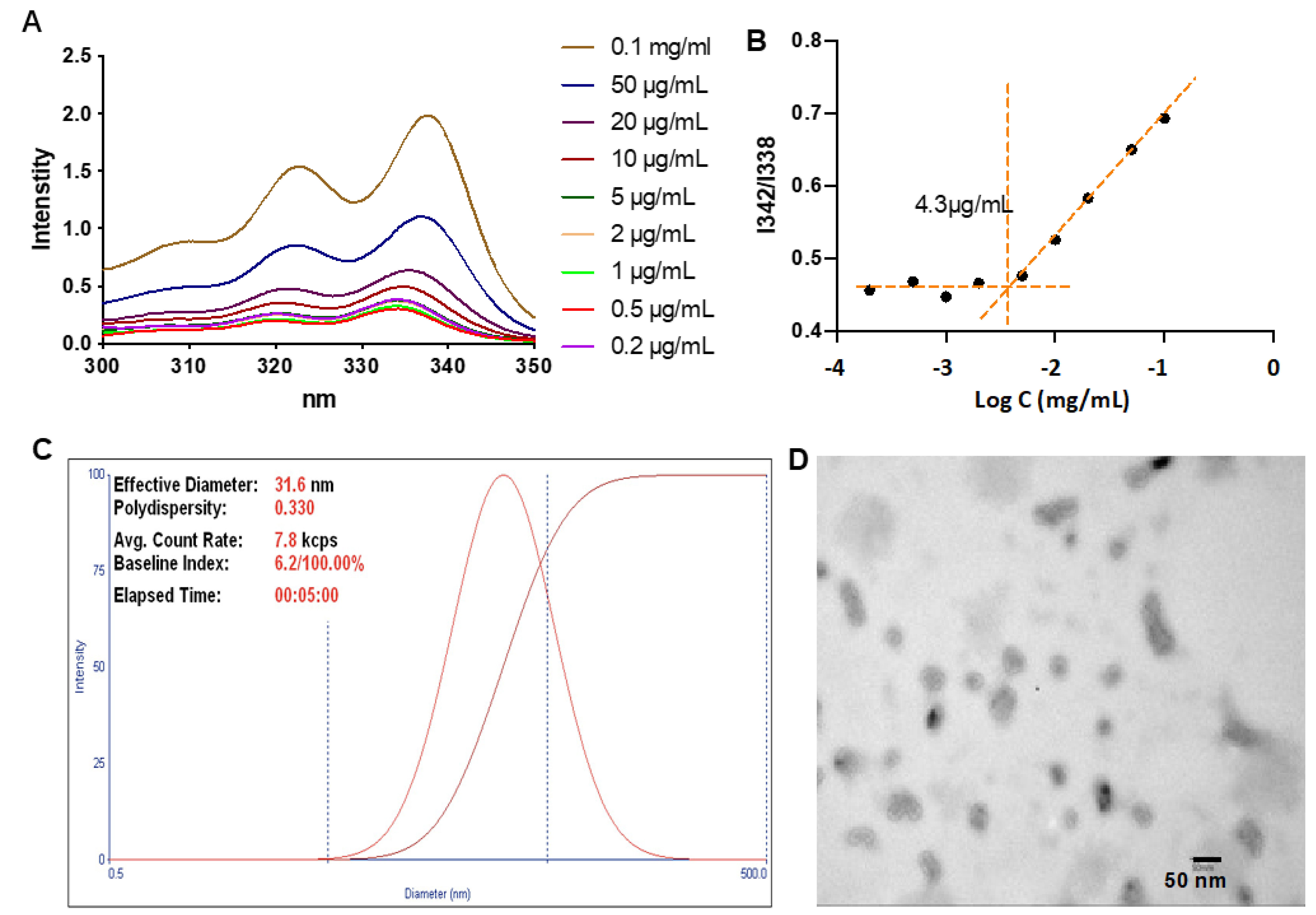
2.7. Transmission Electron Microscopy (TEM) Measurements
2.8. Determination of the Critical Micellar Concentration (CMC)
2.9. Uptake of TLNm
2.10. In Vitro Cell MRI
2.11. Acute Toxicity Studies of TLNm
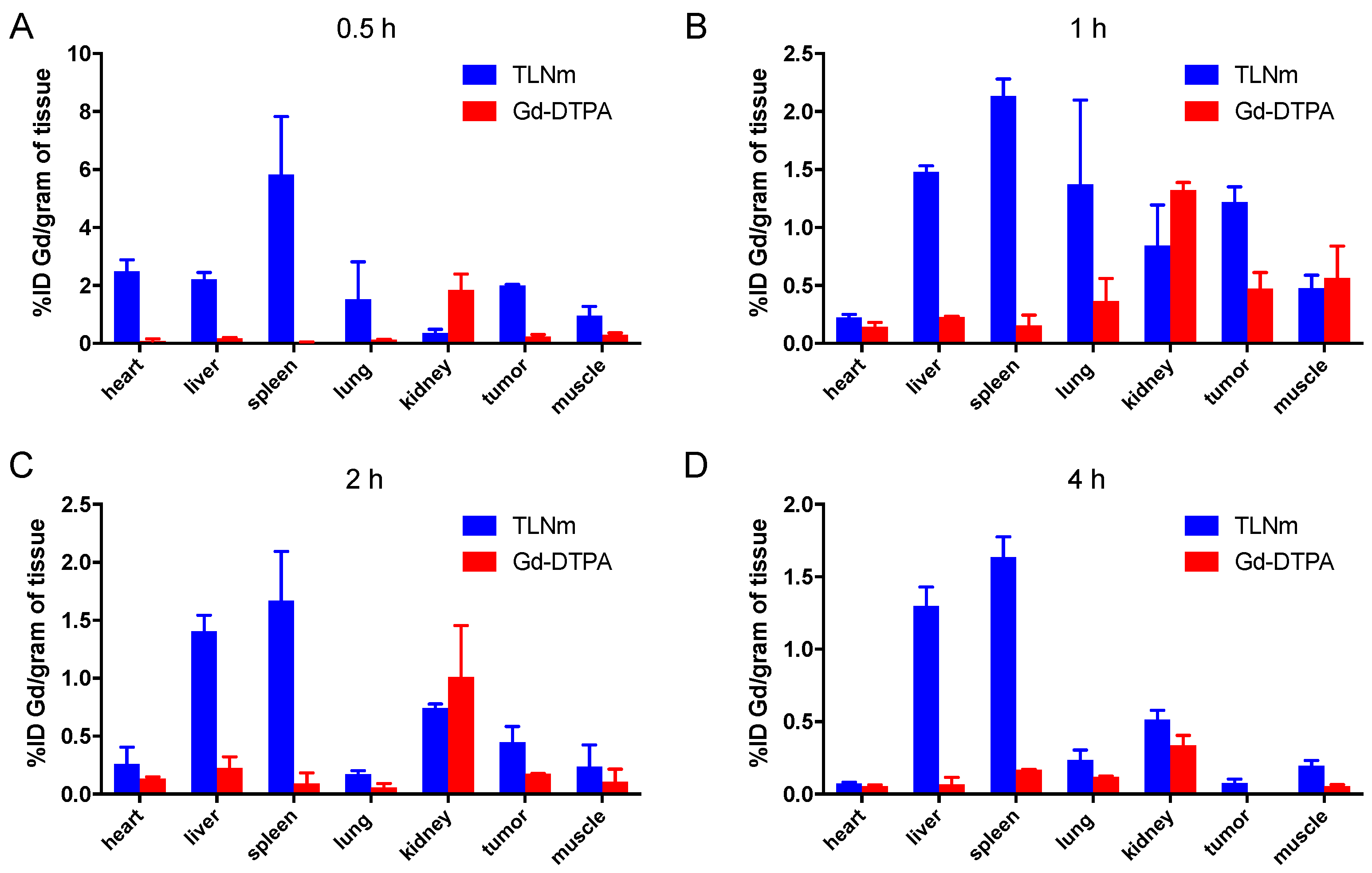
2.12. Biodistribution
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of TPGS-L-NETA-Gd
3.2. Determination of the CMC and Particle Sizes
3.3. Uptake of TLNm
3.4. In Vitro Cell MR
3.5. In Vivo Biodistribution
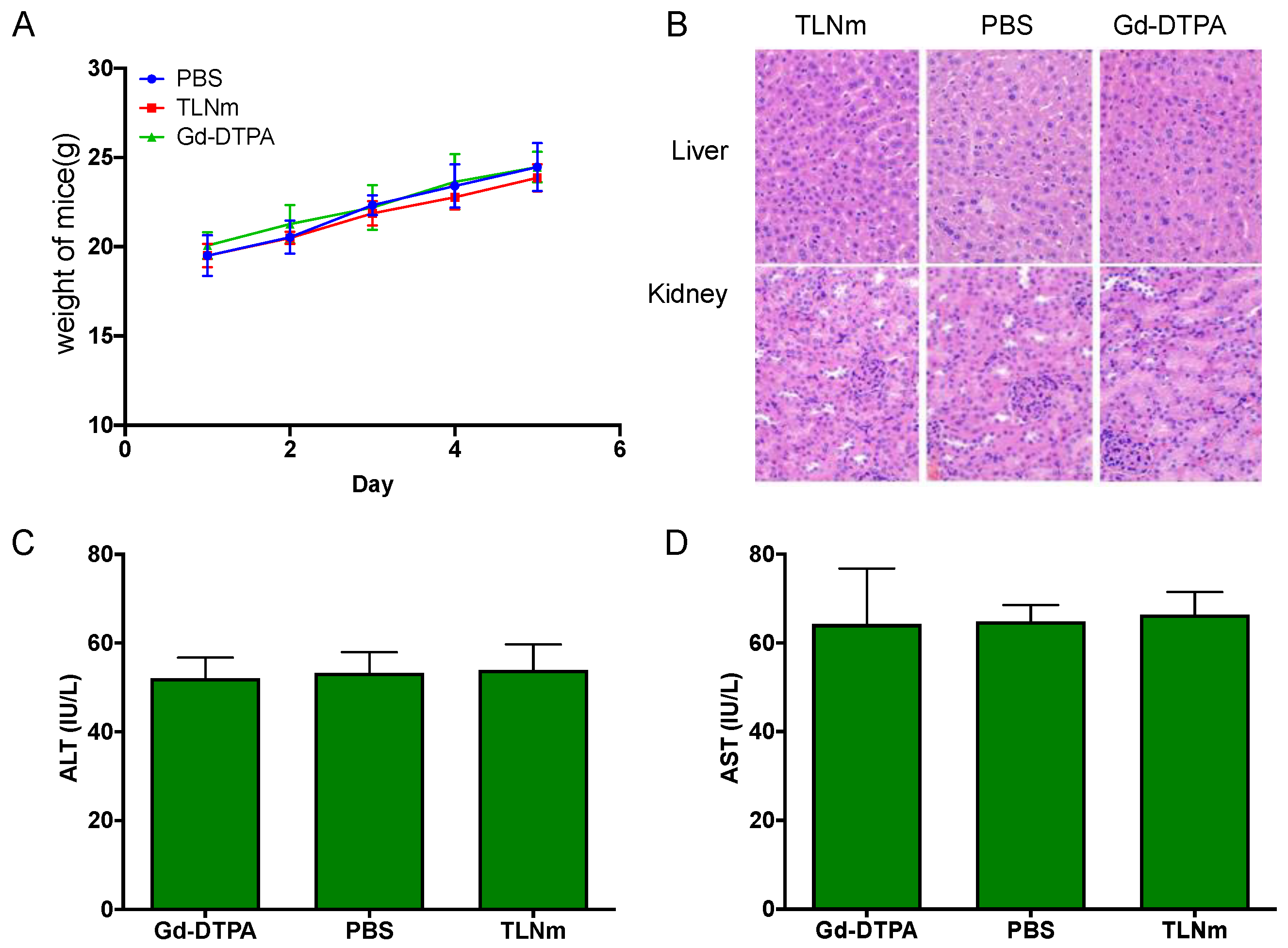
3.6. Acute Toxicity Studies of TLNm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermeulen, I.; Isin, E.M.; Barton, P.; Cillero-Pastor, B.; Heeren, R.M.A. Multimodal molecular imaging in drug discovery and development. Drug Discov. Today 2022, 27, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Sharp, P.S.; Main, M.J.; Simpson, P.B. Advanced molecular imaging for the characterisation of complex medicines. Drug Discov. Today 2022, 27, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, Z.R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 190. [Google Scholar] [CrossRef]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, A.; Van Roosbroeck, R.; Trekker, J.; Stakenborg, T.; Dresselaers, T.; Vande Velde, G.; Struys, T.; Lambrichts, I.; Lammertyn, J.; Lagae, L.; et al. Synthetic Antiferromagnetic Gold Nanoparticles as Bimodal Contrast Agents in MRI and CT-An Experimental In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 1494. [Google Scholar] [CrossRef]
- Gao, L.; Yu, J.; Liu, Y.; Zhou, J.; Sun, L.; Wang, J.; Zhu, J.; Peng, H.; Lu, W.; Yu, L.; et al. Tumor-penetrating Peptide Conjugated and Doxorubicin Loaded T(1)-T(2) Dual Mode MRI Contrast Agents Nanoparticles for Tumor Theranostics. Theranostics 2018, 8, 92–108. [Google Scholar] [CrossRef]
- Elistratova, J.; Akhmadeev, B.; Korenev, V.; Sokolov, M.; Nizameev, I.; Gubaidullin, A.; Voloshina, A.; Mustafina, A. Self-assembly of Gd(3+)-bound keplerate polyanions into nanoparticles as a route for the synthesis of positive MRI contrast agents. Impact of the structure on the magnetic relaxivity. Soft Matter 2018, 14, 7916–7925. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Magerstädt, M.; Gansow, O.A.; Brechbiel, M.W.; Colcher, D.; Baltzer, L.; Knop, R.H.; Girton, M.E.; Naegele, M. Gd(DOTA): An alternative to Gd(DTPA) as a T1,2 relaxation agent for NMR imaging or spectroscopy. Magn. Reson. Med. 1986, 3, 808–812. [Google Scholar] [CrossRef]
- Vorobiev, V.; Adriouach, S.; Crowe, L.A.; Lenglet, S.; Thomas, A.; Chauvin, A.S.; Allemann, E. Vascular-targeted micelles as a specific MRI contrast agent for molecular imaging of fibrin clots and cancer cells. Eur. J. Pharm. Biopharm. 2021, 158, 347–358. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, M.; Zhang, K.; Zu, G.; Kuang, Y.; Tong, X.; Xiong, D.; Pei, R. Poly(glycerol) Used for Constructing Mixed Polymeric Micelles as T(1) MRI Contrast Agent for Tumor-Targeted Imaging. Biomacromolecules 2017, 18, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, R.; Wen, X.; Li, L.; Li, C. Micelles based on biodegradable poly(L-glutamic acid)-b-polylactide with paramagnetic Gd ions chelated to the shell layer as a potential nanoscale MRI-visible delivery system. Biomacromolecules 2008, 9, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Sonali; Agrawal, P.; Singh, R.P.; Rajesh, C.V.; Singh, S.; Vijayakumar, M.R.; Pandey, B.L.; Muthu, M.S. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: Preparation, characterization and brain distribution in rats. Drug Deliv. 2016, 23, 1788–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadoqi, M.; Lau-Cam, C.A.; Wu, S.H. Investigation of the micellar properties of the tocopheryl polyethylene glycol succinate surfactants TPGS 400 and TPGS 1000 by steady state fluorometry. J. Colloid Interface Sci. 2009, 333, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, J.; Zheng, C.; Zhu, J.; Zhang, Y.; You, X.; Cai, F.; Shah, V.; Liu, J.; Ge, L. TPGS modified reduced bovine serum albumin nanoparticles as a lipophilic anticancer drug carrier for overcoming multidrug resistance. J. Mater. Chem. B 2016, 4, 3959–3968. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Arribada, R.G.; Silva, J.O.; Silva-Cunha, A.; Townsend, D.M.; Ferreira, L.A.M.; Barros, A.L.B. In Vitro and In Vivo Effect of pH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics 2022, 14, 2394. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Liu, A.; Zhu, Y.; Gao, D.; Yang, Y.; Sun, J.; Fan, H.; Zhang, X. NIR-to-Red Upconversion Nanoparticles with Minimized Heating Effect for Synchronous Multidrug Resistance Tumor Imaging and Therapy. ACS Appl. Mater. Interfaces 2018, 10, 14378–14388. [Google Scholar] [CrossRef]
- Du, Z.; Mao, Y.; Zhang, P.; Hu, J.; Fu, J.; You, Q.; Yin, J. TPGS-Galactose-Modified Polydopamine Co-delivery Nanoparticles of Nitric Oxide Donor and Doxorubicin for Targeted Chemo-Photothermal Therapy against Drug-Resistant Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2021, 13, 35518–35532. [Google Scholar] [CrossRef]
- Chong, H.S.; Song, H.A.; Ma, X.; Milenic, D.E.; Brady, E.D.; Lim, S.; Lee, H.; Baidoo, K.; Cheng, D.; Brechbiel, M.W. Novel bimodal bifunctional ligands for radioimmunotherapy and targeted MRI. Bioconjug. Chem. 2008, 19, 1439–1447. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.S.; Ma, X.; Le, T.; Kwamena, B.; Milenic, D.E.; Brady, E.D.; Song, H.A.; Brechbiel, M.W. Rational design and generation of a bimodal bifunctional ligand for antibody-targeted radiation cancer therapy. J. Med. Chem. 2008, 51, 118–125. [Google Scholar] [CrossRef]
- Rose, T.A.; Choi, J.W. Intravenous Imaging Contrast Media Complications: The Basics That Every Clinician Needs to Know. Am. J. Med. 2015, 128, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Sun, L.; Lan, X.; Zeng, D.; Xiang, G.; Ma, X. Synthesis and Evaluation of New Bifunctional Chelators with Phosphonic Acid Arms for Gallium-68 Based PET Imaging in Melanoma. Bioconjug. Chem. 2018, 29, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Xiang, G.; Ma, X.; Hui, W.; Ouyang, Q.; Sun, L.; Ding, J.; Sheng, J.; Zeng, D. Universal Molecular Scaffold for Facile Construction of Multivalent and Multimodal Imaging Probes. Bioconjug. Chem. 2016, 27, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gai, Y.; Sun, L.; Hui, W.; Ouyang, Q.; Anderson, C.J.; Xiang, G.; Ma, X.; Zeng, D. New Bifunctional Chelator p-SCN-PhPr-NE3TA for Copper-64: Synthesis, Peptidomimetic Conjugation, Radiolabeling, and Evaluation for PET Imaging. Inorg. Chem. 2016, 55, 6892–6901. [Google Scholar] [CrossRef] [Green Version]
- Gai, Y.; Hu, Z.; Rong, Z.; Ma, X.; Xiang, G. A Practical Route for the Preparation of 1,4,7-Triazacyclononanyl Diacetates with a Hydroxypyridinonate Pendant Arm. Molecules 2015, 20, 19393–19405. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.S.; Song, H.A.; Birch, N.; Le, T.; Lim, S.; Ma, X. Efficient synthesis and evaluation of bimodal ligand NETA. Bioorg. Med. Chem. Lett. 2008, 18, 3436–3439. [Google Scholar] [CrossRef]
- Wang, S.; Gai, Y.; Sun, L.; Lan, X.; Zeng, D.; Xiang, G.; Ma, X. Synthesis and evaluation of novel 1,4,7-triazacyclononane derivatives as Cu(2+) and Ga(3+) chelators. J. Inorg. Biochem. 2022, 229, 111719. [Google Scholar] [CrossRef]
- Wang, S.; Gai, Y.; Li, M.; Fang, H.; Xiang, G.; Ma, X. Synthesis of a new bifunctional NODA for bioconjugation with PSMA ligand and one-step Al(18)F labeling. Bioorg. Med. Chem. 2022, 60, 116687. [Google Scholar] [CrossRef]
- Ludwig, J.M.; Gai, Y.; Sun, L.; Xiang, G.; Zeng, D.; Kim, H.S. SW43-DOX +/- loading onto drug-eluting bead, a potential new targeted drug delivery platform for systemic and locoregional cancer treatment—An in vitro evaluation. Mol. Oncol. 2016, 10, 1133–1145. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Li, M.; Liu, Q.; Gai, Y.; Yuan, L.; Wang, S.; Zhang, X.; Ye, M.; Zhang, Y.; Gao, M.; et al. Ultra-sensitive Nanoprobe Modified with Tumor Cell Membrane for UCL/MRI/PET Multimodality Precise Imaging of Triple-Negative Breast Cancer. Nanomicro Lett. 2020, 12, 62. [Google Scholar] [CrossRef]
- Zhong, X.H.; Shi, W.Y.; Ma, A.T.; Gong, X.C.; Zhai, X.H.; Zhang, T.; Wang, X.D. Effects of Radix scutellariae and Rhizoma atractylodis on LPS-induced abortion and the uterine IL-10 contents in mice. Am. J. Chin. Med. 2008, 36, 141–148. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, Y.; Li, Y.; Wu, S.; Xu, L.; Lu, Y.; Lan, X.; Xiang, G.; Ma, X. Preparation and In Vitro Evaluation of a Gadolinium-Containing Vitamin E TPGS Micelle as a Potential Contrast Agent for MR Imaging. Pharmaceutics 2023, 15, 401. https://doi.org/10.3390/pharmaceutics15020401
Gai Y, Li Y, Wu S, Xu L, Lu Y, Lan X, Xiang G, Ma X. Preparation and In Vitro Evaluation of a Gadolinium-Containing Vitamin E TPGS Micelle as a Potential Contrast Agent for MR Imaging. Pharmaceutics. 2023; 15(2):401. https://doi.org/10.3390/pharmaceutics15020401
Chicago/Turabian StyleGai, Yongkang, Yuying Li, Shuangping Wu, Ling Xu, Yao Lu, Xiaoli Lan, Guangya Xiang, and Xiang Ma. 2023. "Preparation and In Vitro Evaluation of a Gadolinium-Containing Vitamin E TPGS Micelle as a Potential Contrast Agent for MR Imaging" Pharmaceutics 15, no. 2: 401. https://doi.org/10.3390/pharmaceutics15020401






