Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents
Abstract
:1. Introduction
2. Source and Physicochemical Properties of XG
3. Important Properties of XG for Designing Drug Delivery Systems
3.1. Mucoadhesive Properties
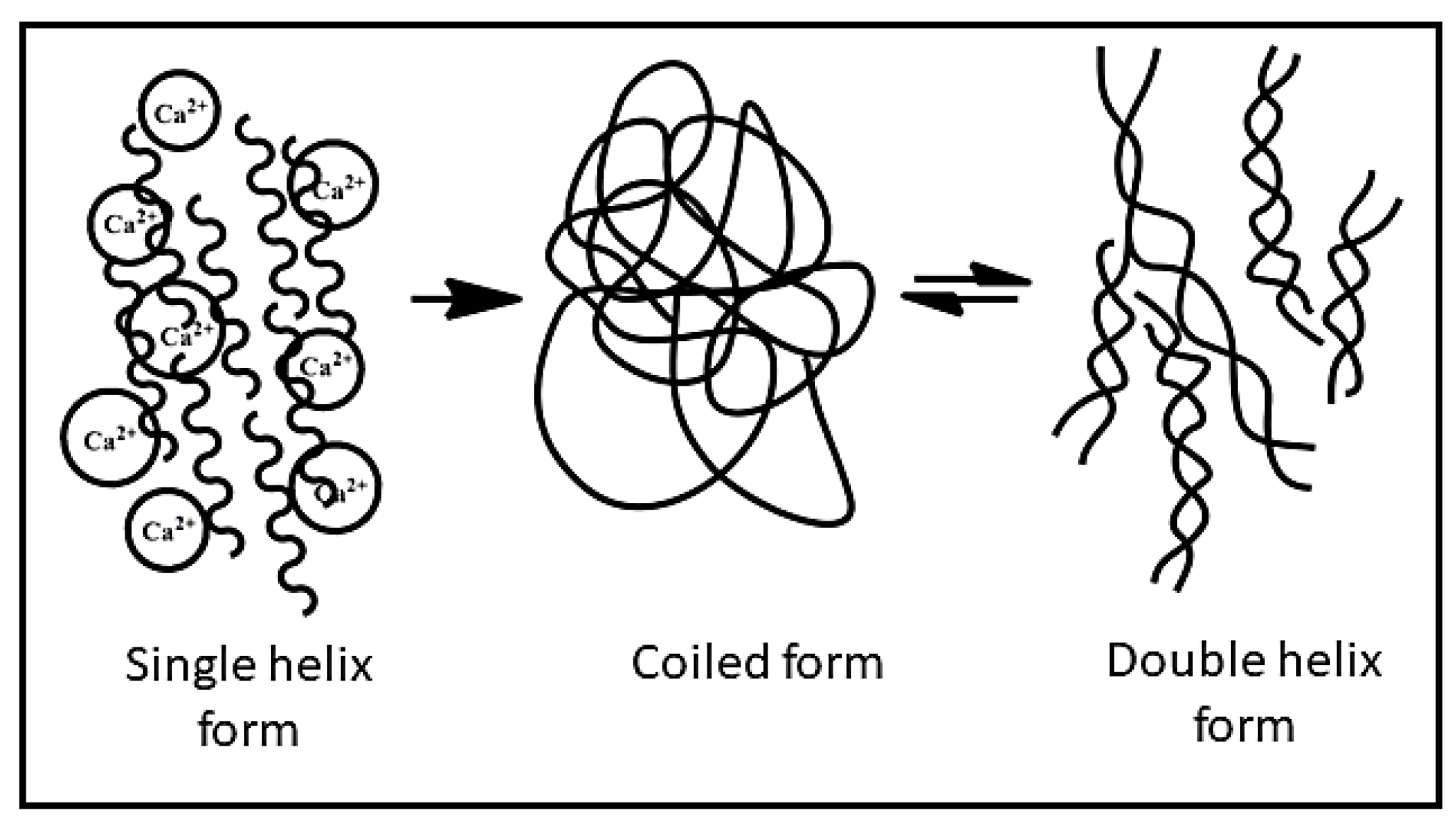
3.2. In Situ Gelling and Rheological Properties
4. Designing Various Forms of Delivery Systems Using XG
4.1. Matrix Systems
4.2. Films
4.3. Hydrogels
5. XG-Based Systems for the Delivery of Drugs
5.1. Delivery of Anti-Microbial Drugs
5.2. Delivery of Chemotherapeutic Agent
5.3. Delivery of Anti-Diabetic Drugs
5.4. Delivery of Drugs for the Treatment of Cardiovascular Diseases
5.5. Anti-Spasmodic Drug Delivery
5.6. Delivery of Drugs for the Treatment of Inflammation, Rheumatoid Arthritis, and Gout
5.7. Delivery of Immunosuppressive Drugs
5.8. Delivery of Drugs for the Treatment of Skin Diseases
5.9. Delivery of Drugs for the Treatment of Central Nervous System-Related Disorders
5.10. Delivery of Drugs for the Treatment of Obesity
5.11. Delivery of Drugs for the Treatment of Glaucoma
5.12. Delivery of Drugs for the Treatment of Pulmonary Diseases
6. XG-Based Systems for the Delivery of Genetic Materials
7. XG-Based Systems for the Delivery of Proteins and Peptides
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, S.K.; Tiwari, A.; Jain, A.; Verma, A.; Saraf, S.; Panda, P.K.; Gour, G. Application potential of polymeric nanoconstructs for colon-specific drug delivery. In Multifunctional Nanocarriers for Contemporary Healthcare Applications; IGI Global: Hershey, PA, USA, 2018; pp. 22–49. [Google Scholar] [CrossRef]
- Grenha, A.; Dionísio, M. Locust bean gum: Exploring its potential for biopharmaceutical applications. J. Pharm. Bioallied Sci. 2012, 4, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Pinheiro, A.C.; Souza, B.W.; Lima, M.; Ribeiro, C.; Miranda, C.; Teixeira, J.A.; Moreira, R.A.; Coimbra, M.A.; Gonçalves, M.P.; et al. Extraction, purification and characterization of galactomannans from non-traditional sources. Carbohydr. Polym. 2009, 75, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Tiwari, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Locust Bean Gum in Drug Delivery Application; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 203–222. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, H.; Van Betteray, B.; Germain, R.; Hiddink, G.; Jones, J.; Oosterhof, J. Development of specific primers for the molecular detection of bacterial spot of pepper and tomato. Acta Hortic. 2009, 808, 99–102. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef] [Green Version]
- Tao, F.; Wang, X.; Ma, C.; Yang, C.; Tang, H.; Gai, Z.; Xu, P. Genome Sequence of Xanthomonas campestris JX, an Industrially Productive Strain for Xanthan Gum. J. Bacteriol. 2012, 194, 4755–4756. [Google Scholar] [CrossRef]
- Elkatatny, S.; Jafarov, T.; Al-Majed, A.; Mahmoud, M. Formation Damage Avoidance by Reducing Invasion with Sodium Silicate-Modified Water-Based Drilling Fluid. Energies 2019, 12, 1485. [Google Scholar] [CrossRef] [Green Version]
- Hui, Y.H.; Corke, H.; de Leyn, I.; Nip, W.-K.; Cross, N. Bakery Products: Science and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Deep, A.; Rani, N.; Kumar, A.; Nandal, R.; Sharma, P.C.; Sharma, A.K. Prospective of Natural Gum Nanoparticulate Against Cardiovascular Disorders. Curr. Chem. Biol. 2019, 13, 197–211. [Google Scholar] [CrossRef]
- Lopes, B.M.; Lessa, V.L.; Silva, B.M.; Carvalho Filho, M.A.S.; Schnitzler, E.; Lacerda, L.G. Xanthan gum: Properties, production conditions, quality and economic perspective. J. Food Nutr. Res. 2015, 54, 185–194. [Google Scholar]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Singhvi, G.; Hans, N.; Shiva, N.; Dubey, S.K. Chapt. 5: Xanthan gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 121–124. [Google Scholar] [CrossRef]
- Benny, I.S.; Gunasekar, V.; Ponnusami, V. Review on application of Xanthan gum in drug delivery. Int. J. PharmTech Res. 2014, 6, 1322–1326. [Google Scholar]
- Philip, A.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef]
- Abu-Huwaij, R.; Obaidat, R.M.; Sweidan, K.; Al-Hiari, Y. Formulation and In Vitro Evaluation of Xanthan Gum or Carbopol 934-Based Mucoadhesive Patches, Loaded with Nicotine. AAPS PharmSciTech 2010, 12, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, K.; Puneeth, K.P.; Tamizh, M.T. Development and evaluation of rosiglitazone maleate floating tablets using natural gums. Int. J. PharmTech Res. 2010, 2, 1662–1669. [Google Scholar]
- El-Gazayerly, O.N. Release of Pentoxifylline from Xanthan Gum Matrix Tablets. Drug Dev. Ind. Pharm. 2003, 29, 241–246. [Google Scholar] [CrossRef]
- Ray, S.; Banerjee, S.; Maiti, S.; Laha, B.; Barik, S.; Sa, B.; Bhattacharyya, U.K. Novel interpenetrating network microspheres of xanthan gum–poly(vinyl alcohol) for the delivery of diclofenac sodium to the intestine—In vitro and in vivo evaluation. Drug Deliv. 2010, 17, 508–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, A.; Campbell, D.; Tighe, B. Chapt. 2: The ageing ocular surface: Challenges for biomaterials design and function. In Biomaterials and Regenerative Medicine in Ophthalmology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 17–43. [Google Scholar] [CrossRef]
- Shiledar, R.R.; Tagalpallewar, A.A.; Kokare, C.R. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr. Polym. 2014, 101, 1234–1242. [Google Scholar] [CrossRef]
- Samia, O.; Hanan, R.; Kamal, E.T. Carbamazepine Mucoadhesive Nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv. 2011, 19, 58–67. [Google Scholar] [CrossRef]
- Dehghan, M.G.; Kazi, M. Lyophilized chitosan/xanthan polyelectrolyte complex based mucoadhesive inserts for nasal delivery of promethazine hydrochloride. Iran. J. Pharm. Res. 2014, 13, 769–784. [Google Scholar] [CrossRef]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J.A. In situ forming polymeric drug delivery systems. Indian J. Pharm. Sci. 2009, 71, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Mazeau, K.; Rinaudo, M. The prediction of the characteristics of some polysaccharides from molecular modeling. Comparison with effective behavior. Food Hydrocoll. 2004, 18, 885–898. [Google Scholar] [CrossRef]
- Mohammed, Z.H.; Haque, A.; Richardson, R.K.; Morris, E.R. Promotion and inhibition of xanthan ‘weak-gel’ rheology by calcium ions. Carbohydr. Polym. 2007, 70, 38–45. [Google Scholar] [CrossRef]
- Bergmann, D.; Furth, G.; Mayer, C. Binding of bivalent cations by xanthan in aqueous solution. Int. J. Biol. Macromol. 2008, 43, 245–251. [Google Scholar] [CrossRef]
- Dário, A.F.; Hortêncio, L.M.A.; Sierakowski, M.R.; Neto, J.C.Q.; Petri, D.F.S. The effect of calcium salts on the viscosity and adsorption behavior of xanthan. Carbohydr. Polym. 2011, 84, 669–676. [Google Scholar] [CrossRef]
- Fitzpatrick, P.; Meadows, J.; Ratcliffe, I.; Williams, P.A. Control of the properties of xanthan/glucomannan mixed gels by varying xanthan fine structure. Carbohydr. Polym. 2013, 92, 1018–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudanavar, P.; Sreeharsha, N.; Murtale, S.; Naveen, N.; Alsanie, W.; Alhomrani, M.; Alamri, A.; Asdaq, S.B.; Anwer, M.; Gharsan, M.; et al. Synthesis and evaluation of grafted xanthan gum as a drug carrier in developing lornoxicam gel formulations. Pharmacogn. Mag. 2022, 18, 271–278. [Google Scholar] [CrossRef]
- Verhoeven, E.; Vervaet, C.; Remon, J. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2006, 63, 320–330. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Patil, S.A.; Aminabhavi, T.M. Evaluation of acrylamide-grafted-xanthan gum copolymer matrix tablets for oral controlled delivery of antihypertensive drugs. Carbohydr. Polym. 2007, 69, 130–141. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.D.; Tran, P.H.L. Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, A.; Bhatt, N. Formulation and evaluation of glibenclamide loaded mouth dissolving films with xanthan gum as film forming polymer. Eur. J. Mol. Clin. Med. 2021, 8, 1016–1025. [Google Scholar]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Lin, Q.; Liu, H.; Shen, C.; Nan, K.; Chen, H. In vitro and in vivo evaluation of xanthan gum–succinic anhydride hydrogels for the ionic strength-sensitive release of antibacterial agents. J. Mater. Chem. B 2016, 4, 1853–1861. [Google Scholar] [CrossRef]
- Shastri, D.H.; Prajapati, S.; Patel, L. Design and Development of Thermoreversible Ophthalmic In Situ Hydrogel of Moxifloxacin HCl. Curr. Drug Deliv. 2010, 7, 238–243. [Google Scholar] [CrossRef]
- Barzic, A.I.; Ioan, S. Chapt. 1: Antibacterial drugs—From basic concepts to complex therapeutic mechanisms of polymer systems. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; IntechOpen: London, UK, 2015; pp. 3–28. [Google Scholar] [CrossRef] [Green Version]
- Kala, S.; Gurudiwan, P.; Juyal, D. Formulation and Evaluation of Besifloxacin Loaded In Situ Gel For Ophthalmic Delivery. Pharm. Biosci. J. 2018, 6, 36–40. [Google Scholar] [CrossRef]
- Garg, R.; Kumar, V.; Sharma, V. Design and Characterization of Flucytosine Loaded Bioadhesive In Situ Ophthalmic Gel for Improved Bioavailability. Pharm. Biosci. J. 2019, 7, 17–20. [Google Scholar] [CrossRef]
- Gangane, P.S.; Pachpute, T.; Mahapatra, D.K.; Mahajan, N.M. HPMC Polymers and Xanthan Gum Assisted Development and Characterization of Stavudine Extended Release Floating Tablets. Indian J. Pharm. Educ. Res. 2021, 55, S681–S692. [Google Scholar] [CrossRef]
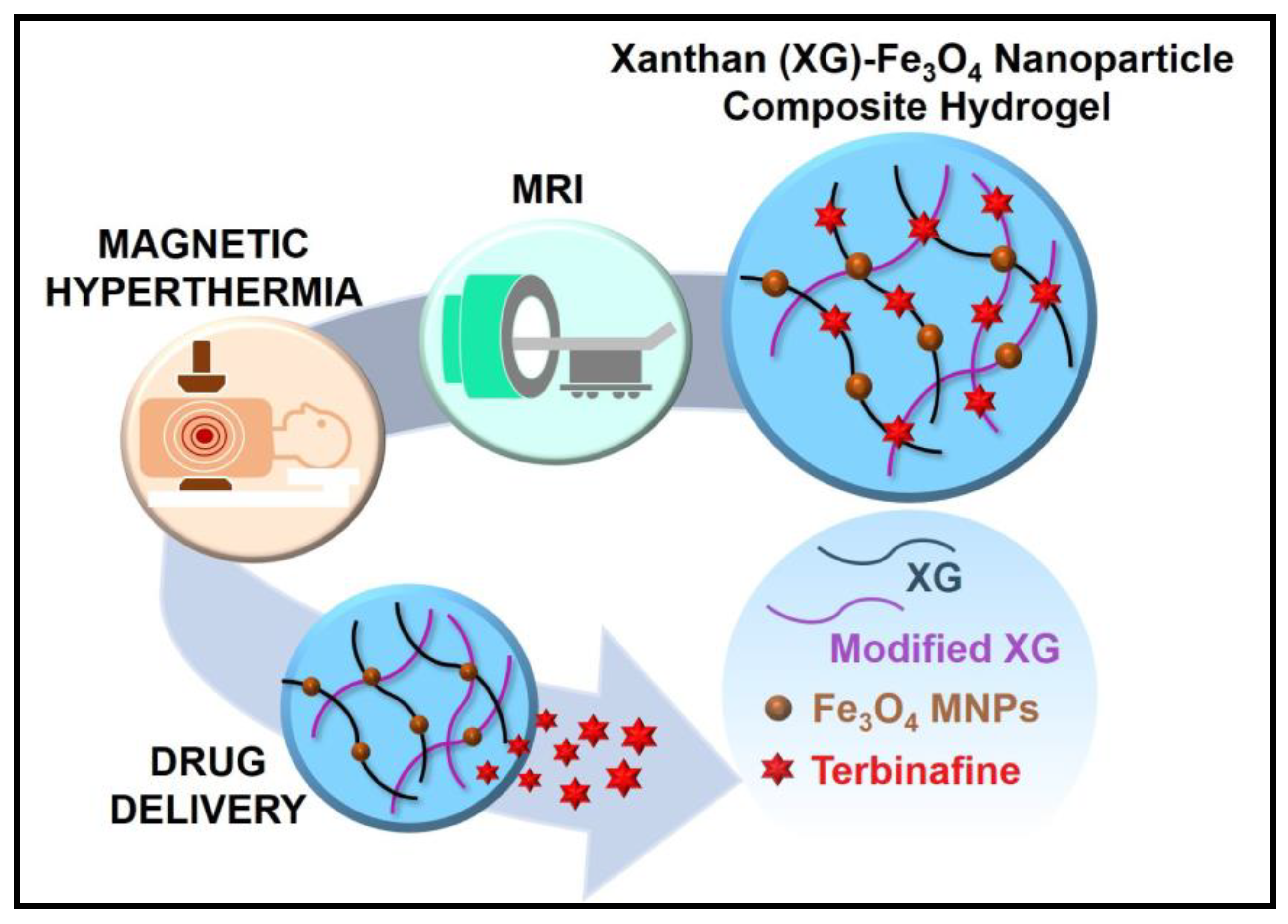
- Ribeiro, M.; Boudoukhani, M.; Belmonte-Reche, E.; Genicio, N.; Sillankorva, S.; Gallo, J.; Rodríguez-Abreu, C.; Moulai-Mostefa, N.; Bañobre-López, M. Xanthan-Fe3O4 Nanoparticle Composite Hydrogels for Non-Invasive Magnetic Resonance Imaging and Magnetically Assisted Drug Delivery. ACS Appl. Nano Mater. 2021, 4, 7712–7729. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/Xanthan Gum Based Hydrogels as Potential Carrier for an Antiviral Drug: Fabrication, Characterization, and Safety Evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.R.; Kumria, R. Binders for colon specific drug delivery: An in vitro evaluation. Int. J. Pharm. 2002, 249, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carbohydr. Polym. 2019, 208, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Singh, S.; Kotla, N.G.; Tomar, S.; Maddiboyina, B.; Sharma, D.; Sunnapu, O. A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int. J. Nanomed. 2015, 10, 7175–7182. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2021, 78, 59–80. [Google Scholar] [CrossRef]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Xu, J.; Ni, C. Preparation of redox responsive modified xanthan gum nanoparticles and the drug controlled release. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 994–1001. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Wen, Q.; Ni, C. Preparation of xanthan gum nanogels and their pH/redox responsiveness in controlled release. J. Appl. Polym. Sci. 2019, 136, 6–11. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alle, M.; Reddy, G.B.; Kim, T.H.; Park, S.H.; Lee, S.-H.; Kim, J.-C. Doxorubicin-carboxymethyl xanthan gum capped gold nanoparticles: Microwave synthesis, characterization, and anti-cancer activity. Carbohydr. Polym. 2020, 229, 115511. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological Perspectives of Diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to Diabetes Mellitus. Adv. Exp. Med. Biol. 2012, 771, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.W.; Plotnick, L. Type 1 Diabetes Mellitus in Pediatrics. Pediatr. Rev. 2008, 29, 374–385. [Google Scholar] [CrossRef]
- Razzaq, F.A.; Asif, M.; Asghar, S.; Iqbal, M.S.; Khan, I.U.; Khan, S.-U.; Irfan, M.; Syed, H.K.; Khames, A.; Mahmood, H.; et al. Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation. Cells 2021, 10, 2404. [Google Scholar] [CrossRef]
- Thakare, E.B.; Malpure, P.S.; Maru, A.D.; More, Y.M. Formulation and Evaluation of Mucoadhesive Buccal Tablet of Repaglinide. J. Drug Deliv. Ther. 2019, 9, 415–424. [Google Scholar] [CrossRef]
- Imran, M.; Hameed, A.; Shafiullah Hafizur, R.M.; Ali, I.; Roome, T.; Shah, M.R. Fabrication of Xanthan stabilized green gold nanoparticles based tolbutamide delivery system for enhanced insulin secretion in mice pancreatic islets. J. Macromol. Sci. Part A 2018, 55, 729–735. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, A.; Ramasamy, S.; Yadav, A.K.; Bijauliya, R.K. Evaluation of Drug Release from Carboxymethyl Starch-Xanthan Gum-HPMC Matrix. J. Drug Deliv. Ther. 2021, 11, 27–32. [Google Scholar] [CrossRef]
- Phaechamud, T.; Ritthidej, G.C. Sustained-release from Layered Matrix System Comprising Chitosan and Xanthan Gum. Drug Dev. Ind. Pharm. 2007, 33, 595–605. [Google Scholar] [CrossRef]
- Ratnam, S.V.; Bhowmik, D.; Yadav, R.; Singh, D. Formulation and evaluation of carvedilol fast dissolving tablets. J. Chem. Pharm. Sci. 2014, 7, 85–88. [Google Scholar]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. Uniquely different PVA-xanthan gum irradiated membranes as transdermal diltiazem delivery device. Carbohydr. Polym. 2013, 95, 252–261. [Google Scholar] [CrossRef]
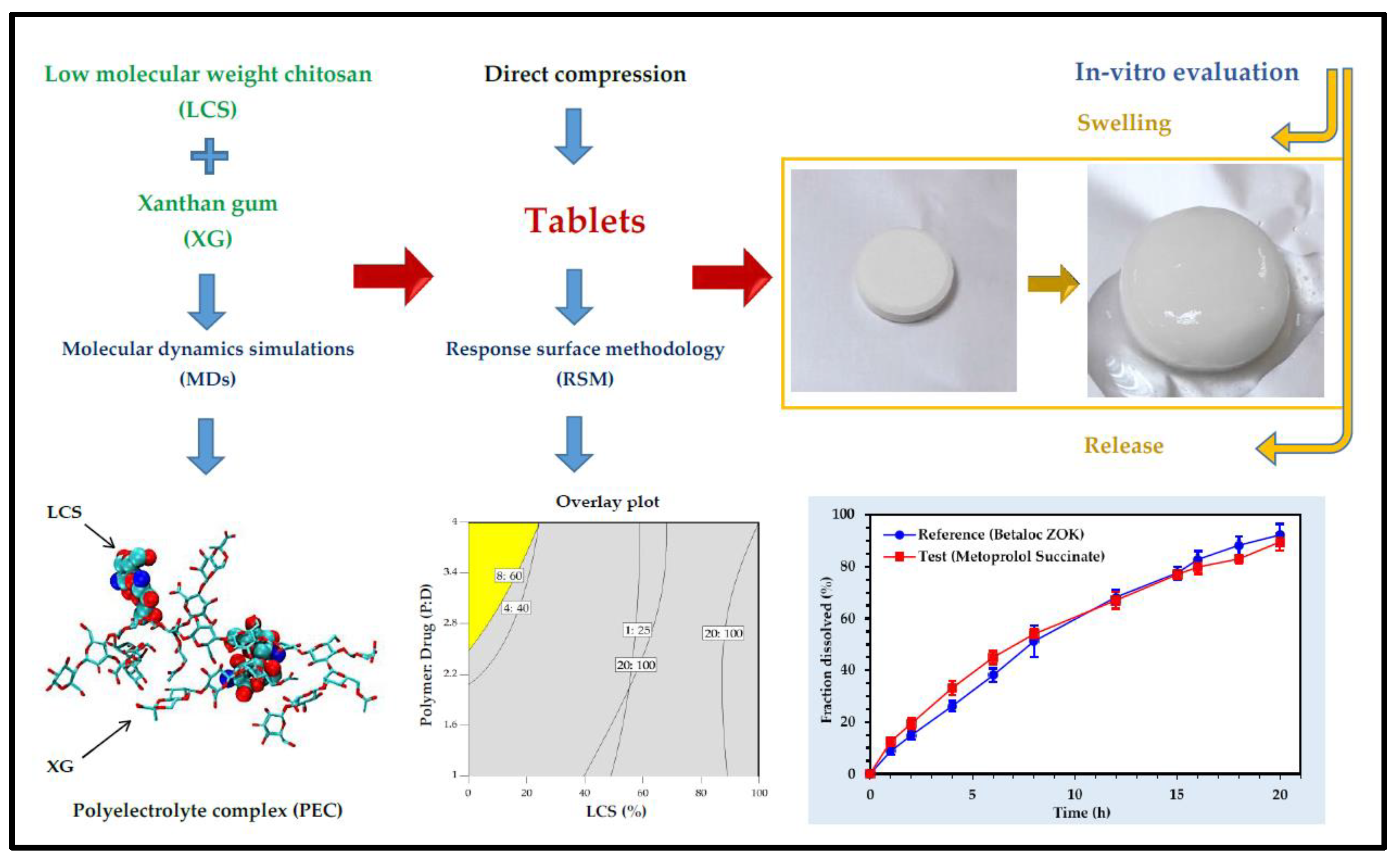
- Dadou, S.M.; El-Barghouthi, M.I.; Antonijevic, M.D.; Chowdhry, B.Z.; Badwan, A.A. Elucidation of the Controlled-Release Behavior of Metoprolol Succinate from Directly Compressed Xanthan Gum/Chitosan Polymers: Computational and Experimental Studies. ACS Biomater. Sci. Eng. 2019, 6, 21–37. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, N. Sex-Gender Differences in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 544–558. [Google Scholar] [CrossRef] [Green Version]
- TarikaPriya, A.; Neeharika, M.S.; Sekhar, C.K. Formulation and Evaluation of Sustained Release Matrix Tablets of Mebeverine Hydrochloride Using Natural and Synthetic Polymers. World J. Pharm. Pharm. Sci. 2014, 3, 1424–1436. [Google Scholar]
- Rashmitha, V.; Madhusudan, R.Y.; Pavani, S. Formulation and evaluation of fenoverine floating tablets. Asian J. Pharm. Clin. Res. 2021, 14, 175–180. [Google Scholar] [CrossRef]
- Olaru, L.; Soong, L.; Dhillon, S.; Yacyshyn, E. Coexistent rheumatoid arthritis and gout: A case series and review of the literature. Clin. Rheumatol. 2017, 36, 2835–2838. [Google Scholar] [CrossRef]
- Del Favero, A. Anti-inflammatory analgesics and drugs used in rheumatoid arthritis and gout. Side Eff. Drugs Annu. 1985, 9, 83–100. [Google Scholar] [CrossRef]
- Li, L.; Zheng, X.; Pan, C.; Pan, H.; Guo, Z.; Liu, B.; Liu, Y. A pH-sensitive and sustained-release oral drug delivery system: The synthesis, characterization, adsorption and release of the xanthan gum-graft-poly(acrylic acid)/GO–DCFP composite hydrogel. RSC Adv. 2021, 11, 26229–26240. [Google Scholar] [CrossRef] [PubMed]
- Stasaid, M.; Boutemak, K.; Ibtissem, L.; Flahaut, E.; Zafour, A.H.-Z. Synthesis of carboxymethyl Xanthan/ double-walled carbon nanotube hybrid hydrogel nanocomposite for transdermal release of drug. Soft Mater. 2021, 20, 168–182. [Google Scholar] [CrossRef]
- Dhage, C.M.; Shinkar, D.M.; Pathan, V.T.; Gage, G.P.; Jadhav, A.G. Formulation and in Vitro Evaluation of Mucoadhesive Buccal Tablet of Febuxostat. LINO. Available online: https://www.sybespharmacy.com/asset/pdf/research-activities/publication/300156_10._dms.pdf (accessed on 3 August 2022).
- Caddeo, C.; Nacher, A.; Díez-Sales, O.; Merino-Sanjuán, M.; Fadda, A.M.; Manconi, M. Chitosan–xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J. Microencapsul. 2014, 31, 694–699. [Google Scholar] [CrossRef]
- Jaya, S.; Divya, S. Formulation and in vitro evaluation of matrix tablets of metoclopramide hydrochloride. Int. J. Appl. Pharm. 2019, 11, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Mutalik, S.; Suthar, N.A.; Managuli, R.S.; Shetty, P.K.; Avadhani, K.; Kalthur, G.; Kulkarni, R.V.; Thomas, R. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int. J. Biol. Macromol. 2016, 86, 709–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Y.; Khan, H. Immunosuppressive Drugs. Encycl. Infect. Immun. 2022, 4, 726–740. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, E.; Kim, J.; Seo, Y.; Lee, K.H.; Hong, J.W.; Gilad, A.A.; Park, H.; Choi, J. Effective delivery of immunosuppressive drug molecules by silica coated iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2016, 142, 290–296. [Google Scholar] [CrossRef]
- Sadeq, Z.A.; Sabri, L.A.; Al-Kinani, K.K. Natural polymer Effect on gelation and rheology of ketotifen-loaded pH-sensitive in situ ocular gel (Carbapol). J. Adv. Pharm. Educ. Res. 2022, 12, 45–50. [Google Scholar] [CrossRef]
- Azari, F.; Ghanbarzadeh, S.; Safdari, R.; Yaqoubi, S.; Adibkia, K.; Hamishehkar, H. Development of a Carrier Free Dry Powder Inhalation Formulation of Ketotifen for Pulmonary Drug Delivery. Drug Res. 2019, 70, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Bhupathyraaj, M.; Pole, S. Study on effect of combination of sodium alginate and xanthan gum on drug release from Tacrolimus microbeads. Eur. J. Mol. Clin. Med. 2020, 7, 4585. [Google Scholar]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Pagano, C.; Puglia, D.; Luzi, F.; Michele, A.; Scuota, S.; Primavilla, S.; Ceccarini, M.; Beccari, T.; Iborra, C.; Ramella, D.; et al. Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment. Pharmaceutics 2021, 13, 324. [Google Scholar] [CrossRef]
- Nance, E.; Pun, S.H.; Saigal, R.; Sellers, D.L. Drug delivery to the central nervous system. Nat. Rev. Mater. 2021, 7, 314–331. [Google Scholar] [CrossRef]
- Mehetre, G.D.; Patki, S.S.; Thenge, R.R.; Shrikhande, V.N. Quetiapine Fumarate Buccoadhesive Tablet-Formulation and In Vitro Evaluation. Res. J. Pharm. Technol. 2020, 13, 5095–5102. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Yarce, C.J.; Moreno, R.A.; Prieto, V.; Recalde, J. Natural gum-type biopolymers as potential modified nonpolar drug release systems. Carbohydr. Polym. 2018, 189, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pandala, S.; Bakshi, V.; Jadi, R.K. Formulation Development and In Vitro Characterization of Zolmitriptan Controlled Release Drug Delivery Systems. INNOSC Theranostics Pharmacol. Sci. 2019, 2, 550. [Google Scholar] [CrossRef] [Green Version]
- Rajput, A.; Bariya, A.; Allam, A.; Othman, S.; Butani, S.B. In situ nanostructured hydrogel of resveratrol for brain targeting: In vitro-in vivo characterization. Drug Deliv. Transl. Res. 2018, 8, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.-H.; Seok, S.H.; Park, E.-S. Anti-obesity effect with reduced adverse effect of the co-administration of mini-tablets containing orlistat and mini-tablets containing xanthan gum: In vitro and in vivo evaluation. Int. J. Pharm. 2020, 591, 119998. [Google Scholar] [CrossRef]
- Morsi, N.; Ibrahim, M.; Refai, H.; El Sorogy, H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur. J. Pharm. Sci. 2017, 104, 302–314. [Google Scholar] [CrossRef]
- Deulker, A.L.; Sancoalcar, A.; Vaidya, S.; Gude, R. Formulation development and evaluation of long acting ophthalmic in-situ gelling system of Dorzolamide Hydrochloride. Int. J. Drug Dev. Res. 2013, 5, 156–163. [Google Scholar]
- Mishra, B.; Singh, J. Chapt. 4: Novel drug delivery systems and significance in respiratory diseases. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 57–95. [Google Scholar] [CrossRef]
- Todoroff, J.; Vanbever, R. Fate of nanomedicines in the lungs. Curr. Opin. Colloid Interface Sci. 2011, 16, 246–254. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, A.; Tripathi, D.K. Biodegradable Hydrogel Bead of Casein and Modified Xanthan Gum for Controlled Delivery of Theophylline. Curr. Drug Ther. 2016, 11, 150–162. [Google Scholar] [CrossRef]
- Lazzari, A.; Kleinebudde, P.; Knop, K. Xanthan gum as a rate-controlling polymer for the development of alcohol resistant matrix tablets and mini-tablets. Int. J. Pharm. 2018, 536, 440–449. [Google Scholar] [CrossRef]
- Bueno, P.V.A.; Hilamatu, K.C.; Carmona-Ribeiro, A.M.; Petri, D.F. Magnetically triggered release of amoxicillin from xanthan/Fe3O4/albumin patches. Int. J. Biol. Macromol. 2018, 115, 792–800. [Google Scholar] [CrossRef]
- Balzli, C.L.; McCormick, C.C.; Caballero, A.R.; Tang, A.; O’Callaghan, R.J. The effectiveness of an improved combination therapy for experimental Staphylococcus aureus keratitis. Adv. Ther. 2010, 27, 933–940. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Patil, S.A.; Agnihotri, S.A.; Aminabhavi, T.M. Development of Polysaccharide-Based Colon Targeted Drug Delivery Systems for the Treatment of Amoebiasis. Drug Dev. Ind. Pharm. 2007, 33, 255–264. [Google Scholar] [CrossRef]
- Hanna, D.H.; Saad, G.R. Encapsulation of ciprofloxacin within modified xanthan gum- chitosan based hydrogel for drug delivery. Bioorg. Chem. 2019, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Mazahir, F.; Banerjee, S.; Verma, A.; Ghosh, A. Preparation and in vitro evaluation of xanthan gum facilitated superabsorbent polymeric microspheres. Carbohydr. Polym. 2013, 98, 64–72. [Google Scholar] [CrossRef]
- Mishra, B.; Sahoo, S.K.; Sahoo, S. Liranaftate loaded Xanthan gum based hydrogel for topical delivery: Physical properties and ex-vivo permeability. Int. J. Biol. Macromol. 2018, 107, 1717–1723. [Google Scholar] [CrossRef]
- Ghosal, K.; Adak, S.; Agatemor, C.; Kalarikkal, P.G.N.; Thomas, S. Novel interpenetrating polymeric network based microbeads for delivery of poorly water soluble drug. J. Polym. Res. 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Zandraa, O.; Patwa, R.; Saha, N.; Capáková, Z.; Saha, P. Self-crosslinked chitosan/dialdehyde xanthan gum blended hypromellose hydrogel for the controlled delivery of ampicillin, minocycline and rifampicin. Int. J. Biol. Macromol. 2021, 167, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, D.; Li, Q.; Zhang, M.; Liu, H.; Wu, X. Preparation and Evaluation of a Xanthan Gum–Containing Linezolid Ophthalmic Solution for Topical Treatment of Experimental Bacterial Keratitis. Pharm. Res. 2021, 38, 347–359. [Google Scholar] [CrossRef]
- Parhi, R.; Reddy, S.S.; Swain, S. Transdermal Delivery of Ondansetron HCl from Thermoreversible Gel Containing Nanocomposite. Curr. Nanomater. 2019, 4, 137–147. [Google Scholar] [CrossRef]
- Chauhan, D.; Patel, A.; Shah, S. Influence of selected natural polymers on in-vitro release of colon targeted mebeverine HCl matrix tablet. Int. J. Drug Dev. Res. 2012, 4, 247–255. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Janes, K.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef] [PubMed]
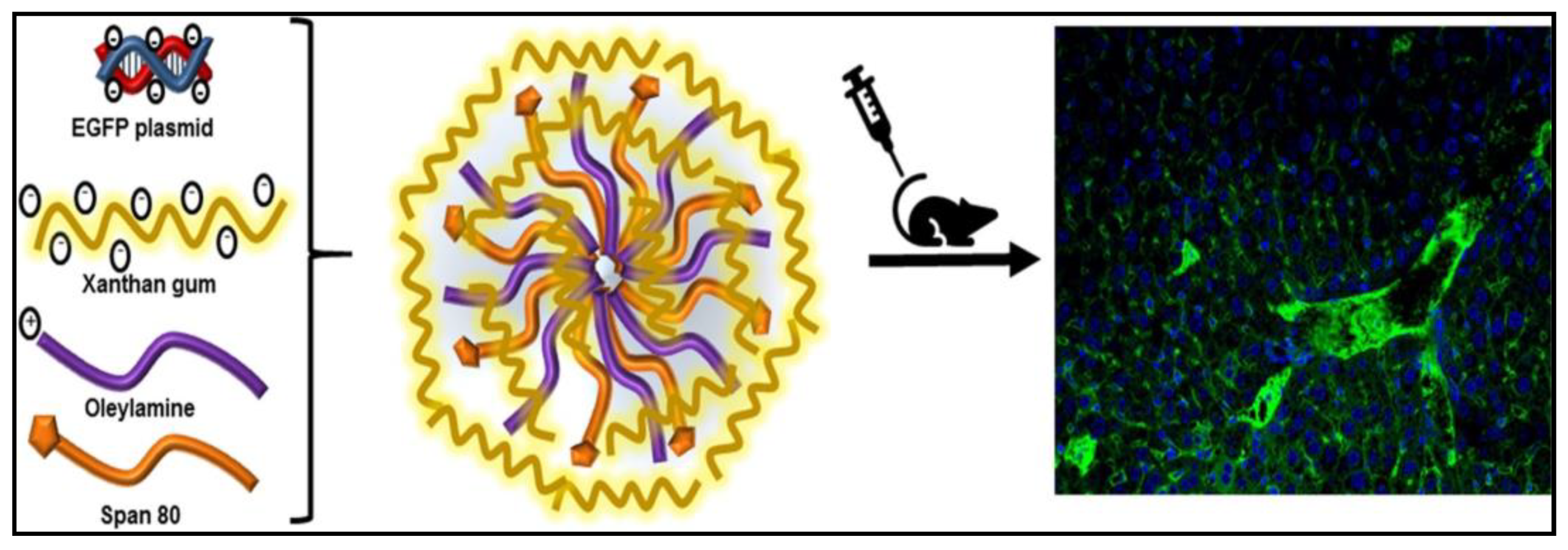
- Fernandez-Piñeiro, I.; Alvarez-Trabado, J.; Márquez, J.; Badiola, I.; Sanchez, A. Xanthan gum-functionalised span nanoparticles for gene targeting to endothelial cells. Colloids Surf. B Biointerfaces 2018, 170, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Giri, N.C. Chapt. 2: Protein and peptide drug delivery. In Smart Drug Delivery; Ahmad, U., Haider, M.F., Akhtar, J., Eds.; IntechOpen: London, UK, 2022; pp. 1–53. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Sabaa, M.W.; Hanna, D.H.; Abdel-Aziz, M.M.; Mohamed, R.R. Antimicrobial pH-sensitive protein carrier based on modified xanthan gum. J. Drug Deliv. Sci. Technol. 2020, 57, 101673. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Hanna, D.H.; Abu Elella, M.H.; Mohamed, R.R. Encapsulation of bovine serum albumin within novel xanthan gum based hydrogel for protein delivery. Mater. Sci. Eng. C 2019, 94, 1044–1055. [Google Scholar] [CrossRef]

| Properties | Value |
|---|---|
| Physical state | Dry-solid powder |
| Colour | Yellow-white |
| Molecular weight | Ranges 1 × 106 to 2 × 106 g/mol |
| pH | 6–7 |
| Flash point | >100 °C |
| Solubility | Water, DMSO, DMF |
| Sr. No. | Polymers | Form | Drug | Remarks | Reference |
|---|---|---|---|---|---|
| Delivery of antimicrobial drugs | |||||
| 1. | XG, sodium alginate and ethyl cellulose | In situ ocular gels | Besifloxacin | The transparent, clear, non-irritant gel was synthesised with sustained drug release (for 8 h) and high drug content capacity. | [44] |
| 2. | XG, HPMC, and sodium alginate | In situ ocular gels | Flucytosine | The drug-loaded formulation showed high drug content (95.2–98.6%), sustained drug release (up to 8 h) and stability at both room and higher temperatures. | [45] |
| 3. | XG, HPMC, and sodium alginate | Floating low-density tablet | Stavudine | Sustained release of the antiviral drug stavudine was achieved with all the pre-compression and post-compression characteristics for the tablet. | [46] |
| 4. | Xanthan gum, bovine serum albumin and magnetic nanoparticles | Magnetic field responsive antimicrobial patch | Amoxicillin | The adsorption of the drug was found to be more on XG and BSA films compared to others. Drug release followed a quasi-Fickian pattern. The formulation was tested for antimicrobial activity, and it showed more efficacy in the presence of an external magnetic field. | [100] |
| 5. | XG | Combination of tobramycin and dexamethasone | The combination of both drugs loaded into xanthan gum was better than the commercially available treatment even when it contains half the amount of steroid. | [101] | |
| 6. | XG coated with Eudragit-L 100 | Tablets | Metronidazole | Coated XG tablets showed delayed release. In the dissolution medium, decreased drug release was observed due to microbial degradation or polymer solubilisation. The release of the drug was greatly affected by the nature of the polymer used and the enteric coating of the tablet. | [102] |
| 7. | XG and Chitosan | Polyelectrolyte hydrogel | Ciprofloxacin | Increased drug encapsulation with an increase in drug concentration was observed, and it followed zero-order drug release kinetics. The drug-loaded hydrogel inhibited the growth of bacteria and showed no effect against fungi. | [103] |
| 8. | XG | Microspheres | Ciprofloxacin | The in-vitro drug release was carried out in both acidic as well as alkaline mediums; the release pattern followed a non-Fickian trend. Based on the results, it was concluded that the synthesised formulation was suitable for the sustained release of ciprofloxacin. | [104] |
| 9. | XG | Hydrogel | Liranaftate | The microemulsion-based drug hydrogel showed increased antifungal activity in comparison to plain drug solution against Candida albicans. The microemulsion proved to increase the skin retention and permeability of the drug. | [105] |
| 10. | XG, HPMC K15, and HPMC K100M | Floating low-density tablet | Stavudine | An increase in the half-life of stavudine in the gastric fluid was noted. Sustained release of the antiviral drug stavudine was achieved with all the pre-compression and post-compression characteristics. | [46] |
| 11. | XG with magnetic nanoparticles (Fe3O4) | Hydrogel | Terbinafine | The hydrogel shows thermally induced controlled drug delivery (3-fold) by magnetic hyperthermia and high efficacy compared to the pure drug for antifungal properties. The incorporation of Fe3O4 nanoparticles helps the hydrogel function as non-invasive monitoring by magnetic resonance imaging (MRI). | [47] |
| 12. | XG, poly(-vinyl alcohol), sodium alginate | Interpenetrating polymer network microbeads | Norfloxacin | Hydrogel showed high encapsulation efficiency. The microbeads had mucoadhesive properties at physiological pH, which was assessed with the use of a caprine intestine. The system showed pH-dependent drug release; it fitted the Higuchi and Korsemeyer–Peppas models. | [106] |
| 13. | XG and Chitosan | Hydrogel | Acyclovir | The drug-loaded formulation showed pH-dependent drug release and swelling behaviour; it fitted the Higuchi and Korsemeyer–Peppas models. It was observed that the hydrogel had a porous structure and was thermally stable. | [49] |
| 14. | XG and Chitosan | Hydrogel scaffold | Ampicillin, Rifampicin, and Minocycline. | Hydrogel showed good swelling and porosity. The in vitro drug release study of all the antibiotics showed fast drug release within 24 h in simulated gastric fluid. The gels prepared were stated to be non-toxic after the in vitro cell cytocompatibility test. Antibacterial activity of the loaded formulation was evaluated. | [107] |
| 15. | XG | Solution | Linezolid | The drug-loaded formulation was analysed against Staphylococcus aureus infections in the ocular tissues. The in vitro antibacterial activity and in vivo evaluation of drug efficacy showed that the linezolid-loaded XG exhibits better topical instillation and ocular permeation with bioadhesion to the precorneal membrane. | [108] |
| Delivery of chemotherapeutic drugs | |||||
| 1. | XG | Microspheres and Hydrogels | α-linolenic acid and Docosahexaenoic acid | The antioxidant activity and anti-neoplastic activity of α-linolenic acid were increased when loaded into the XG-based nanoformulations whereas the activity of docosahexaenoic acid remained the same when treated against colon cancer. | [50] |
| 2. | XG-chitosan | Nanofibers | Curcumin | The developed nanofibers were observed to be stable at pH 6.5 and pH 7.4. The drug-loaded formulation was tested on the colon cancer cell line; it was observed that the loaded polyphenol showed more efficacy than the pure drug. The permeation of the drug was increased by 3.4-fold. | [53] |
| 3. | XG | pH-responsive nanoparticles | Doxorubicin | The nanoparticles showed high drug loading and pH-sensitive drug release. The drug-loaded formulation was biocompatible. | [54] |
| 4. | XG | Nanogels | Doxorubicin | Nanogels were also nontoxic and biocompatible proving to be an ideal carrier for the delivery of chemotherapeutic drugs. | [55] |
| 5. | XG coated Gold nanoparticles | - | Doxorubicin | XG is used as a stabilising and reducing agent. In vitro studies showed that nanoparticles coated with XG showed a 3-fold increase in the efficacy of the drug against the A549 cell line. | [56] |
| 6. | Carboxymethyl XG coated Gold nanoparticles | - | - | Modified XG was used as a stabilising agent. The release of the drug was extensive at acidic pH and physiological pH. In-vitro studies of drug-loaded nanoparticles showed a 4.6-fold increase in the anticancer activity of the drug compared to pure drug. | [57] |
| 7. | XG-poly(-vinylpyrrolidone-co-poly)-acrylic acid | pH-responsive hydrogel | 5-fluorouracil | Controlled release of the drug was observed. It also showed stability in a wide range of pH media and maximum swelling in basic medium confirming its use as a pH-responsive hydrogel. In vivo studies showed acute oral toxicity studies were performed. | [52] |
| 8. | XG and guar gum | Nanoparticles | 5-fluorouracil | In vitro and in vivo studies showed intact microflora of the intestine, which fulfils the aim of the research of overcoming the side effects of 5-fluorouracil by co-administration of probiotics (B. bifidum) | [51] |
| 9. | XG | Thermoreversible gel | Ondansetron hydrochloride | The addition of XG increased the viscosity of the gel, thus decreasing the release of the drug and achieving sustained drug release. The gel was stable at room temperature and freeze temperature for a period of a month. | [109] |
| Delivery of anti-diabetic drugs | |||||
| 1. | XG | Topical nanoemulgel | Glimepiride | XG (3% w/w) was used as a gelling agent. The formulation significantly increased the permeation and bioavailability of the drug. | [62] |
| 2. | XG | Mouth dissolving films | Glibenclamide | The films were formed for the rapid release of the drug and to increase its bioavailability in the body. Stability studies proved the formulation was stable even in extreme conditions. | [37] |
| 3. | XG and HPMC K100M | Mucoadhesive buccal tablet | Repaglinide | The drug release was directly proportional to the concentration of both the polymers used. The stability studies showed that the tablets were stable for up to 2 months without any decrease in the drug content. | [63] |
| 4. | XG stabilised gold nanoparticles | Tolbutamide | The loaded formulation showed high loading efficiency without changing the structure of the drug. Drug-loaded formulation was investigated for its insulin secretion in mice models; it enhanced insulin secretion when compared to the plain drug solution. | [64] | |
| Delivery of drugs for the treatment of cardiovascular disease | |||||
| 1. | XG, HPMC, and corn starch | Matrix | Verapamil hydrochloride | XG-based formulation significantly provided sustained release of the drug. The drug release was dependent upon the concentration of the polymer. | [65] |
| 2. | XG and Chitosan | Tablets | Propranolol hydrochloride | The mixture of both polymers prolonged the time of drug release from the matrix at pH 1.2. Three layered polymer tablet pH-responsive was formed, with an increase in the barrier/middle layer of the matrix, and the lag in the release also increased. | [66] |
| 3. | XG, HPMC K4M, and sodium alginate | Transdermal patches | Carvedilol | The XG-based formulation showed drug release within 12 h. It was stable in various physicochemical parameters and ideal for the delivery of carvedilol. | [67] |
| 4. | XG and poly(-vinyl alcohol) | Membranes | Diltiazem HCl | Mechanical strength, physical properties, and drug release were observed to be dependent on the molecular weight of the polymers used for the preparation of membranes. | [68] |
| Anti-spasmodic drug delivery | |||||
| 1. | XG and sodium alginate | Floating tablets | Fenoverine | The formulation with 15% XG was ideal, with 99.6% drug release in 12 h. It also showed an improved swelling ratio with required drug release kinetics and floating behaviour. | [72] |
| 2. | XG | Matrix Tablet | Mebeverine HCl | XG can hydrate more rapidly than the other three gums used. The resulting drug diffusional path length for XG was the longest. The in vitro drug release studies revealed that the level of the polymer in the matrix tablets played an important role in the modulation of drug release. | [110] |
| Delivery of drugs for the treatment of inflammation, rheumatoid arthritis, and gout | |||||
| 1. | XG and graphene oxide | Hydrogel | Diclofenac potassium | The swelling ratio and drug release certainly increased with the increase in pH. The drug-loaded composite hydrogel was pH-responsive. In vivo studies concluded the bioavailability of the drug increased with an increase in the concentration of the composite hydrogel. | [75] |
| 2. | Carboxylated XG | Hydrogels | Diclofenac | An increase in viscoelasticity was observed due to the presence of XG in the system. The encapsulation efficiency of the drug was increased and the sustained release of the drug was due to the presence of nanotubes. The composite was stable in extreme conditions similar to that may be present on the skin. | [76] |
| 3. | Thiolated XG | Topical gel | Lornoxicam | The physicochemical properties of the gel formed were ideal. The high drug content in the thiolated gel was observed. In-vitro drug release studies showed sustained release up to 24 h. The thiolated gel was ideal for topical drug delivery for the treatment of inflammation and pain. | [33] |
| 4. | XG and Vigna mungo | Mucoadhesive bilayer buccal tablets | Febuxostat | The tablets were used to increase the bioavailability of febuxostat at the mucosal site for a prolonged time. The sustained release of the drug occurred for 8 h. The tablets were stable at room temperature and showed good mucoadhesive properties. | [77] |
| 5. | XG and chitosan | Microparticles | Quercetin | The microparticles were loaded with quercetin and the release was controlled by non-Fickian diffusion of the drug. The microparticles were compressed and converted into tablets with a coating of Eudragit. | [78] |
| 6. | XG and ethyl cellulose | Matrix tablet | Metoclopramide | Pre-compression and post-compression characteristics of the tablets were found to be ideal. The tablets prepared using XG and XG-ethyl cellulose both showed sustained drug release for up to 12 h. | [79] |
| 7. | Polyacrylamide-grafted-XG | pH-responsive nanoparticles | Curcumin | In an alkaline medium, the drug release was excellent, with the presence of rat caecal suggesting microflora-dependent drug release. In vivo studies suggested that the drug-loaded nanoparticles showed better anti-inflammatory activity when compared to the pure drug. | [80] |
| Delivery of immunosuppressive drugs | |||||
| 1. | XG, Carbopol and gellan gum | In-situ ocular gel | Ketotifen fumarate | pH-sensitive ocular gels were prepared for better efficacy than the formulation present in the market. Different concentrations of polymers: xanthan gum, Carbopol®, and gellan gum were taken to form different formulations. Among the optimised formulations, formulation with 0.75% Carbopol and 0.3% xanthan gum was found to be the best. It had a drug encapsulation efficiency of 99.74%, gel-forming strength in 46.6 s, and drug release of up to 8 h. | [83] |
| 2. | XG and sodium alginate | Microbeads | Tacrolimus | The sodium alginate was taken as the main polymer but to increase the drug-loading efficiency of microbeads, xanthan gum was added to the formulation. Using different concentrations of crosslinkers also affects drug release. Also, the drug release is dependent on the concentration of the polymer and coating polymer. It was suggested that this system could be used as an alternative and cost-effective system for the delivery of drugs. | [85] |
| Delivery of drugs for the treatment of skin diseases | |||||
| Sodium alginate and XG | Bioadhesive film | Pycnogenol | The film showed suitable mechanical properties such as high deformability, suggesting easy adaptability to any type of surface. The film shows high bioadhesion on the skin and absorption of exudates from the open wound. The drug-loaded films can inhibit the growth of bacterial strains. | [87] | |
| Delivery of drugs for the treatment of central nervous system-related disorders | |||||
| 1. | XG, Carbopol 934P, HPMC K4M, and polyvinyl pyrrolidone K30 | Mucoadhesive matrix tablet | Quetiapine fumarate | Sustained release (96%) was obtained for a prolonged time (10 h). The permeation in the mucus membrane was 77%. | [89] |
| 2. | XG and tragacanth | Tablets | XG exhibited a swelling mechanism for the controlled release of the drug. The binary tablets formed with XG showed pH-responsive drug release, fitting Higuchi and Korsmeyer–Peppas models. | [90] | |
| 3. | XG, guar gum, karaya gum | Oral tablets | Zolmitriptan | Formulations containing xanthan gum showed an increase in sustained release of the drug with the increase in the concentration of polymer. | [91] |
| 4. | xanthan gum and gellan gum | In-situ gel | Resveratrol | The optimisation of in situ gel resulted in 5-fold higher permeation in the nasal mucosa when compared to pure drug suspension-based gel. The drug-loaded formulation was evaluated using the scopolamine-induced amnesia model in mice. Improvement in memory function was observed. | [92] |
| Delivery of drugs for the treatment of obesity | |||||
| Xanthan gum | Mini-tablets | Orlistat | The mini-tablets formed using XG as an oil-reducing agent might help in the development of many other delivery systems for anti-obesity drugs for reducing their side effects. | [93] | |
| Delivery of drugs for the treatment of glaucoma | |||||
| 1. | Pure gellan gum and in combination with HPMC, XG, and Carbopol | pH-responsive in situ gel | Acetazolamide | A combination of XG with gellan gum proved to be the best formulation among all, increasing mucoadhesive properties and a relative decrease in ocular pressure when compared to commercial oral tablets and eye drops. | [94] |
| 2. | XG and poly acrylic acid | Mucoadhesive in situ gel | Dorzolamide hydrochloride | Formulation with 0.2% w/v XG showed 90.84% of drug release for 12 h. The drug release was also analysed using goat’s cornea; formulation showed similar release kinetics to the in vitro release for up to 9 h. | [96] |
| Delivery of drugs for the treatment of pulmonary diseases | |||||
| 1. | Sodium carboxymethyl XG and casein crosslinked with aluminium chloride and glutaraldehyde | Interpenetrating polymer network-based hydrogel beads | Theophylline | Dissolution studies and drug release carried out in both acidic and alkaline mediums delayed release of the drug was observed. | [98] |
| 2. | XG | Mini-tablets and matrix tablets. | Theophylline | It increased the solubility of the drug by 2.8 times in 40% alcohol. At lower concentrations of XG with large particles, the drug release was observed to be faster when compared to the higher concentration of polymer with finer particles (<75 µm) in dissolution studies. | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402. https://doi.org/10.3390/pharmaceutics15020402
Jadav M, Pooja D, Adams DJ, Kulhari H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics. 2023; 15(2):402. https://doi.org/10.3390/pharmaceutics15020402
Chicago/Turabian StyleJadav, Mahima, Deep Pooja, David J. Adams, and Hitesh Kulhari. 2023. "Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents" Pharmaceutics 15, no. 2: 402. https://doi.org/10.3390/pharmaceutics15020402
APA StyleJadav, M., Pooja, D., Adams, D. J., & Kulhari, H. (2023). Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics, 15(2), 402. https://doi.org/10.3390/pharmaceutics15020402








